Abstract
During episodes of inflammation, neutrophils (polymorphonuclear leukocytes [PMNs]) encounter subendothelial matrix substrates that may require additional signaling pathways as directives for movement through the extracellular space. Using an in vitro endothelial and epithelial model, inhibitors of phosphoinositide 3-kinase (PI3K) were observed to promote chemoattractant-stimulated migration by as much as 8 ± 0.3-fold. Subsequent studies indicated that PMNs respond in a similar manner to RGD-containing matrix substrates and that PMN-matrix interactions are potently inhibited by antibodies directed against β3- but not β1-integrin antibodies, and that PI3K inhibitors block β3-integrin dependence. Biochemical analysis of intracellular β3-integrin uncoupling by PI3K inhibitors revealed diminished β3-integrin tyrosine phosphorylation and decreased association with p72syk. Similarly, the p72sykinhibitor piceatannol promoted PMN transmatrix migration, whereas HIV-tat peptide-facilitated loading of peptides corresponding to the β3-integrin cytoplasmic tail identified the functional tyrosine residues for this activity. These data indicate that PI3K-regulated β3-integrin represents a natural “braking” mechanism for PMNs during transit through the extracellular matrix.
Introduction
Migration of neutrophils (polymorphonuclear leukocytes [PMNs ]) to sites of inflammation requires the coordinated interplay of soluble mediators, extracellular matrix ligands, and cell surface adhesion molecules. To subserve this function, PMNs must traverse endothelial cells lining the inner lumen of blood vessels. This process of transendothelial migration requires engagement and disengagement of a number of surface molecules and has been extensively studied.1 After successful transendothelial migration, PMNs encounter subendothelial extracellular matrices in transit to inflammatory sites. Anchorage of cells to the extracellular matrix is mediated in part by integrins, a large family of heterodimeric cell surface proteins that mediate numerous cell functions, including motility, differentiation, and proliferation.2 Integrin engagement of matrix ligand results in highly regulated signal transduction processes that cooperatively involve both “outside-in” and “inside-out” pathways.3 Importantly, integrin signaling can vary depending on the stimulus and on the cell type,4 the molecular details of which are not fully understood at the present time.
Recent studies have identified an important role for phosphoinositide-3-OH kinase (PI3K) in leukocyte migration.5-7 The 4 known isoforms of PI3K are α, β, γ, and δ, and extracellular ligand coupling through membrane-localized G proteins generates the signaling molecule phosphatidylinositol 3,4,5-triphosphate.8 PI3K coordinates a number of leukocyte effector functions, including leukocyte migration.5-7,9 Although not fully understood at present, it appears that PI3K is central to stimulated chemotaxis. For instance, mice lacking the catalytic domain of leukocyte-specific PI3Kγ (p110γ) manifest chemotactic defects in vitro and in vivo6,7 and PI3K activation results in membrane localization of downstream protein kinases.5
In the present study, we examined the role of PI3K in PMN interactions with endothelia, epithelia, and matrix substrates. Detailed analysis revealed that PI3K activation by PMN chemoattractants results in functional regulation of surface β3-integrin coupling. Further examination identified a candidate kinase (p72syk) in such coupling of functional surface β3-integrin. Taken together, these results identify PI3K-modulated, β3-integrin coupling as an endogenous pathway for regulated PMN-matrix interactions.
Materials and methods
Cell culture
Human microvascular endothelial cells (HMVECs), a microvascular endothelial primary culture isolated from adult dermis, were obtained from Cascade Biologics (Portland, OR) and cultured as previously described.10 Briefly, culture medium was supplemented with heat-inactivated bovine calf serum, penicillin, streptomycin, Hepes, heparin, l-glutamine, and endothelial mitogen factor. For preparation of experimental HMVEC monolayers, confluent endothelial cells (passages 1, 2, or 3) were seeded at about 2 × 105cells/cm2 onto either permeable polycarbonate inserts or 6-well plates precoated with 0.1% gelatin. Endothelial cell purity was assessed by phase microscopic “cobblestone” appearance and uptake of fluorescent acetylated low-density lipoprotein. Oral epithelial cells (KB cells) were obtained from the American Type Tissue Collection (ATTC; Rockville, MD) and cultured as described previously.11
Isolation of human PMNs
The PMNs were freshly isolated from whole blood obtained by venipuncture from human volunteers and anticoagulated with acid-citrate-dextrose.12 Plasma and mononuclear cells were removed by aspiration from the buffy coat following centrifugation (400g for 20 minutes) at 25°C. Erythrocytes were removed using a 2% gelatin sedimentation technique. Residual erythrocytes were removed by lysis in cold NH4Cl buffer. Remaining cells were more than 90% PMNs as assessed by microscopic evaluation. PMNs were studied within 2 hour of isolation.
Chemotaxis assay under agarose
Chemotaxis under agarose was used to assess the impact of PI3K inhibition on PMN migration in isolation, as described previously,13 using human PMNs and indicated concentrations of N-formyl-methionyl-leucyl-phenylalanine (fMLP) as a chemoattractant. PMN migration was measured microscopically by the leading front method14 using an ocular micrometer as described previously.15 From each plate, directed migration (migration toward indicated concentrations of fMLP) and spontaneous migration (adjacent wells without chemoattractant) were measured.
PMN transmigration assay
The PMN transmigration assay has been previously detailed12 and was modified to accommodate endothelia, epithelia, or matrix substrates. For experimental treatment, PMNs were pre-exposed to wortmannin (Calbiochem, La Jolla, CA), LY294002 (Calbiochem), piceatannol (Biomol, Plymouth Meeting, PA) or vehicle (concentration of carrier equivalent to the highest concentration of reagent used) for 15 minutes at 25°C with occasional mixing. Prior to addition of PMNs, confluent endothelial or epithelial monolayers were washed free of media with Hanks balanced saline solution (HBSS) containing Ca++ and Mg++. Transmigration assays were performed by the addition of pretreated PMNs to the upper chambers after chemoattractant (10 nM fMLP unless otherwise noted) was added to the opposing (lower) chambers. At time zero, 1 × 106PMNs were added and transmigration was allowed to proceed for 30 minutes at 37°C. Transmigration was quantified by assaying for the PMN azurophilic granule marker myeloperoxidase (MPO) as described previously.16,17 In subsets of experiments, we assessed PMN migration across matrix-coated substrates. To circumvent issues of matrix-integrin ligand redundancies,18 a general matrix was established. Acellular, matrix-coated permeable support substrates were generated as follows. Bare polycarbonate permeable supports (Corning-Costar, Cambridge, MA, 5μm pore size) were precoated for 30 minutes with 50μL 0.1% gelatin (derived from porcine skin, 175 Bloom, Attachment Factor, Cascade Biologics) followed by addition of media (containing 10% newborn calf serum, Gibco, Grand Island, NY) for an additional 30 minutes. Inserts were rinsed in HBBS and PMN transmigration was assessed using similar conditions as described above.
Antibodies
Functionally inhibitory monoclonal antibodies (mAbs) were as follows: β1-integrin19 (clone LM534, obtained from Chemicon International, Temecula, CA), β2-integrin20 (clone 44a, obtained from ATTC), and β3-integrin21(clone AP3, obtained from GTI, Brookfield WI). A control mAb directed against MHC class I22 (clone W6/32, obtained from ATTC) was used as a nonfunctional PMN binding control. All mAbs were diluted to indicated concentrations in HBSS (containing Ca++ and Mg++, with 10 mM Hepes, pH 7.4, Sigma, St Louis, MO) during functional assays.
Immunoprecipitation/Western blotting
The PMNs (1 × 107 PMN/well) were attached to a matrix substrate prepared on 6-well, tissue culture-treated plastic plates (Corning-Costar). Plates were centrifuged at 400g to uniformly settle PMNs onto the matrix substrate, followed by addition of fMLP (final concentration 10 nM) for indicated periods. Cells were lysed (1% [vol/vol] Triton X-100, 25 mM Tris/HCl, pH 8.1, 5 mM EDTA, 10 μg/mL aprotinin, 100μg/mL phenylmethylsulfonyl fluoride [PMSF], and 10μg/mL chymostatin) and debris removed by centrifugation. Lysates were precleared with 50 μL pre-equilibrated protein-G Sepharose (Pharmacia, Uppsala, Sweden). Immunoprecipitation of β3-integrin was performed by addition of mAb AP3 (5μg/mL) followed by addition of 50 μL pre-equilibrated protein-G Sepharose and overnight incubation. Immunoprecipitates were washed 3 times in lysis buffer, boiled in nonreducing sample buffer (2.5% sodium dodecyl sulfate [SDS], 0.38 M Tris pH 6.8, 20% glycerol, and 0.1% bromophenol blue), separated by nonreducing SDS-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose, and blocked overnight in blocking buffer. Primary antibody to phosphotyrosine (clone PY20 from Transduction Labs, Lexington, KY) or anti-p72syk (rabbit polyclonal generated against amino terminal peptide, Santa Cruz Biotechnology, Santa Cruz, CA), as indicated, was added for 3 hours followed by wash and addition of species-matched, peroxidase-conjugated secondary antibody (Cappel, West Chester, PA). Labeled proteins were visualized by enhanced chemiluminescence (ECL; Amersham, Arlington Heights, IL).
HIV-tat peptide treatment of PMNs
Peptides corresponding to amino acids encompassing β3-integrin cytoplasmic tail tyrosine at position 747 (sequence ANNPLYKEATS, where bold indicates tyrosine 747; single-letter amino acid codes), tyrosine at position 759 (sequence FTNITYRGT, where bold indicates tyrosine 759) or a scrambled peptide control (SPLAQAVRSSSR) were synthesized (Synpep, Dublin, CA) with the HIV-tat peptide as an N-terminal leader sequence to facilitate loading into intact cells.23 Peptides were solubilized in DMSO at stock concentrations of 50 mM and diluted in HBBS. PMNs were preincubated with indicated concentrations of peptide or vehicle control (DMSO at 1:1000 dilution) for 20 minutes at 25°C prior to addition to matrix substrates in a gradient of fMLP. Transmigration was assessed as described above.
For localization of HIV-tat peptides, PMNs were attached to coverslips (2.5 × 103 PMN/mL, 30 minutes, 37°C), loaded with HIV-tat peptides (50 μM in HBSS, 30 minutes, 37°C), washed with HBSS, fixed with paraformaldehyde (1% wt/vol for 10 minutes), and permeabilized or not, as indicated, with Triton X-100 (0.2% vol/vol for 5 minutes). Monolayers were then incubated for 1 hour with anti–HIV-tat mAb (a kind gift from Dr Eric Vives, Institut de Genetique Moleculaire, Montpellier, France, used at 1μg/mL)24 prepared in 1% normal goat serum. After washing, the sections were then incubated with fluorescein isothiocyanate (FITC)-conjugated secondary antibody (Cappel, 1μg/mL) for 30 minutes. The sections were then mounted in phosphate-buffered saline (PBS)–polyvinylalchohol and viewed with a fluorescence microscope (Olympus BH2, Melville, NY). As a control for background labeling, control sections were incubated with secondary antibody only.
To investigate the phosphorylation of synthetic β3-integrin HIV-tat peptides, high-performance liquid chromatography (HPLC) was used as described before.25Briefly, PMNs were loaded with peptide (50 μM in HBSS) and brought to 37°C in the presence of 10 nM fMLP; the reaction was allowed to proceed for 15 minutes (optimized from pilot experiments) before termination by snap freezing in liquid nitrogen. Lysate/peptide mixes were thawed and fractionated through molecular weight cutoff filters (< 5 kd), and the profile of HIV-tat phosphopeptides was determined by HPLC. Peptide levels were measured with a 10%:90% H2O/CH3CN gradient (30 minutes) mobile phase (1 mL/min) on a reverse-phase HPLC column (Luna 5μ C18, 150X 4.6 mm, Phenomonex, Torrence, CA). Absorbance was monitored at 220 nm. UV absorption spectra were collected and peptides were identified by their chromatographic elements (retention time, UV spectra, and coelution with standards).
Data presentation
Time-course data and concentration responses were compared by analysis of variance (ANOVA) for significance and individual comparisons were made by Student t test. All results are presented as the mean and SEM of n experiments.
Results
PI3K inhibitors promote transmigration
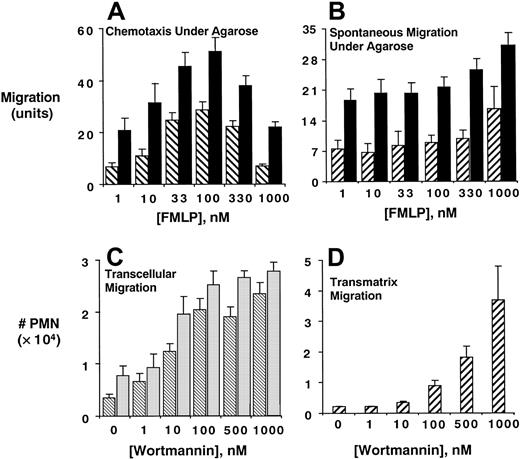
As an initial series of experiments, we examined whether inhibition of PI3K influences PMN migration. Pretreatment of PMNs with the potent and irreversible PI3K inhibitor wortmannin (10 nM) significantly inhibited PMN chemotaxis (Figure1A, P < .01 by ANOVA) as well as spontaneous migration (Figure 1B, P < .01 by ANOVA) under agarose.
Inhibition of PMN PI3K promotes transendothelial, transepithelial, and transmatrix migration.
Isolated human PMNs were examined for chemotaxis (A) or spontaneous migration (B) under agarose following pre-exposure of PMNs to vehicle (▪) or wortmannin (▨, 10 nM, 15 minutes, 22°C) toward indicated concentrations of fMLP. Data are derived from 4 separate experiments with results expressed as mean ± SEM distance migrated. In panels C and D, PMNs were pre-exposed to indicated concentrations of wortmannin and assessed for fMLP-stimulated (10−8 M) transmigration across confluent endothelia (▧, C) or epithelia (░, C) or across matrix-coated substrates (D). Transmigrated PMNs were quantified by determination of MPO content following termination of the assay. Data are derived from 8 to 12 monolayers in each condition from at least 3 separate experiments with results expressed as mean ± SEM number transmigrating PMNs .
Inhibition of PMN PI3K promotes transendothelial, transepithelial, and transmatrix migration.
Isolated human PMNs were examined for chemotaxis (A) or spontaneous migration (B) under agarose following pre-exposure of PMNs to vehicle (▪) or wortmannin (▨, 10 nM, 15 minutes, 22°C) toward indicated concentrations of fMLP. Data are derived from 4 separate experiments with results expressed as mean ± SEM distance migrated. In panels C and D, PMNs were pre-exposed to indicated concentrations of wortmannin and assessed for fMLP-stimulated (10−8 M) transmigration across confluent endothelia (▧, C) or epithelia (░, C) or across matrix-coated substrates (D). Transmigrated PMNs were quantified by determination of MPO content following termination of the assay. Data are derived from 8 to 12 monolayers in each condition from at least 3 separate experiments with results expressed as mean ± SEM number transmigrating PMNs .
We next addressed whether similar observations were apparent in a more physiologically relevant setting (PMN transendothelial and epithelial migration). As shown in Figure 1C, and in stark contrast to results observed under agarose, wortmannin increased fMLP-stimulated PMN transendothelial and transepithelial migration in a concentration-dependent manner (median effective concentration [EC50] ∼10 nM, P < .01 by ANOVA), with concentrations as low as 1 nM resulting in a significant increase (P < .05). Subsequent experiments revealed that endothelial cells were not necessary in this response and that PI3K inhibitors promote PMN transmigration across matrix substrates to a similar extent as in the presence of endothelial cells (14 ± 0.5-fold increase over control, Figure 1D). In addition, these observations were not specific for fMLP, because PMN transmatrix migration in response to LTB4 (10 nM gradient) was similarly increased by inhibition of PI3K (maximal 6 ± 0.4-fold increase with 100 nM wortmannin over untreated controls,P < .001). Importantly, the presence of a chemotactic gradient was necessary, since no differences between wortmannin-treated and untreated PMNs were evident in the absence of fMLP (data not shown). Moreover, these observations were not specific for wortmannin and were also evident using the less potent PI3K inhibitor LY294002 (EC50 ∼2 μM with maximal 7 ± 0.3-fold increase over untreated PMNs , P < .01 by ANOVA).
Original studies with this transmigration model have indicated that PMNs do not degranulate during transit through cell monolayers.16,17,26 Nonetheless, recent studies have suggested that integrin-mediated adhesion may potentate PMN degranulation.27 Because our transmigration assay uses MPO as a biochemical marker, we ruled out that these findings with wortmannin could result from differences in MPO content. To do this, we examined PMN MPO content in the presence and absence of wortmannin before and after transmigration. Following transmigration, PMNs were collected and quantified by hemocytometer and MPO content was compared to resting PMNs. This analysis revealed no differences between wortmannin treatment groups (mean OD absorbance units of 0.26 ± 0.01 and 0.27 ± 0.02/104 PMNs in the presence and absence of 10 nM wortmannin, respectively, n = 4, P = not significant), or between PMNs that had migrated across endothelial (mean OD absorbance units of 0.25 ± 0.03 and 0.27 ± 0.01/104 PMNs before and after transmigration, respectively, n = 4, P = not significant) or epithelial monolayers (mean OD absorbance units of 0.23 ± 0.03 and 0.28 ± 0.04/104 PMNs before and after transmigration, respectively, n = 4, P = not significant), suggesting that MPO is an appropriate biochemical marker for analysis of PMN transmigration. Taken together, we conclude that inhibition of PI3K promotes chemoattractant-stimulated PMN transmatrix migration.
β3-Integrin dependence
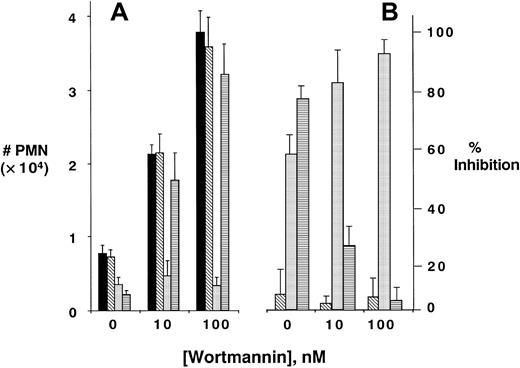
We next determined whether PI3K-dependent promotion of PMN transmigration required surface integrin activity. Initial insight was gained by the observation that matrix proteins were absolutely required for PI3K-dependent increases in PMN transmigration. Indeed, although wortmannin significantly increased PMN migration across a general matrix-coated substrate (Figure 1D), transmigrations of wortmannin-treated PMNs across bare (ie, no matrix coating) polycarbonate substrates were either equivalent to control or even decreased at high concentrations of wortmannin (eg, 31% ± 7% decrease at 500 nM, P < .05); suggesting the necessity for matrix ligands in wortmannin-mediated PMN responses. Based on these data, and numerous previous observations that integrins bind matrix components,2 we screened a panel of functionally inhibitory mAbs directed against β1-, β2-, or β3-integrins (Figure 2). Compared to binding control mAb against major histocompatibility complex (MHC) class I, anti-β1 mAb did not influence PMN transmatrix migration in the presence or absence of any wortmannin concentration tested (P = not significant for all). Anti–β2-integrin mAb blocked PMN transmigration by as much as 88% ± 6.0%, with approximately equal potency in the presence and absence of wortmannin. These findings that PI3K inhibition does not differentially influence β2-integrin function are consistent with previous observations.28
Role of integrins in PI3K-mediated increases in PMN transmatrix migration.
Isolated human PMNs were pre-exposed to indicated concentrations of wortmannin and/or antibodies (10 μg/mL for each) directed against MHC class I (▪), anti–β1-integrin (▧), anti–β2-integrin (░), or anti–β3-integrin (▤) followed by examination of fMLP-stimulated (10−8 M) transmigration across matrix-coated substrates (A). Transmigrated PMNs were quantified by determination of MPO. Panel B represents data converted to percent inhibition relative to control binding antibody. Data are derived from 9 to 12 monolayers in each condition from at least 3 separate experiments with results expressed as mean ± SEM number transmigrating PMNs .
Role of integrins in PI3K-mediated increases in PMN transmatrix migration.
Isolated human PMNs were pre-exposed to indicated concentrations of wortmannin and/or antibodies (10 μg/mL for each) directed against MHC class I (▪), anti–β1-integrin (▧), anti–β2-integrin (░), or anti–β3-integrin (▤) followed by examination of fMLP-stimulated (10−8 M) transmigration across matrix-coated substrates (A). Transmigrated PMNs were quantified by determination of MPO. Panel B represents data converted to percent inhibition relative to control binding antibody. Data are derived from 9 to 12 monolayers in each condition from at least 3 separate experiments with results expressed as mean ± SEM number transmigrating PMNs .
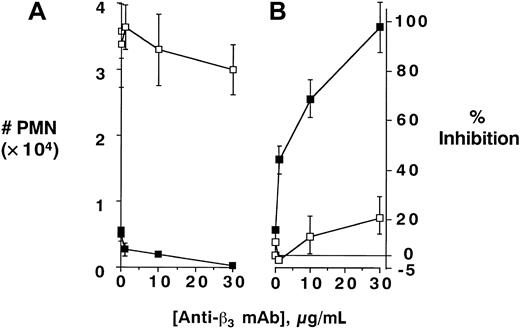
In contrast to these findings with anti–β1- or β2-integrin antibodies, differential responses were evident in the presence of functionally inhibitory anti–β3-integrin antibodies. Although anti-β3 mAb significantly blocked PMN transmatrix migration in untreated PMNs (79% ± 5.2%, compared to binding controls, P < .01), the inhibitory influence of anti-β3 mAb was lost under conditions of inhibited PI3K activity (P = not significantly different from binding control in PMNs pretreated with either 10 or 100 nM wortmannin). The anti–β3-integrin dose-response curve is shown in Figure3 and demonstrates a nearly complete loss of anti-β3 inhibition in PMNs pre-exposed to 100 nM wortmannin (P < .01 by ANOVA). Important in this regard, wortmannin did not influence β3-integrin expression (assessed by flow cytometry and immunoprecipitation of surface biotinylated PMN β3, data not shown). These data suggest that blockade of PI3K inhibits surface β3 function during stimulated PMN-matrix interactions.
PI3K-mediated uncoupling of β3-integrin–mediated inhibition of PMN transmatrix migration; antibody dose response.
Isolated human PMNs were pre-exposed to wortmannin (100 nM, ■) or vehicle (▪) and/or indicated concentrations of antibody directed against β3-integrin followed by examination of fMLP-stimulated (10−8 M) transmigration across matrix-coated substrates (A). Transmigrated PMNs were quantified by determination of MPO content following termination of the assay. Panel B represents data converted to percent inhibition relative to no antibody controls. Data are derived from 8 to 10 monolayers in each condition from at least 3 separate experiments with results expressed as mean ± SEM number transmigrating PMNs .
PI3K-mediated uncoupling of β3-integrin–mediated inhibition of PMN transmatrix migration; antibody dose response.
Isolated human PMNs were pre-exposed to wortmannin (100 nM, ■) or vehicle (▪) and/or indicated concentrations of antibody directed against β3-integrin followed by examination of fMLP-stimulated (10−8 M) transmigration across matrix-coated substrates (A). Transmigrated PMNs were quantified by determination of MPO content following termination of the assay. Panel B represents data converted to percent inhibition relative to no antibody controls. Data are derived from 8 to 10 monolayers in each condition from at least 3 separate experiments with results expressed as mean ± SEM number transmigrating PMNs .
PI3K inhibition blocks β3-integrin tyrosine phosphorylation
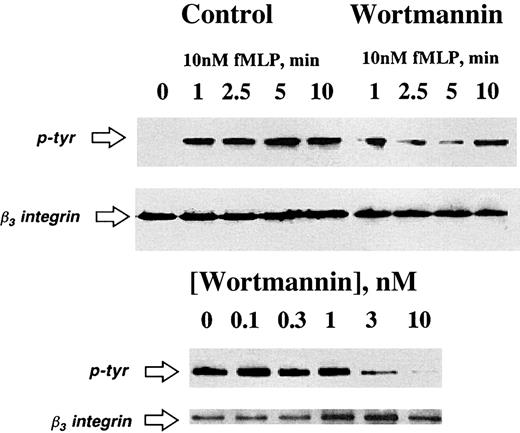
The above results indicate that PI3K inhibitors functionally block β3-integrin during PMN interactions with matrix. The short cytoplasmic tail of β3-integrin contains tyrosine residues at positions 747 and 759, which serve as functional kinase substrates.29 Thus, we next addressed whether PI3K was important in β3-integrin tyrosine phosphorylation. To do this, PMNs were attached to a matrix substrate and activated for various periods of time with fMLP (10 nM). The β3-integrin immunoprecipitates were resolved by SDS-PAGE and Western blots were probed with antiphosphotyrosine. As shown in Figure 4, PI3K inhibition blocked fMLP-stimulated tyrosine phosphorylation in a time-dependent (10 nM fMLP) and concentration-dependent fashion. In the presence of wortmannin, a slight increase in tyrosine phosphorylation was consistently observed at 10 minutes, although analysis beyond this time point did not reveal additional phosphorylation (data not shown). These findings were not a result of decreased PMN adhesion to matrix substrates, as determined morphologically and by biochemical quantification of PMN adhesion (using the marker MPO, data not shown). Moreover, these findings were not a result of decreased PMN β3-integrin content (refer to control β3-integrin Western blots adjacent to phosphotyrosine blots in Figure 4). These data suggest a role for PI3K in chemoattractant-stimulated β3-integrin tyrosine phosphorylation.
Inhibition of PI3K blocks fMLP-activated β3-integrin tyrosine phosphorylation.
Isolated human PMNs were pre-exposed to vehicle or wortmannin (3 nM in the top panel or indicated concentration in the bottom panel) followed by adhesion to matrix substrates. Adherent PMNs were exposed to fMLP (10 nM) for indicated periods of time (top panel) or for 10 minutes (bottom panel). Cells were lysed and β3-integrin was immunoprecipitated and resolved by SDS-PAGE. Tyrosine phosphorylation was assessed by immunoblot. Data are representative from 3 separate experiments.
Inhibition of PI3K blocks fMLP-activated β3-integrin tyrosine phosphorylation.
Isolated human PMNs were pre-exposed to vehicle or wortmannin (3 nM in the top panel or indicated concentration in the bottom panel) followed by adhesion to matrix substrates. Adherent PMNs were exposed to fMLP (10 nM) for indicated periods of time (top panel) or for 10 minutes (bottom panel). Cells were lysed and β3-integrin was immunoprecipitated and resolved by SDS-PAGE. Tyrosine phosphorylation was assessed by immunoblot. Data are representative from 3 separate experiments.
Role for β3-integrin cytoplasmic tail
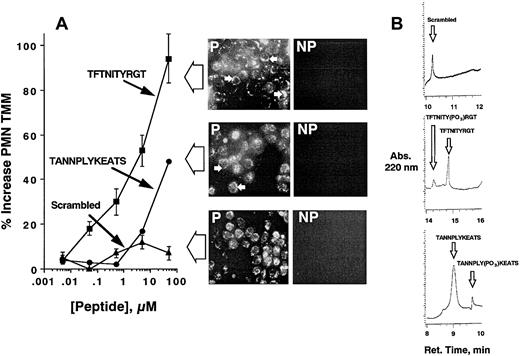
Previous studies have indicated that both of the β3-integrin cytoplasmic tail tyrosines (at positions 747 and 759) can be functionally important, depending on the cell type.29 Thus, we addressed whether functional inhibition of β3-integrin cytoplasmic tail tyrosines would result in responses similar to PI3K inhibition (ie, enhanced PMN transmatrix migration). To do this, intact PMNs were loaded with peptides corresponding to amino acids spanning the β3-integrin cytoplasmic tail tyrosine at position 747 (ANNPLYKEATS) or position 759 (FTNITYRGT). Loading of intact PMNs was accomplished by synthesizing peptides with the 12 amino acid HIV-tat peptide motif (YGRKKRRQRRRG) at the N-terminus, a maneuver that for unknown reasons significantly facilitates peptide uptake across intact plasma membranes.23 Incubation of HIV-tat–linked peptides with PMNs followed by assessment of PMN transmatrix migration revealed a predominant role for tyrosine 759 (Figure5). Indeed, the HIV-tat-FTNITYRGT peptide increased PMN migration in a concentration-dependent manner with a 97% ± 6.2% increase at 50 μM. HIV-tat–linked peptide corresponding to tyrosine 747 also increased transmigration (maximal 44% ± 0.5% at 50 μM, P < .025), but to a lesser extent than those targeting tyrosine 759 (P < .025 compared to those targeting tyrosine 747). A scrambled peptide synthesized with HIV-tat linker was used as a control for these experiments and did not influence transmigration at any concentration tested (P = not significant at all concentrations). As a control for these experiments, localization of HIV-tat peptide in adherent, permeabilized PMNs revealed equivalent loading between different peptides (Figure 5, inset), with an obvious increase in membrane localization of peptides targeting the β3-integrin cytoplasmic tail (ANNPLYKEATS and FTNITYRGT) but not in the scrambled peptide. Such data suggest that these peptides localize to an appropriate compartment within the cell (ie, membrane proximal) and that differential loading does not explain the functional results shown in Figure 5A.
HIV-tat peptide–mediated loading of peptides corresponding to β3-integrin cytoplasmic tail promotes PMN transmatrix migration.
In panel A, isolated human PMNs were pre-exposed to indicated concentrations of HIV-tat peptides motifs corresponding to β3-integrin cytoplasmic tyrosine 747 (closed circles), tyrosine 759 (closed squares), or scrambled peptide for 20 minutes at room temperature followed by examination of fMLP-stimulated (10−8 M) transmigration across matrix-coated substrates. The immunofluorescence insets for each peptide localize HIV-tat peptide within permeabilized (P) and nonpermeabilized (NP) PMNs following peptide loading (see “Materials and methods”). Arrowheads demonstrate predominant localization of peptides at the cytoplasmic face of PMNs. Transmigrated PMNs were quantified by determination of MPO. Data are derived from 9 monolayers in each condition from 4 separate experiments with results expressed as mean ± SEM number transmigrating PMNs. (B) The β3-integrin cytoplasmic sequence is a kinase substrate. Coincubation of HIV-tat peptides coupled to a scrambled peptide (top panel), the FTNITYRGT peptide (middle panel), or the ANNPLYKEATS peptide (bottom panel) with fMLP-activated PMNs were analyzed for phosphorylation by HPLC (representative tracings of 2 experiments are shown). Arrows indicate position of unphosphorylated or phosphorylated standards.
HIV-tat peptide–mediated loading of peptides corresponding to β3-integrin cytoplasmic tail promotes PMN transmatrix migration.
In panel A, isolated human PMNs were pre-exposed to indicated concentrations of HIV-tat peptides motifs corresponding to β3-integrin cytoplasmic tyrosine 747 (closed circles), tyrosine 759 (closed squares), or scrambled peptide for 20 minutes at room temperature followed by examination of fMLP-stimulated (10−8 M) transmigration across matrix-coated substrates. The immunofluorescence insets for each peptide localize HIV-tat peptide within permeabilized (P) and nonpermeabilized (NP) PMNs following peptide loading (see “Materials and methods”). Arrowheads demonstrate predominant localization of peptides at the cytoplasmic face of PMNs. Transmigrated PMNs were quantified by determination of MPO. Data are derived from 9 monolayers in each condition from 4 separate experiments with results expressed as mean ± SEM number transmigrating PMNs. (B) The β3-integrin cytoplasmic sequence is a kinase substrate. Coincubation of HIV-tat peptides coupled to a scrambled peptide (top panel), the FTNITYRGT peptide (middle panel), or the ANNPLYKEATS peptide (bottom panel) with fMLP-activated PMNs were analyzed for phosphorylation by HPLC (representative tracings of 2 experiments are shown). Arrows indicate position of unphosphorylated or phosphorylated standards.
As additional evidence that these HIV-tat peptides are functional, we examined the direct phosphorylation of synthetic phosphopeptides by fMLP-stimulated PMNs using a recently described HPLC assay. As shown in Figure 5B, this analysis revealed significant phosphorylating activity of the ANNPLYKEATS peptide (mean 49% conversion in 5 minutes, n = 2) as well as the FTNITYRGT peptide (mean 68.6% conversion in 5 minutes, n = 2). These data indicate that synthetic peptides targeting the β3-integrin cytoplasmic tail serve as substrates for tyrosine kinase(s). No detectable phosphorylation was evident in scrambled peptide exposed to similar conditions. Taken together, these data suggest that inhibition of β3-integrin cytoplasmic tail tyrosines results in a similar functional response as that of PI3K inhibition.
Role of p72syk in PMN transmatrix migration
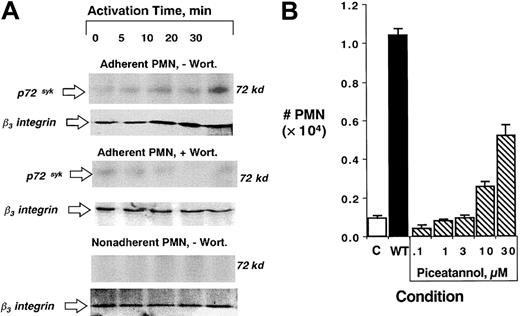
Based on the observations that PI3K inhibition blocks surface β3-integrin function, that inhibitors of PI3K block tyrosine phosphorylation, and that HIV-tat peptides targeting inhibition of β3-integrin cytoplasmic tyrosines promote PMN transmatrix migration, we sought to gain insight into kinases important in tyrosine phosphorylation. Recent studies indicated that p72syk, a kinase expressed predominantly in myeloid-derived cells,30 coordinates integrin function in platelets.31 Thus, we examined the influence of PI3K inhibition on p72syk interactions with β3-integrin in PMNs . As shown in Figure6 (left panel), activation of matrix-adherent PMNs with fMLP (10 nM), and subsequent β3-integrin immunoprecipitation/p72sykWestern blot resulted in a time-dependent association between p72syk and β3-integrin. This response was evident by 5 minutes of activation and dominant by 30 minutes. Similar analysis from PMNs pretreated with wortmannin (100 nM) revealed a nearly complete abolition in p72syk–β3-integrin association (Figure 6). Importantly, as shown in Figure 6, p72syk–β3-integrin association was not observed in nonadherent PMNs exposed to fMLP (10 nM), suggesting that PMN adherence to matrix substrates provides an “outside-in” signal for such associations. Such findings were not a result of decreased PMN β3-integrin content (see control β3-integrin Western blots adjacent to phosphotyrosine blots in Figure 6).
Role of p72syk in PI3K-mediated uncoupling of β3-integrin–mediated transmatrix migration.
(A) Isolated human PMNs were pre-exposed to vehicle (−Wort) or wortmannin (+Wort, 100 nM) and adhered to matrix substrates (top 2 blots) or left in suspension. PMNs were exposed to fMLP (10 nM) for indicated times (0-30 minutes). Cells were lysed and β3-integrin was immunoprecipitated and resolved by SDS-PAGE. Association with p72syk was assessed by immunoblot . Data are representative from 3 separate experiments. (B) PMNs were pre-exposed to vehicle (C), wortmannin (WT, 100 nM) or indicated concentrations of the p72syk inhibitor piceatannol for 20 minutes at room temperature followed by examination of fMLP-stimulated (10−8 M) transmigration across matrix-coated substrates. Transmigrated PMNs were quantified by determination of MPO content following termination of the assay. Data are derived from 8 to 10 monolayers in each condition from 3 separate experiments with results expressed as mean ± SEM number transmigrating PMNs .
Role of p72syk in PI3K-mediated uncoupling of β3-integrin–mediated transmatrix migration.
(A) Isolated human PMNs were pre-exposed to vehicle (−Wort) or wortmannin (+Wort, 100 nM) and adhered to matrix substrates (top 2 blots) or left in suspension. PMNs were exposed to fMLP (10 nM) for indicated times (0-30 minutes). Cells were lysed and β3-integrin was immunoprecipitated and resolved by SDS-PAGE. Association with p72syk was assessed by immunoblot . Data are representative from 3 separate experiments. (B) PMNs were pre-exposed to vehicle (C), wortmannin (WT, 100 nM) or indicated concentrations of the p72syk inhibitor piceatannol for 20 minutes at room temperature followed by examination of fMLP-stimulated (10−8 M) transmigration across matrix-coated substrates. Transmigrated PMNs were quantified by determination of MPO content following termination of the assay. Data are derived from 8 to 10 monolayers in each condition from 3 separate experiments with results expressed as mean ± SEM number transmigrating PMNs .
Based on these data, we thus predicted that the relatively selective p72syk inhibitor piceatannol32 should block surface β3-integrin function in a similar manner as PI3K inhibition and should manifest as increased PMN transmatrix migration (Figure 1). As shown in Figure 6 (right panel), pretreatment of PMNs with piceatannol, but not vehicle control, resulted in a concentration-dependent increase (P < .025 by ANOVA) in PMN transmatrix migration (5.2 ± 0.3-fold increase at 30 μM piceatannol). We next compared the influence of piceatannol on PMN transendothelial and transepithelial migration with bare insert migration. This analysis revealed that piceatannol (30 μM pretreatment of PMNs for 30 minutes) significantly enhanced PMN migration across endothelial (210% ± 65% increase over untreated controls, n = 3, P < .025) and epithelial monolayers (160% ± 50% increase over untreated controls, n = 3,P < .05) but did not influence migration across bare inserts (10% ± 2% increase over untreated controls, n = 3,P = not significant), thus confirming our hypothesis that matrix ligands are critical for this response. These data suggest that the p72syk-selective inhibitor piceatannol, like the PI3K inhibitor wortmannin, functionally blocks β3-integrin function during PMN interactions with matrix. Taken together, such data provide evidence that PI3K modulation of surface β3-integrin activity functions as an endogenous “braking mechanism” during chemoattractant-stimulated PMN migration across matrix substrates.
Discussion
The process of PMN transmigration occurs through a series of steps orchestrated by the interplay of surface adhesion molecules and soluble mediators derived from the local milieu. Following transendothelial migration, PMNs encounter subendothelial matrix substrates, and at present, the molecular mechanism(s) used by PMNs to traverse the extracellular matrix are not fully understood. In these studies, we hypothesized a role for PMN PI3K in regulation of PMN trafficking through vascular endothelial cells. We observed a seemingly paradoxical enhancement in PMN migration under conditions of inhibited PI3K activity, and a series of experiments identified PI3K uncoupling of β3-integrin as a mechanism for this response. These results unveil 2 novel concepts with regard to basic mechanisms of leukocyte function. First, PI3K is central to chemoattractant-stimulated PMN-matrix interactions via modulation of the cytoplasmic tail of β3-integrin, and second, these studies define PMN β3-integrins as an endogenous regulatory mechanism during leukocyte trafficking, the uncoupling of which results in enhanced leukocyte movement though matrix substrates.
Recent studies have clearly defined PI3K as an important molecule in acute inflammatory processes. Transgenic knockout mice lacking the p110γ subunit of PI3K manifest defects in some PMN responses, including protein kinase Akt activation, formation of 3′-phosphorylated lipids, superoxide anion generation, and chemotaxis,6,7,33and that the p110γ subunit may be the major PI3K activated during chemoattractant stimulation of PMNs.34 Although it is difficult to compare our results to these in the mouse, consistent with our studies using the under agarose assay and bare insert experiments, decreased in vitro chemotaxis was observed in PI3K knockout mice PMNs when migrating across polycarbonate membranes not imprinted with matrix proteins.6,7 It should also be noted that some controversy exists regarding the immediate role of PI3K in PMN migration on nonmatrix substrates. Although we (see “Results”) and others35-38 have observed that inhibition of PI3K decreases PMN motility on nonphysiologic substrates (ie, plastic), other studies have suggested only partial or selective inhibition39-41 or no influence of PI3K inhibitors on PMN chemotaxis.42 Similar to our experiments with PI3K inhibition (see “Results”), no differences were observed in matrix-mediated adhesion in PMNs derived from PI3K-deficient mice.6,7 Thus, it is likely that significant variability exists between these different methods and that mechanisms to explain similarities and differences are likely complicated and difficult to resolve. Additionally, it is difficult to directly compare our results to those in the p110γ null mice, because human PMNs express all 4 known PI3K subunits (α, β, γ, and δ) and wortmannin inhibits all forms of PI3K.43 Nonetheless, our present observations using more physiologic substrates (endothelia, epithelia, or matrix) indicate the necessity for matrix ligands as a “second signal” in PMN motility under conditions of inhibited PI3K, as would be the case in vivo. Of note in this regard, in vivo defects associated with PI3K deficiency included neutrophilia and decreased peritoneal elicitation of PMNs and macrophages.6,7 Although the role of β3-integrins in leukocyte extravasation has only been indirectly addressed in vivo, the inflammatory phenotype is clearly similar to that of PI3K deficiency. Indeed, Lindberg and colleagues demonstrated that targeted disruption of integrin-associated protein (IAP) results in defective PMN accumulation and PMNs that show impaired β3-integrin–dependent ligand binding.44Thus, it is possible that PI3K-coupled β3-integrin could contribute to the inflammatory defect associated with the PI3K-null phenotype during inflammatory processes.
Our studies provide unique insight into a prominent role for β3-integrins in PMN interactions with postendothelial matrix substrates. It is unlikely that PMN β3-integrins directly interact with endothelial surface proteins because stripping endothelial cells did not influence PMN migration (see “Results”). Rather, it appears that PMNs use β3-integrins as a postendothelial, matrix adhesive mechanism, likely via RGD-containing peptides.45 Previous studies addressing the role of β3-integrins in leukocyte transmigration have focused primarily on monocytes,46 and revealed that the β3-chain of αvβ3 enhanced monocyte migration through endothelia via a cooperative mechanism involving β2- and β3-integrins. These studies with monocytes did not address whether such responses could be mediated by direct β3-integrin–matrix interaction, but it is unlikely that a similar mechanism is important in PMN β3-integrin function. For example, although anti–β2-integrin mAb potently blocks PMN transmatrix migration, no differences were evident in β2-integrin function under conditions of PI3K inhibition (ie, wortmannin did not block this response, Figure 2). These findings are consistent with previous studies demonstrating no impact of PI3K inhibitors on β2-integrin function in PMNs.28 Similarly, we did not observe an appreciable influence of functionally inhibitory anti–β1-integrin antibodies in the presence or absence of PI3K inhibitors. Taken together, these data identify β3-integrin as an important, and previously unappreciated, molecule in the PMN transmigratory cascade. Of note in this regard, we have not characterized the matrix in detail. The matrices used here required precoating with both gelatin and newborn calf serum (see “Materials and methods”), and because calf serum contains relatively high levels of vitronectin and fibinogen,47 PMN β3-integrins presumably use these serum factors as ligands for transmatrix migration. We have not, however, specifically determined the immediate role of each serum component in this response.
The present studies provide direct evidence that cytoplasmic tail tyrosine residue 759, and to a lesser extent residue 747, mediate PMN β3-integrin function and that the tyrosine kinase p72syk contributes to these observations in this transmatrix migration model. Because PMNs are not amenable to transfection or mutation-based experiments, HIV-tat peptide-facilitated loading of consensus peptides corresponding to the cytoplasmic domains were used. These results revealed that peptides targeting tyrosine residue 759 were substantially more potent (10- to 50-fold) than those targeting residue 747, which is within the well conserved NPXY motif found in a number of integrins,48 including both β1- and β3-integrin studied here (Figures 2and 3). Important in this regard, the β2-integrin cytoplasmic tail does not express a membrane proximal NPXY motif,29 and although anti–β2-integrin antibodies significantly inhibit PMN transmatrix migration (Figure 2), no differences were observed with PI3K inhibition.
Consistent with previous mutational analysis,49 our data using HIV-tat peptide-coupled consensus motifs suggest that tyrosine 759 may be relatively more important for cytoskeletal linkage than tyrosine residue 747, and that phosphorylation of the membrane proximal position 747 may mediate a generalized postligand binding event common to a number of integrin β chains.48 It is also likely that the membrane distal cytoplasmic domain consensus (NITY) is a better substrate for tyrosine kinase p72syk and functions directly through PI3K signaling, because p72sykhas a demonstrated importance in the sustained phosphorylation of B-cell Akt following PI3K activation.50 Moreover, formation of the functional p72syk–β3-integrin complex likely requires integrin ligation because the association was not evident in nonadherent PMNs, and the relatively specific p72sykinhibitor piceatannol induced a functional response consistent with PI3K uncoupling of β3-integrin (ie, increased fMLP-stimulated transmigration). These findings support those of Sarkar and coworkers31 and Gao and colleagues,51which revealed that p72syk activation required β3-integrin occupancy. Thus, it is possible that loading PMNs with peptides corresponding to tyrosine residue 759 indirectly regulates Akt phosphorylation through p72syk-β3 interactions. It is also possible that p72syk is a substrate for Akt. The minimal sequence for efficient phosphorylation by Akt is RXX(S/T)X* where R is arginine, X any amino acid, S/T is serine/threonine, and X* is a hydrophobic residue.43 In fact, our examination of the published p72syk amino acid sequence52 revealed 3 previously unappreciated binding sites for Akt (RVLTV at amino positions 253-257, RIKSY at positions 292-296, and RKSSP at positions 304-308), providing at least the possibility that p72syk is an Akt substrate.
In conclusion, as PMNs respond to chemoattractants and surface molecule engage extracellular matrix ligands, phosphorylation of β3-integrin in association with p72sykdefines a PI3K-dependent modulation of PMN transmatrix migration. Future studies are necessary to define the exact sequence of activation and the coordination of “outside-in” and “inside-out” components for this modulation.
We authors wish to thank Ms Kristin Synnestvedt for technical assistance.
Supported by National Institutes of Health grants DK50189, HL60569, project 3 of PO-1 DE13499, and by a grant from the Crohn's and Colitis Foundation of America.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Sean P. Colgan, Center for Experimental Therapeutics and Reperfusion Injury, Brigham and Women's Hospital, Thorn Bldg 704, 75 Francis St, Boston, MA 02115; e-mail:colgan@zeus.bwh.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal