Abstract
There is substantial evidence that Kaposi sarcoma–associated herpesvirus (KSHV) plays an important role in the pathogenesis of all forms of Kaposi sarcoma (KS). It has been noted that KS commonly occurs in locations, such as the feet, where tissue may be poorly oxygenated. On the basis of this observation, the potential role of hypoxia in the reactivation of KSHV replication was explored by studying 2 KSHV-infected primary effusion lymphoma B-cell lines (BC-3 and BCBL-1) latently infected with KSHV. Acute and chronic exposure of these cells to hypoxia (1% O2) induced KSHV lytic replication, as indicated by an increase in intracellular lytic protein expression and detection of virus in cell supernatants by Western immunoblotting. In addition, hypoxia increased the levels of secreted viral interleukin-6. Moreover, hypoxia enhanced the lytic replication initiated by the viral inducer 12-O-tetradecanoylphorbol-13-acetate. Desferoxamine and cobalt chloride, 2 compounds that increase the intracellular levels of hypoxia-inducible factor 1, were also able to induce KSHV lytic replication. These studies suggest that hypoxia is an inducer of KSHV replication. This process may play an important role in the pathogenesis of KS.
Introduction
Kaposi sarcoma (KS) is a multifocal proliferative disease of vascular origin. KS lesions are characterized by proliferation and growth of spindle-shaped neoplastic cells that appear to be of lymphatic endothelial cell origin.1-3 KS is the most common neoplasm in human immunodeficiency virus type 1–infected individuals.1 Even before the advent of acquired immunodeficiency syndrome (AIDS), KS was known to be more prevalent in certain geographic areas and groups of patients (classic and endemic KS). A classic form of KS is described as occurring in elderly men in southern Italy and other Mediterranean countries,4 and an endemic form of KS has been observed to occur in sub-Saharan Africa.5,6 In addition, transplant recipients and other immunosuppressed patients are at risk for developing KS.4
Since the discovery of Kaposi sarcoma–associated herpesvirus (KSHV), also called human herpesvirus-8 (HHV-8), in 1994,7substantial evidence has been accumulated that implicates it as an essential factor in the pathogenesis of all forms of KS.8,9 It is also involved in the pathogenesis of primary effusion lymphoma (PEL) and certain cases of multicentric Castleman disease.10 KSHV DNA is detected in nearly all KS lesions.7,11,12 As with other human herpesviruses, infection with KSHV can be latent or lytic. Activation of viral replication in latently infected B cells, endothelial cells, or other target cells may be responsible for viral spread and contribute to the development of KS.4 KSHV encodes for several cellular gene mimics that are produced during lytic replication and that have proangiogenic activity.13-16 The virus also encodes several cellular protein mimics with oncogenic potential, such asv-cyc, a D-type cyclin, and a member of the interferon regulatory factor family.16 The production of these viral factors during KSHV lytic replication may contribute to the development of KS. Cells latently infected with KSHV can be activated to undergo lytic replication by phorbol esters. However, relatively little is presently known about the physiologic signals that serve to activate KSHV in infected patients.
In his original description of KS in 1872, Moritz Kaposi noted that the painful edema and characteristic lesions of the condition occurred primarily on the feet of his patients, and we have been struck by a predilection of KS to involve the feet in both classic and AIDS-associated forms.17 The lower extremities often have relatively low tissue oxygen (O2) concentrations,18 and we hypothesized that such hypoxic conditions might reactivate KSHV and lead to the subsequent production of proangiogenic factors, thus favoring the development of KS. In this report, we investigated a possible role for hypoxia in the re-activation of KSHV in 2 KSHV-infected PEL cell lines.
Materials and methods
Cell culture at 21% and 1% O2
The KSHV-positive and Epstein-Barr virus–negative PEL cell lines BCBL-119 (National Institutes of Health AIDS Research and Reagent Program, Rockville, MD) and BC-320(American Type Culture Collection, Rockville MD) were grown in complete medium (RPMI 1640 medium containing 15% fetal calf serum, 2 mm/L glutamine, 50 U/mL penicillin, and 50 μg/mL streptomycin; all from Gibco BRL [Gaithersburg, MD]). Cells were cultured in either a standard incubator in atmospheric O2or an incubator at 1% O2 by means of the Proox model 110 O2 controller (which controls O2 levels from 1% to 99%) (Reming Bioinstruments, Redfield, NY). The CO2was maintained at 5% in both chambers, and the air in the low-O2 incubator was displaced with nitrogen and continually monitored to maintain 1% O2. Cells were induced with 12-O-tetradecanoylphorbol-13-acetate (TPA) (Sigma, St Louis, MO). Two other compounds tested as inducers of KSHV lytic replication, desferoxamine mesylate (Dfx) (10 mM and 5 mM) (Sigma) and cobalt chloride (CoCl2) (100 mM and 5 mM) (Sigma), were dissolved in phosphate-buffered salined (PBS), filter-sterilized, and stored as stock solutions at −20°C until use.
