Abstract
The pathogenesis of thrombosis in heparin-induced thrombocytopenia (HIT) was studied by investigating whether antibodies to heparin-platelet factor 4 (H-PF4) induced tissue factor (TF) synthesis by monocytes. Plasma from 5 patients with HIT containing IgG to H-PF4 was incubated with peripheral blood mononuclear cells without or with purified PF4 and heparin. Significant TF-dependent procoagulant activity (PCA) expressed by monocytes, measured with a factor Xa-based chromogenic assay, was induced after incubation of each HIT plasma sample. This monocyte PCA required the presence of PF4 and was inhibited by high concentrations of heparin. Furthermore, purified HIT IgG added to whole blood with PF4 and heparin also provoked significant synthesis of TF mRNA by monocytes, demonstrated by RT-PCR, and this effect was not observed with normal IgG. These findings strongly support the hypothesis that antibodies to PF4 developed in HIT trigger the production of tissue factor by monocytes, and this effect could account in vivo for hypercoagulability and thrombotic complications in affected patients.
Introduction
Heparin-induced thrombocytopenia (HIT) is a common complication of unfractionated heparin (UFH) therapy, often associated with arterial or venous thrombosis.1 Such thrombocytopenia results from platelet activation caused by antibodies specific for complexes composed of heparin and platelet factor 4 (FP4).2-5 The platelet activation involves the binding of HIT IgG to specific Fc receptors (FcγRIIA),6 partly explaining why HIT differs from most other types of drug-induced immune thrombocytopenia, which are mainly complicated by hemorrhage.
The most frequent manifestation in HIT is thrombosis, sometimes associated with disseminated intravascular coagulation, and one of the mechanisms proposed to explain the hypercoagulability in HIT is the production of phospholipid platelet microparticles after platelet activation.7 However, HIT antibodies might also bind to endothelial cells and trigger the synthesis of tissue factor (TF),4,8,9 a transmembrane glycoprotein that initiates the coagulation pathway.10,11 TF can be produced by monocytes when activated by lipopolysaccharides, cytokines, or immune complexes,12 and it can be triggered in the presence of activated platelets.13 We therefore investigated whether antibodies to PF4 developed in patients with HIT to induce the synthesis of TF by human monocytes.
Study design
Plasma and purification of immunoglobulin G
Plasma from 5 patients with definite HIT complicated by venous (n = 5) and arterial (n = 1) thromboses was studied. Every sample contained significant levels of antibodies to H-PF4 complexes (mean A492, 1.7; range, 1.0-2.1) (Asserachrom HPIA, Stago; Asnières, France)14 able to induce heparin-dependent platelet activation in serotonin release assay (SRA).15Normal human plasma without antibodies to H-PF4 complexes (mean A492 < 0.2) was collected from 5 healthy volunteers not receiving heparin. All samples were heated at 56°C for 1 hour, centrifuged for 30 minutes twice at 1500g, filtered through 0.45 μm Millex-GV (Millipore, Molsheim, France), and stored at −80°C until study.
The IgG fraction from each HIT plasma sample was purified by affinity chromatography using a Hi-Trap protein G column (Pharmacia LKB Biotechnology, Uppsala, Sweden), and antibody purity was evaluated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis. Each IgG fraction was tested by enzyme-linked immunosorbent assay and serotonin release assay to check that heparin-dependent antibodies were effectively purified and retained their ability to activate platelets in the presence of heparin. Normal IgG was similarly purified from the plasma of 2 healthy volunteers, and all IgG fractions were kept at 4°C until tested.
Chromogenic assay for procoagulant activity of monocytes
Peripheral blood mononuclear cells (PBMCs) were obtained from healthy donors by venipuncture, using EDTA as an anticoagulant. PBMCs were isolated by Ficoll-Hypaque density gradient centrifugation (MSL 1077; Eurobio, Les Ullis, France),16 washed in 5 mM phosphate-buffered saline (PBS) EDTA, and resuspended in endotoxin-free RPMI-1640 medium (Gibco, Paisley, United Kingdom) supplemented with 2 mM L-glutamine (ICN Biomedicals, Irvine Ayrshire, Scotland), 100 IU/mL penicillin, 100 μg/mL streptomycin (Boehringer Mannheim, Mannheim, Germany), and 25 mM HEPES (Eurobio).
After isolation, PBMCs (5 × 104 cells per well of a microtiter plate) were incubated with each HIT or control plasma sample diluted to 10% in culture medium. Each sample was tested in the absence and in the presence of human PF4 (Hyphen Biomed, Andresy, France) at 0.5 μg/mL supplemented or not with unfractionated heparin (0.0125 IU/mL or 1.25 IU/mL). Each test was performed in duplicate, and cells were incubated for 4 hours at 37°C in an atmosphere of 95% air and 5% CO2. A positive stimulation control was performed for each experiment with 1 μg/mL lipopolysaccharide (LPS) fromEscherichia coli serotype 026:B6 (Sigma Aldrich Chimie, St Quentin Fallavier, France). Procoagulant activity of monocytes was then evaluated by an amidolytic assay adapted from a previously described procedure, and the results were expressed as mU factor Xa/well.17
Detection of monocyte TF mRNA synthesis by RT-PCR
HIT or normal IgG (135-170 μL to obtain a final concentration of 500 μg/mL) was added either with or without heparin (0.25 IU/mL) and PF4 (10 μg/mL) to 1 mL whole blood collected from healthy donors on 0.129 M sodium citrate (9:1). LPS was also tested at 1 μg/mL as a positive control in each experiment, and heparin and PF4 without IgG were incubated in whole blood as a negative control.
After 90 minutes of incubation at 37°C in Teflon tubes (Skatron tubes; Polylabo, Strasbourg, France), whole blood cooled to 4°C was added to 1 mL cold PBS with fetal calf serum (FCS; 2%) and then incubated with 16 × 106 Dynabeads CD14 (Dynal France, Compiègne, France) for 30 minutes at 4°C with gentle shaking. Dynabeads-monocyte complexes were then isolated and washed with cold 2% FCS-PBS, monocytes were lysed, and Dynabeads CD14 were removed.
Statistics
Paired Student t test was performed using Stat View (SAS Institute, Cary, NC) for Power Macintosh.
Results and discussion
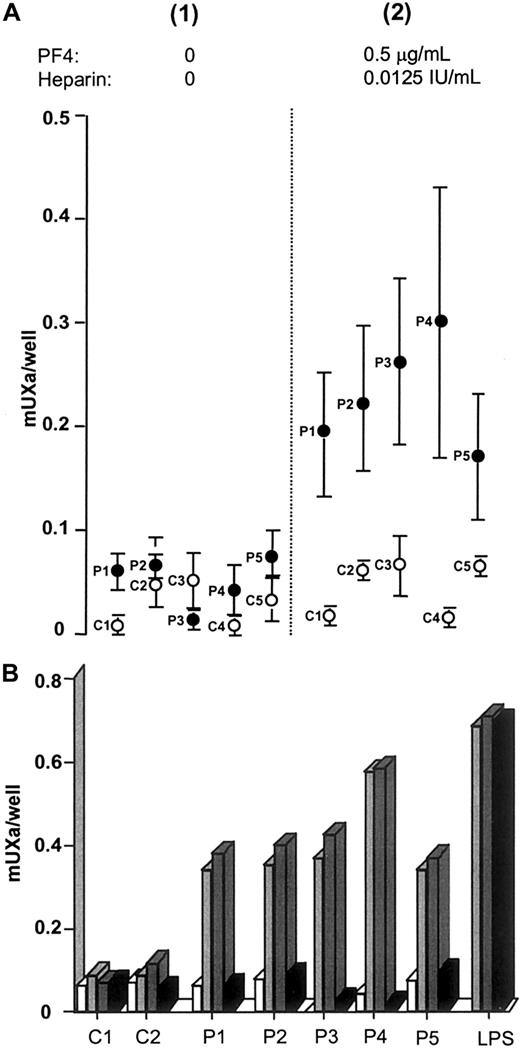
We first looked for the expression of monocyte PCA induced by HIT plasma using a TF-specific and -sensitive chromogenic assay. HIT plasma was incubated with PBMCs in the presence of heparin at 0.0125 IU/mL and with 0.5 μg/mL PF4, and these concentrations were chosen for a ratio recognized as optimal for the binding of HIT antibodies (ie, 1 IU heparin to 40 μg PF4).3 In these conditions, a significant increase in the generation of FXa was measured (P = .006; paired t test) (Figure1A), supporting TF expression on the basis of the high specificity of the assay.17 In contrast, no significant monocyte PCA was measured when normal plasma was added to PBMCs in similar conditions. When tested alone, plasma from the 5 patients with HIT did not induce significant monocyte PCA; thus, factor Xa generation remained below 0.1 mU Xa/well, with values identical to those measured when PBMCs were incubated with normal plasma. Monocyte PCA was thus only induced by patient plasma and was dependent on PF4, strongly indicating that this effect is specific to HIT antibodies.
Monocyte procoagulant activity.
(A) Monocyte procoagulant activity induced by samples of HIT (●) or control (○) plasma diluted to 10% without (1) or with (2) purified PF4 and heparin. Error bars indicate mean ± 1 SEM of 3 separate experiments. (B) Monocyte procoagulant activity induced by samples of HIT plasma (P1 to P5) or normal plasma (C1 and C2), tested alone (■) or in the presence of PF4 (0.5 μg/mL) without ( ) or with 2 different concentrations of heparin (0.0125 IU/mL,
) or with 2 different concentrations of heparin (0.0125 IU/mL, , and 1.25 IU/mL, ▪). Data correspond to the mean of triplicate values in one experiment.
, and 1.25 IU/mL, ▪). Data correspond to the mean of triplicate values in one experiment.
Monocyte procoagulant activity.
(A) Monocyte procoagulant activity induced by samples of HIT (●) or control (○) plasma diluted to 10% without (1) or with (2) purified PF4 and heparin. Error bars indicate mean ± 1 SEM of 3 separate experiments. (B) Monocyte procoagulant activity induced by samples of HIT plasma (P1 to P5) or normal plasma (C1 and C2), tested alone (■) or in the presence of PF4 (0.5 μg/mL) without ( ) or with 2 different concentrations of heparin (0.0125 IU/mL,
) or with 2 different concentrations of heparin (0.0125 IU/mL, , and 1.25 IU/mL, ▪). Data correspond to the mean of triplicate values in one experiment.
, and 1.25 IU/mL, ▪). Data correspond to the mean of triplicate values in one experiment.
In addition, when heparin concentration was 100-fold higher (ie, 1.25 IU/mL) with identical amounts of PF4 (0.5 μg/mL), monocyte PCA was no longer induced by HIT plasma. This inhibition, which was not observed when monocytes were stimulated by endotoxin (Figure 1B), is a typical feature of HIT antibodies, probably resulting from the dissociation of macromolecular heparin/PF4 complexes.20
Immune complexes have never been detected in patients with HIT,6 and cell activation induced by antibodies could result from a process mainly occurring in the microenvironment of the cell surface, as suggested by Newman and Chong.21 As for platelets, cell activation might also involve the direct binding of HIT IgG to monocytes through the interaction of IgG Fc fragments with specific receptors.
We demonstrated in separate experiments that monocyte PCA was also triggered by HIT plasma in the presence of PF4, but without heparin, and that it remained unchanged with a low concentration of UFH (0.0125 IU/mL) (Figure 1B). The role of chondroitin sulphate proteoglycans can be evoked to explain this result because these molecules, which are expressed in large amounts in human monocytes,22 represent specific receptors for tetramer PF423 and might allow the membrane binding of HIT antibodies if relatively high concentrations of PF4 are present.
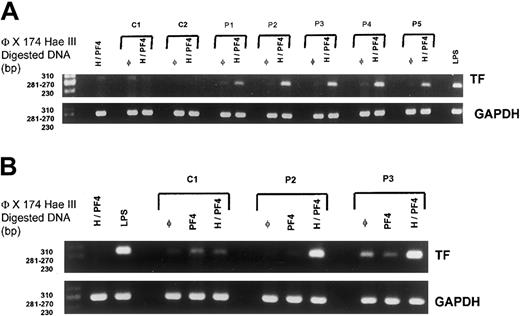
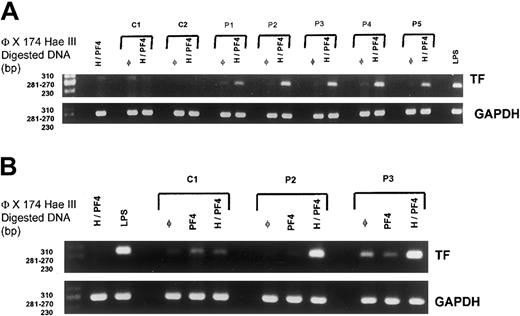
The synthesis of tissue factor by monocytes could also be triggered by activated platelets, and this prompted us to study the effect of HIT purified IgG added to whole blood on TF gene expression. When HIT IgG were incubated in whole blood together with PF4 and heparin, a single band of the expected size (ie, 282 bp) was clearly visualized after RT-PCR performed on mRNA isolated from purified monocytes. This band, corresponding to TF cDNA, was similar to bands obtained after the stimulation of cells with LPS (Figure 2A) and either could not be detected or was very faint (with IgG from P1 and P4) when HIT IgG was added to whole blood without PF4 and heparin. In contrast, no significant TF-specific PCR product was obtained when normal IgG was added to whole blood, regardless of whether PF4 and UFH were present (Figure 2A). In all experiments, RT-PCR products specific for GAPDH were obtained as a single band of 265 bp, and the expression of this control gene in monocytes was never modified by HIT or control IgG in the presence of PF4 and heparin.
Monocyte TF in RNA synthesis.
(A) Tissue factor mRNA synthesis in immunopurified monocytes after the incubation of normal (C1-C2) or HIT IgG (P1-P5) in whole blood at 500 μg/mL in the absence (φ) or the presence of PF4 (10 μg/mL) with heparin (0.25 IU/mL) (H/PF4). Whole blood was also incubated in the presence of H-PF4 complexes without IgG or in the presence of LPS as a positive stimulation control (LPS). (B) Effects of IgG purified from one sample of normal plasma (C1) and from 2 selected patients (P2 and P3) tested alone (φ) or in the presence of PF4 without (PF4) or with (H/PF4) heparin.
Monocyte TF in RNA synthesis.
(A) Tissue factor mRNA synthesis in immunopurified monocytes after the incubation of normal (C1-C2) or HIT IgG (P1-P5) in whole blood at 500 μg/mL in the absence (φ) or the presence of PF4 (10 μg/mL) with heparin (0.25 IU/mL) (H/PF4). Whole blood was also incubated in the presence of H-PF4 complexes without IgG or in the presence of LPS as a positive stimulation control (LPS). (B) Effects of IgG purified from one sample of normal plasma (C1) and from 2 selected patients (P2 and P3) tested alone (φ) or in the presence of PF4 without (PF4) or with (H/PF4) heparin.
TF mRNA synthesis was thus only elicited by HIT IgG, and, in agreement with the findings recorded on chromogenic assay, this effect was dependent on the presence of PF4 at relatively high concentrations. Plasma PF4 levels are also probably increased in patients with HIT and thus are compatible with the formation of heparin/PF4 complexes able to bind IgG antibodies before cell activation.21
Heparin was also necessary in whole blood with PF4 to allow HIT IgG to trigger TF monocyte mRNA synthesis (Figure 2B). This result apparently disagreed with findings obtained with the chromogenic assay, but the role of activated platelets during HIT IgG-induced TF synthesis might be critical in whole blood and involve P-selectin, which interacts with PSGL-1 on the surfaces of monocytes.13
In conclusion, our study clearly demonstrates that HIT antibodies can trigger TF synthesis by monocytes, and this process could contribute to the thrombotic complications observed in our patients in vivo. However, this hypothesis warrants further studies including a larger HIT patient population, both without and with thrombosis.
We thank Hendra Setiadi (University of Oklahoma Health Sciences Center, Oklahoma City) for valuable suggestions and Doreen Raine for editing the English version of this paper.
Supported by the Institut pour la Recherche en Hémostase et Thrombose.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Yves Gruel, Laboratoire d'Hémostase, EA Cellules hématopoiétiques, Hémostase et Greffe, Faculté de Médecine, 2 Bis Bd Tonnellé, 37032 Tours Cedex, France; e-mail: gruel@med.univ-tours.fr.



 ) or with 2 different concentrations of heparin (0.0125 IU/mL,
) or with 2 different concentrations of heparin (0.0125 IU/mL, , and 1.25 IU/mL, ▪). Data correspond to the mean of triplicate values in one experiment.
, and 1.25 IU/mL, ▪). Data correspond to the mean of triplicate values in one experiment.