Analysis of KSHV lytic protein expression by flow cytometry
Cells were seeded at 300 000 viable cells per milliliter and incubated for 2 to 3 days as indicated. When inducers were used, cells were incubated overnight, followed by the addition of the inducer, and incubated for another 2 to 3 days under the same conditions. The intracellular expression of processivity factor 8 (PF-8, an early-lytic protein) and glycoprotein 8.1 (gpK8.1, a late-lytic protein) in the viable cells was then determined by flow cytometric analysis as described.21
Western blot analysis for the viral minor capsid protein
The level of virus in the cellular supernatants of KSHV-infected cells was determined by means of a peptide affinity–purified rabbit polyclonal antibody directed toward a 17–amino acid peptide of the open reading frame 26 (ORF 26) minor capsid protein (mCP) of KSHV as described previously.22 To determine if we could use this assay to quantitatively measure the level of virus in the media, we measured the mCP band intensity for increasing concentrations of sucrose-purified KSHV (ABI, Columbia, MD) by Western blot. We found that the mCP band intensity, as determined by means of Un-Scan-It (Silk Scientific, Orem, UT), was directly proportional to the amount of virus loaded from 1 μg (equivalent to 3.3 × 107 viral particles) to 16 μg of viral lysate protein loaded. Cell culture samples were first centrifuged (10 minutes at 1000g) to remove the cells. The supernatant was ultracentrifuged in 1 to 1.5 mL aliquots at 100 000g for 35 minutes at 4°C. The pelleted material was washed with 1 mL cold PBS, pelleted as above, and then resuspended in sodium dodecyl sulfate sample buffer (Novex, San Diego, CA) and boiled at 70°C for 10 minutes. Samples that represented equal volumes of pelleted supernatant were electrophoresed on 10% or on 4% to 12% bis-Tris polyacrylamide gels with 2-(N-morphilino) ethanesulfonic acid running buffer, by means of the NuPage system (Novex). Proteins were electroblotted onto nitrocellulose, and the mCP was detected with a peptide affinity–purified rabbit antibody as previously described.22 Briefly, blots were incubated with anti-mCP rabbit antibody for 2 hours at room temperature and then for 30 minutes with an antirabbit secondary antibody conjugated to alkaline phosphatase. Bands were detected by means of the Western Blue–stabilized substrate for alkaline phosphatase (Promega, Madison, WI). The band was identified by comparison with a viral standard (ABI) containing the mCP band that migrated to an approximate molecular mass of 34 kd as determined by comparison with the migration positions of SeeBlue-prestained markers (Novex). Immunoblots were scanned, and densitometry analyses of the scanned images were performed.
Enzyme-linked immunosorbent assays for viral interleukin 6 and vascular endothelial growth factor
Levels of viral interleukin 6 (vIL-6) in culture supernatants were determined by means of a specific enzyme-linked immunosorbent assay as previously described.23 Briefly, polystyrene plates (Immunol 1B; Dynex Technologies, Chantilly, VA) were coated with mouse monoclonal antivIL-6 antibody (v6m12.1.1, 4 μg/mL in carbonate buffer, pH 9) overnight at 4°C. After washing the plates with PBS containing 0.05% Tween 20 (PBS-T) and blocking with SuperBlock (Pierce, Rockford, IL), test samples were added in duplicate to the wells. These samples were diluted in advance with the same volume of PBS-T containing 10% fetal bovine serum (FBS); final concentration of FBS was 10% for both samples and standards. Plates were incubated overnight at room temperature and washed with PBS-T. Maltose-binding protein (MBP)–absorbed rabbit polyclonal anti–vIL-6 antibody (0.5 μg/mL) was added to the wells in PBS-T containing 0.5% bovine serum albumin (BSA) (PBS-T/BSA). Plates were incubated for 2 hours at room temperature and washed. Affinity-purified human serum protein–absorbed goat antirabbit immunoglobulin G antibody conjugated to alkaline phosphatase (Sigma) (1:400 dilution in PBS-T/BSA) was added. Plates were incubated at room temperature for 1.5 hours and washed with PBS-T. A p-nitrophenolphosphate substrate (Sigma) was added to the wells, and the plates were read at 405 nm with λ correction at 595 nm. A purified fusion protein of MBP (42.7 kd) and amino acids 22 through 204 of vIL-6 (21.6 kd) were used as standards. The concentration of vIL-6 was calculated from absorbance values in relation to the standard curve, corrected for the presence of the MBP fusion protein in the vIL-6 standard (vIL-6 corresponds to 33.6% of MBP vIL-6). The assay sensitivity is approximately 30 pg/mL for vIL-6. Vascular endothelial growth factor (VEGF) was determined in the supernatant of cells by means of the Human VEGF kit from Accucyte (College Park, MD) with a dynamic range of 0.195 to 50 ng/mL VEGF.
Statistical analysis
The exact Wilcoxon rank sum test was used to compare expression of KSHV-encoded antigens in cells cultured under normoxia and cells cultured under chronic hypoxia. The Wilcoxon signed rank test was used to compare parallel cultures established under normoxia and acute hypoxia at the same time.
Results
Hypoxia increases KSHV lytic protein expression in KSHV-infected PEL B-cell lines
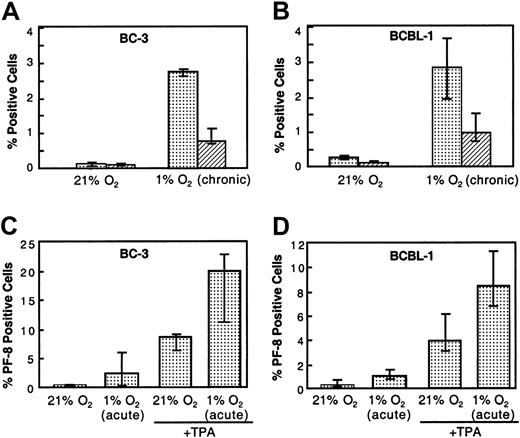
BC-3 and BCBL-1 PEL B-cell lines were used to study the role of hypoxia in KSHV reactivation. Viral reactivation was determined, in part, by studying the expression of 2 lytic proteins, PF-8 and gpK8.1, by flow cytometry. In normoxia (21% O2), the percentage of viable cells expressing the early-lytic protein PF-8 was very low: 0.12% and 0.23% for BC-3 and BCBL-1 cells, respectively. Also, the median percentage of cells expressing the late-lytic protein gpK8.1 was very low, 0.04% and 0.09% for BC-3 and BCBL-1 cells, respectively (Figure 1). However, when BC-3 cells were maintained under chronic hypoxic conditions (1% O2 for more than 3 cell passages), the median percentage of viable BC-3 cells expressing PF-8 increased to 2.7%, a 22-fold increase (P = .012) (Figure 1A). A similar increase in PF-8 expression from 0.23% to 2.86% was seen in BCBL-1 cells, representing a 12-fold increase (P = .004) (Figure 1B). Late-lytic gpK8.1 protein expression was also greater under hypoxic conditions, with BC-3 cells having a median increase from 0.04% to 0.55% (13-fold increase) (P = .016) and BCBL-1 cells having a median increase from 0.09% to 0.94% (10-fold increase) (P = .028) (Figure 1A,B). It was observed throughout these experiments that the cells grown in chronic hypoxia had a somewhat lower cell growth rate and viability. This decrease in viability seen in chronic hypoxia ranged from 10% to 25% compared with cells grown in normoxia.
Effect of chronic hypoxia (1% O2), acute hypoxia (1% O2), and acute hypoxia plus TPA on KSHV intracellular lytic protein expression in BC-3 and BCBL-1 cells 48 to 72 hours after treatment.
Cells were seeded at 300 000 viable cells per milliliter. (A, B) Cells were maintained in either 21% O2 or 1% O2 for at least 3 passages before being used in experiments (chronic exposure). The plotted values for PF-8 (░) and gpK8.1 (▨) expression represent the median of at least 3 independent experiments ± the 25% and 75% quartiles. Exact Wilcoxon rank sum test (unpaired) for panel A: (BC-3, PF-8 21% O2 vs 1% O2) p2 =0 .0012, and (BC-3, gpK8.1 21% O2 vs 1% O2) p2 = 0.016. Exact Wilcoxon rank sum test (unpaired) for panel B: (BCBl-1, PF-8 21% O2 vs 1% O2) p2 = 0.004, and (BCBL-1, gpK8.1 21% O2 vs 1% O2)p2 = 0.028. (C, D) Cells grown in normoxia were incubated in 21% O2 or 1% O2 overnight. They were then exposed to TPA (6.4 nM) or to media control and returned to their respective conditions. The plotted values for PF-8 expression is the median percentage of positive cells for 6 independent experiments ± the 25% and 75% quartiles. Wilcoxon signed rank test (paired) for panel C: (BC-3, PF-8 21% O2 vs 1% O2)p2 = 0.031, and (BC-3/TPA, PF-8 21% O2 vs 1% O2) p2 = 0.031. Wilcoxon signed rank test (paired) for panel D: (BCBL-1, PF-8 21% O2 vs 1% O2) p2 = 0.031, and (BCBL-1/TPA, PF-8 21% O2 vs 1% O2) p2 = 0.031.
Effect of chronic hypoxia (1% O2), acute hypoxia (1% O2), and acute hypoxia plus TPA on KSHV intracellular lytic protein expression in BC-3 and BCBL-1 cells 48 to 72 hours after treatment.
Cells were seeded at 300 000 viable cells per milliliter. (A, B) Cells were maintained in either 21% O2 or 1% O2 for at least 3 passages before being used in experiments (chronic exposure). The plotted values for PF-8 (░) and gpK8.1 (▨) expression represent the median of at least 3 independent experiments ± the 25% and 75% quartiles. Exact Wilcoxon rank sum test (unpaired) for panel A: (BC-3, PF-8 21% O2 vs 1% O2) p2 =0 .0012, and (BC-3, gpK8.1 21% O2 vs 1% O2) p2 = 0.016. Exact Wilcoxon rank sum test (unpaired) for panel B: (BCBl-1, PF-8 21% O2 vs 1% O2) p2 = 0.004, and (BCBL-1, gpK8.1 21% O2 vs 1% O2)p2 = 0.028. (C, D) Cells grown in normoxia were incubated in 21% O2 or 1% O2 overnight. They were then exposed to TPA (6.4 nM) or to media control and returned to their respective conditions. The plotted values for PF-8 expression is the median percentage of positive cells for 6 independent experiments ± the 25% and 75% quartiles. Wilcoxon signed rank test (paired) for panel C: (BC-3, PF-8 21% O2 vs 1% O2)p2 = 0.031, and (BC-3/TPA, PF-8 21% O2 vs 1% O2) p2 = 0.031. Wilcoxon signed rank test (paired) for panel D: (BCBL-1, PF-8 21% O2 vs 1% O2) p2 = 0.031, and (BCBL-1/TPA, PF-8 21% O2 vs 1% O2) p2 = 0.031.
The effect of acute hypoxia on KSHV lytic replication was also investigated. An increase in the expression of both lytic proteins, similar to that observed in chronic hypoxia, occurred in the B-cell lines when acutely exposed to hypoxia (Figure 1C,D). However, the extent of the increase in PF-8 expression as a result of acute hypoxic exposure varied somewhat from experiment to experiment, ranging from a 7.7- to a 34-fold induction in BC-3 (median increase, 9.7-fold) (Figure1C,D). Similarly, there was a 3- to 12-fold induction of gpK8.1 (median increase, 6-fold) in BC-3 cells (not shown). Acute hypoxia also increased PF-8 expression in BCBL-1 cells, but to a lesser degree than that seen for BC-3 cells (Figure 1C,D).
Hypoxia enhances TPA induction of KSHV lytic replication
TPA is an inducer of KSHV lytic replication in PEL cell lines infected with KSHV.19,24 It was previously shown that the maximum percentages of cells that could be induced to undergo lytic replication (based on PF-8 expression quantitated by flow cytometry) by 30 nM TPA 3 days after treatment in BCBL-1 and BC-3 cells were approximately 12% and 20%, respectively.21 We were interested in determining whether hypoxia would affect lytic induction by TPA in the 2 cell lines used. When cells exposed to chronic hypoxia were treated with 30 nM TPA, substantial toxicity (less than 40% viability) occurred in both of the cell lines. A viability of less than 40% was our cutoff for assaying the level of lytic protein expression, and protein expression was therefore not determined for this concentration of TPA. Therefore, the effect of chronic hypoxia on viral reactivation by TPA was assessed at lower TPA concentrations. When cells were grown in normoxia and treated with a suboptimal concentration of TPA (6.4 nM), the median percentage of cells expressing PF-8 was 8.6% for BC-3 and 3.9% for BCBL-1 cells as determined by flow cytometry (Figure 1C,D). However, the median level of PF-8 expression more than doubled when these TPA-treated cells were exposed to acute hypoxia, resulting in 10.3% expression for BCBL-1 cells and 20.0% expression for BC-3 cells (Figure 1C,D).
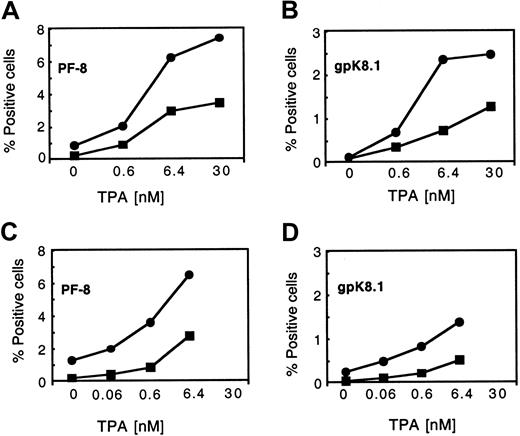
We then assessed the effect of both acute and chronic hypoxia on the lytic protein expression induced by various doses of TPA. As expected, TPA increased PF-8 and gpK8.1 expression in a dose-dependent manner 2 days after treatment in normoxic conditions (Figure2). However, the level of lytic protein expression in response to TPA was significantly increased in cells exposed to both acute and chronic hypoxia (Figure 2). Acute hypoxia was more effective than chronic hypoxia at enhancing the induction of PF-8 and gpK8.1 expression in the presence of TPA for both PEL cell lines.
Dose-dependent expression of PF-8 and gpK8.1 in TPA-stimulated BCBL-1 cells in normoxia and hypoxia (acute and chronic exposure).
BCBL-1 cells that had been cultured in normoxia (21% O2) or hypoxia (1% O2) were seeded at 300 000 viable cells per milliliter and incubated overnight at the indicated conditions. Following the overnight incubation, the cells were treated with increasing concentrations of TPA and maintained at the indicated conditions following TPA treatment for 48 hours. In each panel, the values represent 1 of 2 similar experiments. (A) PF-8 expression in BCBL-1 cells in normoxia (▪) or acute hypoxia (●). (B) gpK8.1 expression in BCBL-1 cells in normoxia (▪) or acute hypoxia (●). (C) PF-8 expression in BCBL-1 cells in normoxia (▪) or chronic hypoxia (●). (D) gpK8.1 expression in BCBL-1 cells in normoxia (▪) or chronic hypoxia (●).
Dose-dependent expression of PF-8 and gpK8.1 in TPA-stimulated BCBL-1 cells in normoxia and hypoxia (acute and chronic exposure).
BCBL-1 cells that had been cultured in normoxia (21% O2) or hypoxia (1% O2) were seeded at 300 000 viable cells per milliliter and incubated overnight at the indicated conditions. Following the overnight incubation, the cells were treated with increasing concentrations of TPA and maintained at the indicated conditions following TPA treatment for 48 hours. In each panel, the values represent 1 of 2 similar experiments. (A) PF-8 expression in BCBL-1 cells in normoxia (▪) or acute hypoxia (●). (B) gpK8.1 expression in BCBL-1 cells in normoxia (▪) or acute hypoxia (●). (C) PF-8 expression in BCBL-1 cells in normoxia (▪) or chronic hypoxia (●). (D) gpK8.1 expression in BCBL-1 cells in normoxia (▪) or chronic hypoxia (●).
Detection of KSHV mCP under normoxic and hypoxic conditions
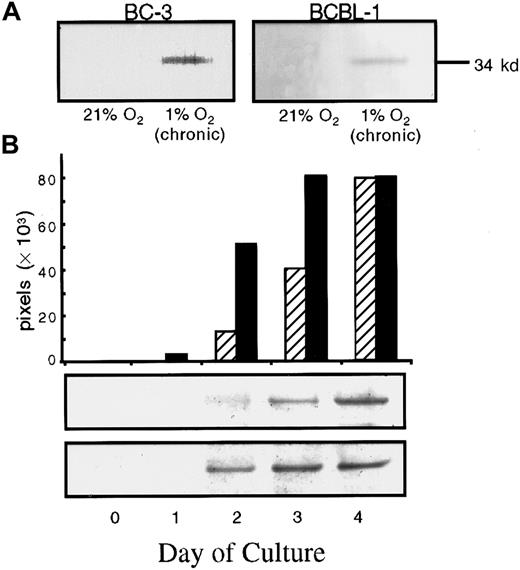
To determine whether exposure to 1% O2(hypoxia) also affected virus production from KSHV-infected cells, we assayed for mCP in culture supernatants under these conditions. Under normoxic conditions, we could not detect mCP in pellets from 1 to 1.5 mL supernatants from BCBL-1 or BC-3 cells. However, mCP was detected in supernatants from BCBL-1 and BC-3 cells exposed to chronic hypoxia (Figure 3A). BCBL-1 cells cultured in 5% O2 also produced increased levels of mCP, although to a lesser degree than that seen at 1% O2 (data not shown). In addition, BCBL-1 and BC-3 cells exposed to a suboptimal concentration of TPA produced higher levels of mCP when exposed to acute hypoxia (Figure 3B). A low level of the mCP was detected 2 days after 6.4-nM TPA treatment in the supernatant of cells grown in 21% O2, and the level further increased over the next 2 days, reaching a maximum on day 4 (Figure 3B). When cells were exposed to acute hypoxia and 6.4 nM TPA, there was a 5-fold increase in the level of mCP observed 2 days after transfer to hypoxic conditions, as compared with TPA-treated cells in normoxia. In addition, when cells were exposed to hypoxia, the level of mCP reached a maximum a day earlier than for cells in normoxia (Figure 3B). Qualitatively similar results were obtained with BC-3 cells (data not shown). We also performed a TPA dose-response experiment to determine the effect of chronic hypoxia on TPA induction of virus. Again, the cells grown in chronic hypoxia produced detectable mCP even in the absence of TPA induction; those grown in normoxia did not. TPA treatment at all concentrations further increased mCP production in cells exposed to hypoxia; cells exposed to normoxia produced measurable virus only at the 2 highest TPA concentrations (6.4 and 30 nM) (results not shown).
Western blot detection of the mCP of KSHV from supernatants of KSHV-infected cells in normoxia (21% O2) and hypoxia (1% O2) in the absence or presence of TPA induction.
(A) 96-hour supernatants were collected from BC-3 or BCBL-1 cells that had been cultured for at least 3 passages at 21% O2 or 1% O2, washed, seeded at 300 000 cells per milliliter, and returned to their respective conditions. For BC-3, the band represents approximately 5.5 × 107 viral particles per milliliter of supernatant. For BCBL-1, the band represents approximately 10 × 107 viral particles per milliliter. These values are based on comparison with a viral standard electrophoresed on the same blot. (B) BCBL-1 cells cultured at 300 000 cells per milliliter in normoxia (21% O2) or acute hypoxia (1% O2) were then treated with 6.4 nM TPA. The time indicated represents the time following TPA addition after incubation of cells overnight. Each band was scanned, and the relative band intensity expressed as total pixels was determined and plotted for samples in normoxia (▨ and top band) and hypoxia (▪ and bottom band).
Western blot detection of the mCP of KSHV from supernatants of KSHV-infected cells in normoxia (21% O2) and hypoxia (1% O2) in the absence or presence of TPA induction.
(A) 96-hour supernatants were collected from BC-3 or BCBL-1 cells that had been cultured for at least 3 passages at 21% O2 or 1% O2, washed, seeded at 300 000 cells per milliliter, and returned to their respective conditions. For BC-3, the band represents approximately 5.5 × 107 viral particles per milliliter of supernatant. For BCBL-1, the band represents approximately 10 × 107 viral particles per milliliter. These values are based on comparison with a viral standard electrophoresed on the same blot. (B) BCBL-1 cells cultured at 300 000 cells per milliliter in normoxia (21% O2) or acute hypoxia (1% O2) were then treated with 6.4 nM TPA. The time indicated represents the time following TPA addition after incubation of cells overnight. Each band was scanned, and the relative band intensity expressed as total pixels was determined and plotted for samples in normoxia (▨ and top band) and hypoxia (▪ and bottom band).
Hypoxia is known to induce the production of VEGF in normal cells, and we considered whether VEGF production induced by hypoxia in the PEL cells might have acted in an autocrine manner to reactive KSHV replication. Consistent with a previous report,25 BC-3 cells produced substantial VEGF (1.43 ng/106 cells; 2.8 ng/mL media) after 3 days of culture under normoxic conditions. However, although there was a small increase in the VEGF produced per cell when these cells were exposed to 1% O2 (to 2.5 ng/106 cells), there was no net change in the VEGF released into the media (2.75 ng/mL media). Also, addition of VEGF at 10 or 100 ng/mL for 3 days had no substantial effect on the level of expression of PF-8 or gpK8.1 by BC-3 or BCBL-1 cells over that seen in control cells. Taken together, these results suggest that hypoxia-induced KSHV reactivation was not the result of an up-regulation of VEGF in these cells by hypoxia.
Effect of hypoxia on the induction of KSHV vIL-6 production
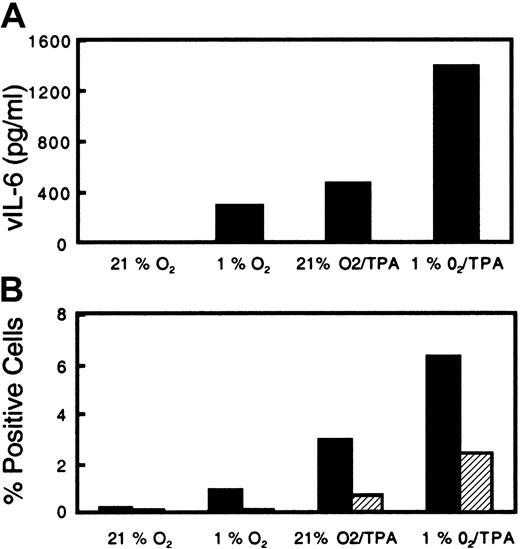
Viral IL-6 is a cytokine thought to play a role in the pathogenesis of KS by stimulating spindle cell growth and activating the production of angiogenic factors from these cells.15 26 Acute exposure of BCBL-1 cells to hypoxia alone was found to induce vIL-6 production approaching the levels induced with 6.4 nM TPA (Figure 4A). When cells were exposed to hypoxia and treated with 6.4 nM TPA, there was a further increase in vIL-6 production over that caused by each treatment alone (Figure 4A). The level of vIL-6 increased in parallel with the extent of lytic intracellular protein expression as determined by flow cytometry (Figure 4B). In 3 different experiments with BC-3 cells, the level of vIL-6 increased in response to acute hypoxia from an average of 245 pg/mL in normoxia to 611 pg/mL in hypoxia.
Effect of acute hypoxia and TPA on secreted vIL-6 production and intracellular PF-8 expression from KSHV-infected BCBL-1 cells.
BCBL-1 cells were seeded at 300 000 cells per milliliter and then incubated in normoxia or hypoxia (1% O2). Following incubation overnight, cells were either left untreated or treated with TPA (6.4 nM) and returned to their respective conditions. (A) After incubation for 72 hours, the cells were harvested and analyzed for vIL-6 in the supernatant. (B) PF-8 (▪) and gpK8.1 (▨) lytic protein expression in the viable cells.
Effect of acute hypoxia and TPA on secreted vIL-6 production and intracellular PF-8 expression from KSHV-infected BCBL-1 cells.
BCBL-1 cells were seeded at 300 000 cells per milliliter and then incubated in normoxia or hypoxia (1% O2). Following incubation overnight, cells were either left untreated or treated with TPA (6.4 nM) and returned to their respective conditions. (A) After incubation for 72 hours, the cells were harvested and analyzed for vIL-6 in the supernatant. (B) PF-8 (▪) and gpK8.1 (▨) lytic protein expression in the viable cells.
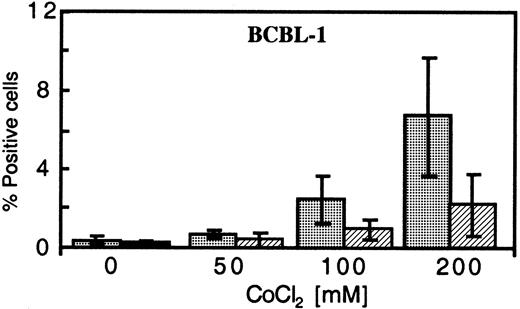
Effect of hypoxia-inducible-factor–inducing agents Dfx and CoCl2 on KSHV protein expression
One of the primary mediators of the response of cells to low O2 is hypoxia-inducible factor 1 alpha (HIF-1α). Under normal conditions, HIF-1α is rapidly degraded, but this process is inhibited under hypoxic conditions.27 To investigate the ability of increased HIF-1α to reactivate KSHV replication, we tested the effects of 2 commonly used inducers of HIF-1α, Dfx and CoCl2, on KSHV-infected cells.28 In a dose-dependent manner, CoCl2 treatment in normoxic conditions increased the percentage of BCBL-1 cells expressing PF-8 and gpK8.1 (Figure 5); at 200 μM CoCL2, the level of antigen expression was close to that obtained with cells exposed to 30 nM TPA21 (compare Figure2). CoCl2 also enhanced the lytic protein expression induced by 6.4 nM TPA up to 2-fold and increased vIL-6 levels in culture supernatants (data not shown). Although less effective than CoCl2, treatment of BCBL-1 cells with Dfx increased PF-8 protein expression from an average of 0.43% for untreated cells to 0.88% and 1.2% for concentrations of 5 μM and 10 μM, respectively. Concentrations above 200 μM CoCl2 and 20 μM Dfx led to substantial toxicity (less than 40% viability), and therefore samples were not measured for lytic protein expression.
Dose-dependent expression of PF-8 and gpK8.1 in CoCl2-treated BCBL-1 cells in normoxia.
BCBL-1 cells, treated with increasing concentrations of CoCl2 and harvested 72 hours after seeding, were analyzed for PF-8 (░) and gpK8.1 (▨) protein expression. The plotted values represent the mean percentage of positive cells ± SD of 3 experiments.
Dose-dependent expression of PF-8 and gpK8.1 in CoCl2-treated BCBL-1 cells in normoxia.
BCBL-1 cells, treated with increasing concentrations of CoCl2 and harvested 72 hours after seeding, were analyzed for PF-8 (░) and gpK8.1 (▨) protein expression. The plotted values represent the mean percentage of positive cells ± SD of 3 experiments.
Discussion
Although KSHV can be activated by phorbol esters in the laboratory, the physiologic stimuli involved in the reactivation of KSHV from its latent state in B cells, endothelial cells, and other target cells in the body are poorly understood. Our studies indicate that hypoxia (1% O2) can activate lytic replication of KSHV in 2 different PEL B-cell lines and increase the expression of the proangiogenic factor vIL-6. Some activation was observed with a higher O2 saturation (5% O2). An O2saturation as low as 2.5% can be found in certain tissues under physiological conditions,29 and a saturation of 1% or lower can be found under certain pathologic conditions,30,31 including vascular ischemia of the legs.32 This suggests that hypoxia-induced KSHV activation may be important in the life cycle of this virus and contribute to KS pathogenesis. In addition, we found that hypoxia potentiates TPA-induced KSHV activation, suggesting that this stimulus may work in concert with other inducers of KSHV replication. We have not determined if endothelial cells infected with HHV-8 would respond similarly to hypoxia, and therefore future studies on these cells will be required to determine the extent of involvement of hypoxia in HHV-8 pathogenesis.
Although the percentage of cells induced to undergo lytic replication by hypoxia is less than the maximal level induced by phorbol esters, it represents a median increase of up to 22-fold over the background replication obtained under normoxic conditions. It is worth noting that our data, as well as previous studies,21suggest that there may be a limit to the percentage of cells that can be activated into the lytic phase. It was previously reported that a maximum of approximately 15% live BCBL-1 cells can be induced into the lytic phase and that for BC-3 cells the maximum is closer to 20% to 25%. The reason a substantially higher percentage of live cells do not undergo lytic replication with hypoxia alone is unclear, but could be related to a cell-cycle–related restriction of KSHV reactivation. Also, although hypoxia enhanced TPA-induced replication, we found that combining a high level of TPA (eg, 30 nM) and hypoxia led to significant cellular toxicity. To what extent this represents direct cellular toxicity as a result of the combined effects of hypoxia and TPA, as opposed to cell death from increased KSHV lytic replication under these conditions, is not clear at this time.
Hypoxia induces a number of effects through an increase in the intracellular levels of HIF-1α. Under normoxic conditions, this protein is degraded by the ubiquitin proteosome system in conjunction with the von Hippel–Lindau gene product, but under hypoxic conditions, this process is inhibited.33,34 We hypothesized that increased levels of HIF-1α might mediate the reactivation of KSHV. This hypothesis was supported by our results showing that 2 different chemicals, Dfx and CoCl2, that are known to mimic hypoxia by raising levels of HIF-1α were both found to induce KSHV replication when added to cells grown under standard laboratory conditions. Moreover, the level of induction by CoCl2exceeded the level observed in cells exposed to 1% O2. It is known that HIF-1α reaches one half of maximal levels in cells exposed to 1% O2, with higher levels being reached as the O2 level is lowered below 1%.29 Thus, O2 levels below 1% might further increase the level of KSHV lytic induction. HIF-1α mediates cellular effects by binding to hypoxia-responsive elements (HREs) in the cellular genome. Analysis of the KSHV genome reveals the existence of several regions that contain the HRE consensus sequence (G/C/T)ACGTGC(G/T)35 upstream of KSHV ORFs. In some regions of the genome, there is also a sequence with similarity to the identified HIF-enhancer sequence TCCTCTT36 between the HREs and the coding region. These regions include the immediate early protein encoded by ORF 57 and the lytic genes encoded by ORF 26, 29b, K10, and 64. In addition, the HRE consensus sequence is found upstream of ORF 50, a gene whose expression has been reported to be required for reactivation of HHV-8 lytic replication in B-cells.37 It therefore remains possible that the direct binding of HIF-1 activates these genes and/or others leading to HHV-8 lytic replication. This matter is currently under investigation. Alternatively, it is possible that KSHV is indirectly activated by a cellular response to hypoxia, such as VEGF production. However, we did not detect KSHV activation when VEGF was exogenously added to the PEL cells. Also, hypoxia did not result in a substantial increase in the already high constitutive level of VEGF production by the BC-3 cells. Taken together, these results suggest that the increase in KSHV reactivation induced by hypoxia was not mediated through stimulation of VEGF under these conditions. However, it remains possible that other indirect mechanisms may be mediating this effect.
KSHV encodes a number of proangiogenic human factors, including vIL-6, viral macrophage inflammatory protein 1 (vMIP-I), and vMIP-II, that are thought to be involved in the pathogenesis of KS. In addition, ORF 74 of KSHV encodes a constitutively active G-protein–coupled receptor that stimulates the production of cellular proangiogenic factors, and by these means, the virus could induce the proliferation of vascular endothelial cells.38,39 These viral-encoded proteins are all produced or increased during lytic KSHV replication.24Thus, hypoxic conditions such as may occur at the edges of a wound may induce both lytic KSHV replication and the growth of target endothelial cells. This may provide a mechanism to aid the establishment of infection if this virus enters the body through a cut in the skin or mucosal tissue. Hypoxic conditions may activate the virus and contribute to the development of KS after localized infection of cells by KSHV.
There is some evidence to indicate that hypoxia may affect the replication of certain other viruses as well.40 In particular, it has been reported that replication of herpes simplex virus type 2 can be increased 3- to 5-fold by exposure of infected cells to low O2 concentrations.40 Also, there is some evidence that production of Coxsackie virus can be either decreased or increased by exposure to hypoxia and that expression of HBx protein by hepatitis B–infected hepatoma cells is enhanced by hypoxia.41 What is noteworthy about KSHV, however, is the combination of viral induction by hypoxia and hypoxia-induced production of proangiogenic factors that could promote the growth of endothelial target cells.
The findings in this study may also explain certain clinical and epidemiological features of KS. Although there are reports that advanced KS may involve a monoclonal proliferation,42 the best available evidence suggests that spindle cell proliferation induced directly and/or indirectly by KSHV-encoded genes plays an important role in the development and progression of this condition.43-45 Hypoxia-induced KSHV reactivation may contribute to the increased prevalence of KS in patients with diabetes mellitus,46 47 as this condition is often associated with vascular disease and poor circulation. It may also contribute to the tendency of KS to occur on the lower legs, especially in the elderly men who develop classic KS. Hypoxic tissues may also produce VEGF as a direct result of hypoxia, and this may work in tandem with hypoxia-induced KSHV replication to promote spindle cell growth. Additional studies will be needed to further understand how these factors may interact in the pathogenesis of KS.
Activation of lytic KSHV infection by hypoxia may also help explain the pattern of distribution of KSHV infection and KS in the world. Unlike other human herpesviruses, which infect almost all individuals worldwide, the prevalence of KSHV infection varies widely from region to region.9,48,49 Interestingly, the prevalence of KSHV infection and KS is especially high in areas of the world where malaria is still endemic (eg, sub-Saharan Africa) or where a subset of the population has hereditary anemias, such as thalassemia, that confer partial protection against malaria (eg, southern Italy and other Mediterranean areas).4,50 51 It is possible that tissue hypoxia in patients with malaria or hereditary animas leads to increased shedding of KSHV and spread of the virus to other individuals.
In summary, our study provides evidence that KSHV can be activated by exposure of latently infected B-cell lines to hypoxia and that hypoxia can potentiate activation of KSHV by TPA. This finding may be important in the epidemiology and life cycle of KSHV and in the pathogenesis of KS. Further study of this phenomenon may lead to strategies to prevent or treat KSHV-related diseases.
We thank Isaac Rodriguez-Chavez for critical reading of the manuscript and Seth M. Steinberg and Jodi Black for assistance and advice.
D.A.D and A.S.R. contributed equally to this manuscript.
This is a US goverment work. There are no restrictions on its use.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
David A. Davis, HIV and AIDS Malignancy Branch, National Cancer Institute, National Institutes of Health, Bldg 10, Rm 10S255, MSC 1868, 9000 Rockville Pike, Bethesda, MD 20892; e-mail:dadavis@helix.nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal