Imexon is a cyanoaziridine derivative that has antitumor activity in multiple myeloma. Previous studies have shown that imexon induces oxidative stress and apoptosis in the RPMI 8226 myeloma cell line. This study reports that imexon has cytotoxic activity in other malignant cell lines including NCI-H929 myeloma cells and NB-4 acute promyelocytic leukemia cells, whereas normal lymphocytes and U266 myeloma cells are substantially less sensitive. Flow cytometric experiments have shown that imexon treatment is associated with the formation of reactive oxygen species (ROS) and the loss of mitochondrial membrane potential (Δψm) in imexon-sensitive myeloma cell lines and NB-4 cells. In contrast, reduction of Δψm and increased levels of ROS were not observed in imexon-resistant U266 cells. Treatment of imexon-sensitive RPMI 8226 cells with the antioxidant N-acetyl-l-cysteine (NAC) protects cells against these effects of imexon. Mitochondrial swelling was observed by electron microscopy in RPMI 8226 myeloma cells treated with 180 μM imexon as early as 4 hours. Damage to mitochondrial DNA was detected by a semiquantitative polymerase chain reaction assay in imexon-treated RPMI 8226 cells; however, nuclear DNA was not affected. Finally, partial protection of RPMI 8226 cells against the imexon effects was achieved by treatment with theonyltrifluoroacetone, an inhibitor of superoxide production at mitochondrial complex II. These changes are consistent with mitochondrial oxidation and apoptotic signaling as mediators of the growth inhibitory effects of imexon. Interestingly, oxidative damage and decrease of Δψm induced by imexon highly correlates with sensitivity to imexon in several myeloma cell lines and an acute promyelocytic leukemia cell line.
Introduction
Imexon (4-imino-1, 3-diazabicyclo-[3.1.0] hexan-one) is a 2-cyanoaziridine derivative that has been extensively studied as an immunomodulator and an anticancer agent. Imexon was shown to be active in a variety of animal tumor models, in tumor cell lines, and in humans.1,2 Among 10 fresh human tumor types, multiple myeloma was the most sensitive to imexon with a median inhibitory concentration of 50% (IC50) of 1μM at 10 to 14 days using colony forming assays.3 Importantly, in pilot phase I trials imexon was well tolerated by cancer patients.2 No myelosuppression, renal dysfunction, or elevation of hepatic enzyme was observed after imexon treatment in humans.2 In the absence of antiemetics, nausea and vomiting were the major toxicities associated with intravenous administration of imexon.2 The lack of myelosuppression after imexon treatment was confirmed also in animal studies with mice and dogs.1
We have recently shown that the cytotoxic mechanism of imexon action in RPMI 8226 myeloma cells involves thiol depletion, oxidative stress, and apoptosis.4 This activity requires an aziridine ring. The activation of imexon is believed to involve aziridine ring opening and subsequent binding to sulfhydryl groups of cysteine residues.5 This results in the depletion of cellular thiols, the induction of oxidative stress, and apoptosis.4Whether thiol depletion is causal or a marker of activity is not known. Interestingly, imexon also induces gross alterations in mitochondrial ultrastructure, but not in other cellular organelles.4Moreover, oxidative damage of DNA was observed primarily in the cytoplasm and not in the nucleus, suggesting that mitochondria could be targets of the drug.
It is well established that mitochondria are important regulators of apoptosis and undergo major changes during apoptotic cell death.6-10 These changes include opening of the mitochondrial megachannel known as the permeability transition pore, leading to disruption of the mitochondrial membrane potential (Δψm), and the release of cytochrome c from the mitochondria to the cytosol.8,11,12 Changes in cellular redox potential due to enhanced generation of reactive oxygen species (ROS), a decrease in their detoxification, or the depletion of reduced glutathione (GSH) are sufficient to induce opening of the mitochonrial permeability transition pore and, subsequently, apoptosis in a number of cell types.13-15
Based on these data, the major goal of the current studies was to investigate whether imexon induces mitochondrial ultrastructural and biochemical alterations that are characteristic of the apoptotic cell death pathway in RPMI 8226 myeloma cells. We also investigated whether inhibition of normal superoxide production in mitochondria inhibits imexon-induced cytotoxicity. In addition, we investigated the effects of imexon on peripheral blood lymphocytes, acute promyelocytic leukemia NB-4 cells, and NCI-H929 and U266 myeloma cell lines. The results show that imexon induces changes in mitochondrial morphology, a reduction of the mitochondrial membrane potential, and cytochrome c release. These changes are consistent with drug-induced mitochondrial oxidation and apoptotic signaling.
Materials and methods
Chemicals
Imexon, theonyltrifluoroacetone (TTFA), N-acetyl-l-cysteine (NAC), and diazzaquone (AZQ) were obtained from Sigma Chemical (St Louis, MO). MitoTracker Red (CMXRos), dihydroethidium (Hydroethidine, HE) and PicoGreen dsDNA quantification kit were purchased from Molecular Probes (Eugene, OR). The imexon stock solution (1 mg/mL) was prepared in phosphate-buffered saline (PBS), filter sterilized, and stored at −80°C. A stock solution of NAC (200 mM) was prepared in PBS, titrated with NaOH to pH 7.2, and filter sterilized. The solution of TTFA was prepared in dimethyl sulfoxide (DMSO; 10 mM), diluted in PBS to 1 mM concentration, and filter sterilized. All other chemicals were the highest purity available and were obtained from Sigma unless noted otherwise.
Cell cultures and viability assays
The human myeloma cells (RPMI 8226, NCI-H929, U266) and the promyelocytic leukemia NB-4 cell line were obtained from the American Type Culture Collection (Rockville, MD). All cell lines were cultured at 37°C in 5% CO2 in RPMI 1640 media (Gibco-BRL Products, Grand Island, NY) supplemented with 10% (v/v) heat inactivated bovine calf serum (Hyclone Laboratories, Logan, UT), 2 mMl-glutamine, penicillin (100 U/mL), and streptomycin (100 μg/mL). Peripheral blood lymphocytes were separated from the blood of normal donors according to the method of Edelson and Cohn.16 Lymphocytes were maintained in RPMI 1640 media in similar conditions as cell lines. To stimulate lymphocytes, the cells were cultured in the presence of phytohemagglutinin-M (PHA, 20 μg/mL) for 3 days and then incubated with various concentrations of imexon for 48 hours.
Cellular dehydrogenase activity, which is considered to reflect mitochondrial function and cell viability, was measured by a microculture tetrazolium (MTT) assay that is based on the ability of normally functioning mitochondria to reduce the dye, MTT (3-(4,5,-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide), to a blue formazan.17
Bright-field microscopy studies
For morphologic studies, the RPMI 8226 cells were pretreated with 50 μM TTFA, an inhibitor of complex II,18 for 16 hours. The cells were then treated with 50 μM TTFA and 180 μM imexon simultaneously for 48 hours. Untreated RPMI 8226 myeloma cells and cells treated with 50 μM TTFA or 180 μM imexon only were included as controls. The cells were cytospun on slides using a Cytospin 2 centrifuge (Shandon, Pittsburgh, PA), then fixed with 100% methanol for 2 minutes at room temperature, air-dried, and then stained with DiffQuick stain (Gibco-BRL Products). The cells were morphologically evaluated for apoptosis by bright-field microscopy (100 × oil immersion). The criteria used to identify apoptotic cells included chromatin condensation, formation of apoptotic bodies, and cellular shrinkage as described by Payne and coworkers.19
Transmission electron microscopy and morphometric studies
Mitochondrial morphologic changes and effects of imexon on cellular organelles were evaluated by transmission electron microscopy of RPMI 8226 cells. After treatment with 180 μM imexon for various time periods, the cells (1 × 106) were fixed with 3% glutaraldehyde made up in 0.1 M cacodylate buffer (pH 7.2). The cells were then postfixed in 1% osmium tetroxide, dehydrated in a graded series of ethanols, and embedded in epoxy resin. Ultrathin sections were evaluated for mitochondrial morphologic changes using a Philips CM12 transmission electron microscope (Eindhoven, The Netherlands).
Cytofluorometric determination of Δψm and ROS
In these experiments, 3 different myeloma cell lines, NB-4 cells, and normal blood lymphocytes were evaluated for the changes in Δψm and ROS levels after imexon treatment. Also, RPMI 8226 cells pretreated with 10 mM NAC for 3 hours and then incubated with 180 μM imexon for 48 hours were included.
The lipophilic cationic dye, MitoTracker Red (CMXRos), which is concentrated in intact mitochondria was used along with flow cytometry analysis to detect changes in the Δψm.20 21 Cells (0.5 × 106/mL) were stained with a final concentration of 100 nM CMXRos for 30 minutes at 37°C. The cells were then centrifuged for 5 minutes at 750g, the supernatant was removed, and the cells were resuspended in 500 μL PBS and kept on ice. The cells were then analyzed on a flow cytometer (Becton Dickinson FACScan, San Jose, CA) using excitation at 488 nm and emission at 600 nm.
Oxidative damage in the imexon-treated cells was assessed by staining with the membrane permeable dye, dihydroethidium (HE), which is oxidized to the fluorescent intercalator, ethidium, by cellular oxidants, particularly superoxide radicals.22 The oxidative conversion of HE to ethidium is then measured by flow cytometry. Cells (0.5 × 106/mL) were stained at a final concentration of 2 μM HE for 30 minutes at 37°C. The cells were then centrifuged for 5 minutes at 750g, the supernatant was removed, and the cells were resuspended in 500 μL PBS and kept on ice. The cells were then analyzed on a flow cytometer (BD FACScan, excitation: 488 nm, emission: 620 nm).
Preparation of S-100 fraction and Western blot analysis for cytochrome c
Cytosolic fractions were isolated according to the method of Vander Heiden and coworkers.23 Briefly, RPMI 8226 cells (2 × 108) were resuspended in 0.24 mL ice-cold buffer A (20 mM Hepes, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA,1 mM DTT, 17 μg/mL phenylmethylsulfonyl fluoride [pH = 7.4]). Cells were incubated on ice and after 30 minutes, sucrose solution (1 M) was added to achieve a final sucrose concentration of 250 mM. Cells were then immediately homogenized in a ground glass homogenizer (Kontes Glass, Vinaland, NJ) and centrifuged for 10 minutes at 750g to remove unlysed cells and nuclei. The supernatant was then centrifuged at 10 000g for 25 minutes. The resulting pellet containing the mitochondrial fraction was resuspended in buffer A containing 250 mM sucrose. The 10 000g supernatant was then centrifuged at 100 000g for 60 minutes to yield the cytosolic fraction in the resulting supernatant. The protein concentrations were determined according to the method of Smith and colleagues.24 A Laemmli sample buffer25 was then added to samples and boiled for 5 minutes. Protein aliquots were loaded (30 μg/lane) on 15% sodium dodecyl sulfate-polyacrylamide gel for size fractionation by electrophoresis. The proteins were then blotted onto Immobilon-P PVDF transfer membrane (Millipore, Bedford, MA) at 100 mA overnight. Membranes were blocked with 5% milk proteins in Tris buffer saline/0.05% Tween (TBST) and immunostained with mouse anticytochrome c monoclonal antibody (1:500, Pharmingen, San Diego, CA). The membranes were washed and incubated with goat antimouse IgG antibody conjugated to horseradish peroxidase (1:40 000, Pierce, Rockford, IL). Antibody complexes were detected using the enhanced chemiluminescence detection system (Amersham, Pharmacia Biotech, Piscataway, NJ). To estimate the apparent molecular mass of proteins, kaleidoscope prestained standards from Biorad (Biorad Laboratories, Richmond, CA) were used. Individual protein band densities were analyzed by the Eagle Eye II Video Still System (Stratagene, La Jolla, CA).
Semiquantitative polymerase chain reaction method
DNA damage in mitochondrial DNA and the nuclear hprt(hypoxanthine phosphoribosyltransferase) gene was assessed using a semiquantitative polymerase chain reaction (PCR) according to the previously described method of Yakes and associates.26 DNA was isolated from imexon-treated RPMI 8226 cells with the QIAamp isolation kit (Qiagen, Valencia, CA) according to the protocol supplied by the manufacturer with the following modifications. The RPMI 8226 cells were washed with PBS, resuspended in 200 μL PBS, and lysed at 50°C in the presence of proteinase K and the buffer provided with the Qiagen kit. The concentrations of total DNA were determined using a Picogreen dsDNA quantification kit and a fluorescent plate reader (Fluorolite FPM-2, Jolley Consulting and Research, Grayslake, IL).
Semiquantitative PCRs were performed in a Mastercycler gradient 5331 (Eppendorf Scientific, Hamburg, Germany) with a GeneAMP-XL PCR kit (PerkinElmer, Norwalk, CT). Reaction mixtures contained 20 ng DNA, 1 × XL buffer II, 100 ng/μL bovine serum albumin (BSA; Boehringer Mannheim, Mannheim, Germany), 1.2 mM Mg(AOC)2, 0.4 μM primers, and 200 μM dNTPs. The total volume of the reaction mixture was 50 μL. The mock tube without DNA was added as a control. The reaction was initiated by adding 1 U rTth polymerase XL, (recombinant DNA polymerase blend of Thermus thermophilusand Thermus litoralis DNA polymerases,Perkin-Elmer) when samples had reached a temperature of 75°C. The thermocycler profile was: initial denaturation for 2 minutes at 94°C followed by 32 cycles of 94°C denaturation for 17 seconds, and then 65.8°C primer extension for 12 minutes. A final extension was performed at 72°C for 10 minutes. The nuclear region analyzed for DNA damage included a 10.4- kb fragment from the hprt gene (GenBank accession no. J00205) encompassing exons 2-5 amplified by using primers, 5′-TGG GAT TAC ACG TGT GAA CCA ACC-3′ (sense), and primer, 5′-GCT CTA CC TCT CCT CTA CCG TCC-3′ (antisense). The mitochondrial DNA region analyzed for damage included an 8.9-kb fragment of the mitochondrial genome (GenBank no. J01415) amplified by using primers, 5′-TCT AAG CCT TAT TCG AGC CGA-3′ (sense) and 5′-TTT AT GCG GAG ATG TTG GAT GG-3′ (antisense). Aliquots of PCR products were resolved on a 1% agar gel. The gel was stained with ethidium bromide and the band densities were evaluated with the Eagle Eye II Still Video System (Stratagene).
Results
Cytotoxicity in malignant cell lines and normal lymphocytes
Imexon reduced viability in all malignant cell lines examined, but different cell lines exhibited distinct sensitivities to imexon. The IC50 of imexon measured by MTT at 48 hours was in the 30- to 40-μM range in RPMI 8226, NCI-H929 myeloma cells, and NB-4 acute promyelocytic leukemia cell line. However, the U266 myeloma cell line was not affected by imexon at these concentrations (Table1). The IC50 of imexon in U266 cells was 419 ± 36.8 μM.
To evaluate the effects of imexon on lymphocytes, we studied cytotoxic effects of imexon in human unstimulated lymphocytes and in lymphocytes stimulated with PHA for 3 days. Unstimulated lymphocytes as well as lymphocytes stimulated with PHA are partially protected against imexon cytotoxic effects. The IC50 of imexon at 48 hours measured by MTT assay was 125.8 ± 12.5 μM and 75.3 ± 6.5 μM for unstimulated lymphocytes and lymphocytes stimulated with PHA, respectively.
Morphologic changes in myeloma cells after imexon treatment
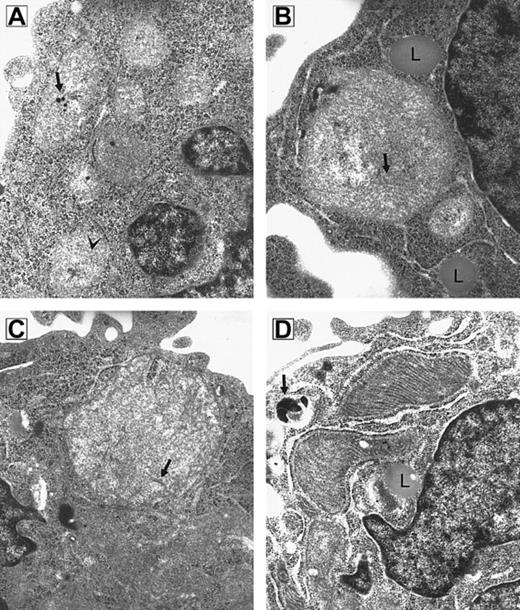
Because RPMI 8226 myeloma cells are known to be sensitive to imexon, this cell line was used to test whether imexon treatment is associated with time-dependent morphologic changes in mitochondria. Transmission electron microscopy revealed that mitochondria of imexon-treated cells are enlarged compared to control cells.4 To investigate the time course of morphologic changes induced by imexon, the cells were treated with 180 μM imexon for 0, 4, 8, 16, 24, and 48 hours (Figure1). In untreated cells normal mitochondria with cristae and calcific bodies are found. Treatment of RPMI 8226 cells with 180 μM imexon for 4 hours (Figure 1B) and 8 hours (Figure 1C) leads to the formation of megamitochondria; however, no damage is observed in other cellular organelles. Also, no calcific bodies are detected after imexon treatment. The enlargement of mitochondria was not consistent with swelling that accompanies classic necrosis, because no flocculent densities were observed in the mitochondrial matrix and no evidence of other organelle swelling was observed.27 28 The formation of lipid droplets (Figure1B,D) in association with mitochondria was also observed after imexon treatment. Mitochondria of cells treated with 180 μM imexon for 16 hours were electron dense with abundant cristae (Figure 1D) indicating high cellular adenosine triphosphate (ATP) demand and associated oxidative phosphorylation. When RPMI 8226 myeloma cells were exposed to 180 μM imexon for 24 and 48 hours, typical features of apoptotic cell death were detected in the majority of cells (data not shown).
RPMI 8226 myeloma cells.
Electron micrograph composite of an untreated RPMI 8226 myeloma cell (A) and cells treated for 4 hours (B), 8 hours (C), and 16 hours (D) with 180 μM imexon (original magnification × 49 500). (A) Small mitochondria with sparse cristae (arrowhead) and calcific bodies (arrow) are seen. (B) Megamitochondrion in association with lipid droplets (L); cristae are indicated by arrow. (C) Megamitochondrion; arrow indicates cristae. (D) Mitochondria are electron dense with abundant cristae. A lipid droplet (L) is seen in proximity to mitochondria. A secondary lysosome is indicated by the arrow.
RPMI 8226 myeloma cells.
Electron micrograph composite of an untreated RPMI 8226 myeloma cell (A) and cells treated for 4 hours (B), 8 hours (C), and 16 hours (D) with 180 μM imexon (original magnification × 49 500). (A) Small mitochondria with sparse cristae (arrowhead) and calcific bodies (arrow) are seen. (B) Megamitochondrion in association with lipid droplets (L); cristae are indicated by arrow. (C) Megamitochondrion; arrow indicates cristae. (D) Mitochondria are electron dense with abundant cristae. A lipid droplet (L) is seen in proximity to mitochondria. A secondary lysosome is indicated by the arrow.
Release of cytochrome c from the mitochondria into the cytoplasm in imexon-treated RPMI 8226 cells
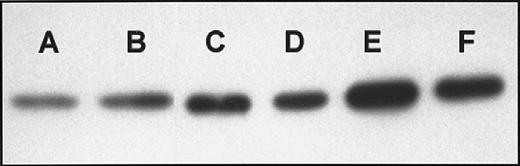
Immunoblots of cytosolic cytochrome c from imexon-treated RPMI 8226 cells indicate that imexon induces a substantial release of cytochrome c from mitochondria into the cytosol in a time-dependent manner (Figure 2). Continuous treatment of RPMI 8226 myeloma cells with 180 μM imexon caused release of cytochrome c into the cytoplasm, first observed at 8 hours and continuing to increase up to 24 hours after imexon was added (Figure 2).
Immunoblots of cytosolic cytochrome c.
Western blot analysis of cytochrome c was performed in the cytosolic fraction of RPMI 8226 cells treated in the absence (A) or presence of 180 μM imexon for 4 hours (B), 8 hours (C), 16 hours (D), 24 hours (E), and 48 hours (F).
Immunoblots of cytosolic cytochrome c.
Western blot analysis of cytochrome c was performed in the cytosolic fraction of RPMI 8226 cells treated in the absence (A) or presence of 180 μM imexon for 4 hours (B), 8 hours (C), 16 hours (D), 24 hours (E), and 48 hours (F).
Changes in the Δψm and formation of ROS in myeloma and leukemia cells after imexon treatment
It is well established that apoptosis induced by some agents is associated with the perturbation of mitochondrial functions and the formation of ROS.10,14 29-33 In the current studies, we tested whether imexon treatment induced a decrease in the Δψm and an increase in cellular oxidants and whether these changes are correlated with sensitivity to imexon.
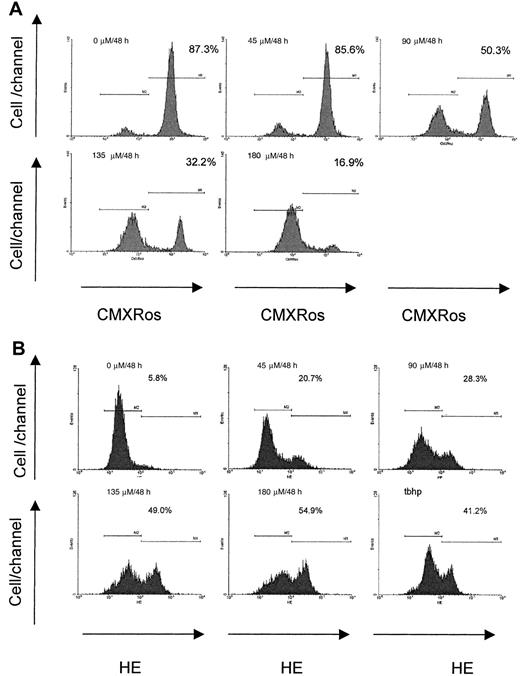
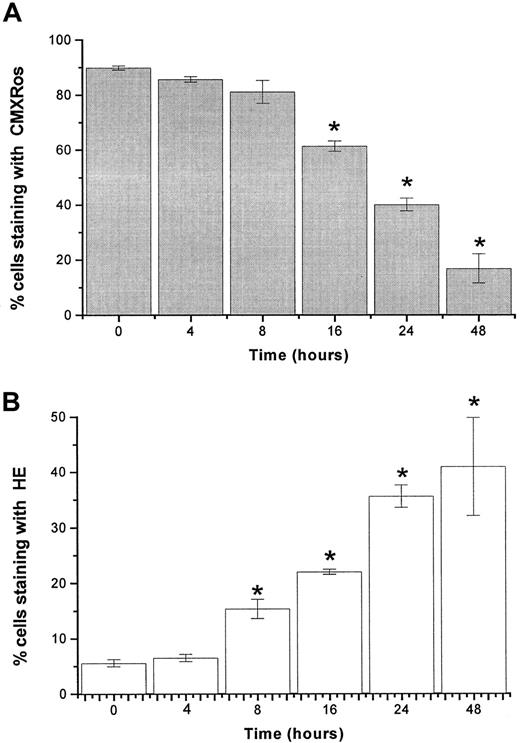
The lipophilic cation, CMXRos, accumulates in the mitochondrial matrix by the electrochemical gradient according to the physicochemical principle of the Nernst equation. In control cells, the concentration of cations will be 2 to 3 logs higher in the mitochondrial matrix than in the cytosol.34 The staining of RPMI 8226 myeloma cells with CMXRos revealed that imexon induced disruption of the Δψm in a concentration-dependent as well as a time-dependent manner. Figure 3 displays data from flow cytometry analyses of RPMI 8226 myeloma cells treated with different concentrations of imexon for 48 hours. The fraction of cells with an intact Δψm decreased significantly after treatment with 90 μM imexon. The more profound decrease of cells with intact Δψm was observed in the myeloma cells treated with 135 μM imexon, and a near complete loss of Δψmwas observed after exposure of myeloma cells to 180 μM imexon (Figure3A). The decrease in Δψm after imexon treatment is also time dependent as demonstrated in Figure4A. Myeloma RPMI 8226 cells treated for 4 or 8 hours with 180 μM imexon did not display significant changes in the Δψm (P > .05). After 16 hours of exposure to 180 μM imexon the fraction of cells with intact mitochondrial membrane potential decreased to 61% ± 2% and continued to decrease up to 48 hours after treatment (Figure 4A).
Concentration-dependent changes in mitochondrial membrane potential and levels of ROS after imexon treatment.
Reduction of the mitochondrial membrane potential (Δψm, A) and formation of reactive oxygen species (ROS, B) in RPMI 8226 myeloma cells treated with imexon for 48 hours as measured by CMXRos or HE staining and flow cytometry. Treatment with 200 μM tbhp for 30 minutes was used as positive control. Data are representative of 3 experiments. The fraction of cells staining with CMXRos having high Δψm and the fraction of cells staining with dihydroethidium (HE) corresponding to cells with increased levels of ROS are indicated.
Concentration-dependent changes in mitochondrial membrane potential and levels of ROS after imexon treatment.
Reduction of the mitochondrial membrane potential (Δψm, A) and formation of reactive oxygen species (ROS, B) in RPMI 8226 myeloma cells treated with imexon for 48 hours as measured by CMXRos or HE staining and flow cytometry. Treatment with 200 μM tbhp for 30 minutes was used as positive control. Data are representative of 3 experiments. The fraction of cells staining with CMXRos having high Δψm and the fraction of cells staining with dihydroethidium (HE) corresponding to cells with increased levels of ROS are indicated.
Time-dependent changes in mitochondrial membrane potential and levels of ROS after imexon treatment.
The loss of mitochondrial membrane potential Δψm (A) and induction of ROS (B) in RPMI 8226 myeloma cells exposed to 180 μM imexon for various time periods. The fractions of cells that have high Δψm accumulate CMXRos, whereas the fraction of cells producing ROS is indicated by the enhanced fluorescence of the oxidized form of HE. Data represent mean ± SE of 3 experiments. The stars indicate statistically significant differences from control untreated cells (P < .05).
Time-dependent changes in mitochondrial membrane potential and levels of ROS after imexon treatment.
The loss of mitochondrial membrane potential Δψm (A) and induction of ROS (B) in RPMI 8226 myeloma cells exposed to 180 μM imexon for various time periods. The fractions of cells that have high Δψm accumulate CMXRos, whereas the fraction of cells producing ROS is indicated by the enhanced fluorescence of the oxidized form of HE. Data represent mean ± SE of 3 experiments. The stars indicate statistically significant differences from control untreated cells (P < .05).
Imexon treatment is also associated with an increase in the levels of ROS.4 Figure 3B shows a representative flow cytometry experiment of RPMI 8226 human myeloma cells stained with HE to detect ROS (primarily superoxide) after treatment with imexon for 48 hours. Myeloma cells exposed to 200 μM tert-butylhydroperoxide (tbhp) for 30 minutes were included as a positive control. An increased fraction of cells staining with HE was observed after treatment with 45 μM imexon and this fraction expanded as the concentration of imexon was increased (Figure 3B). Similarly, treatment with 180 μM imexon for various time periods resulted in a time-dependent increase in the fraction of cells experiencing oxidative stress (Figure 4B). At 4 hours there was no change in ROS, but at 8 hours a significantly increased fraction of cells staining with HE was observed (P < .05). Longer treatments with imexon resulted in commensurately increased levels of ROS.
Mitochondrial membrane potential was also significantly reduced after treatment with 180 μM imexon for 48 hours in the NCI-H929 and NB-4 cells (Figure 5A). These cell lines were shown to be highly sensitive to imexon effects. In contrast, treatment with 180 μM imexon for 48 hours did not induce dramatic loss of Δψm in imexon-resistant U266 myeloma cells and in the RPMI 8226 cells pretreated with antioxidant, NAC (10 mM), for 3 hours and then treated simultaneously with 180 μM imexon and 10 mM NAC (Figure 5A). The data from flow cytometry experiments also have shown increased levels of ROS in other imexon-sensitive cell lines (NCI-H929 myeloma cell line, NB-4 acute promyelocytic leukemia cell line, and lymphocytes) after treatment with 180 μM imexon for 48 hours (Figure5B). On the other hand, ROS were not detected in imexon-resistant U266 cells nor in RPMI 8226 cells incubated with 10 mM NAC and 180 μM imexon simultaneously (Figure 5B).
Changes in mitochondrial membrane potential and levels of ROS in malignant cells and normal lymphocytes.
Imexon effect on Δψm (A) and levels of ROS (B) in other malignant cells, normal lymphocytes, and RPMI 8226 cells treated with 10 mM NAC. Data are representative of 3 experiments. The solid gray area represents cells treated with 180 μM imexon for 48 hours; the open area represents untreated cells.
Changes in mitochondrial membrane potential and levels of ROS in malignant cells and normal lymphocytes.
Imexon effect on Δψm (A) and levels of ROS (B) in other malignant cells, normal lymphocytes, and RPMI 8226 cells treated with 10 mM NAC. Data are representative of 3 experiments. The solid gray area represents cells treated with 180 μM imexon for 48 hours; the open area represents untreated cells.
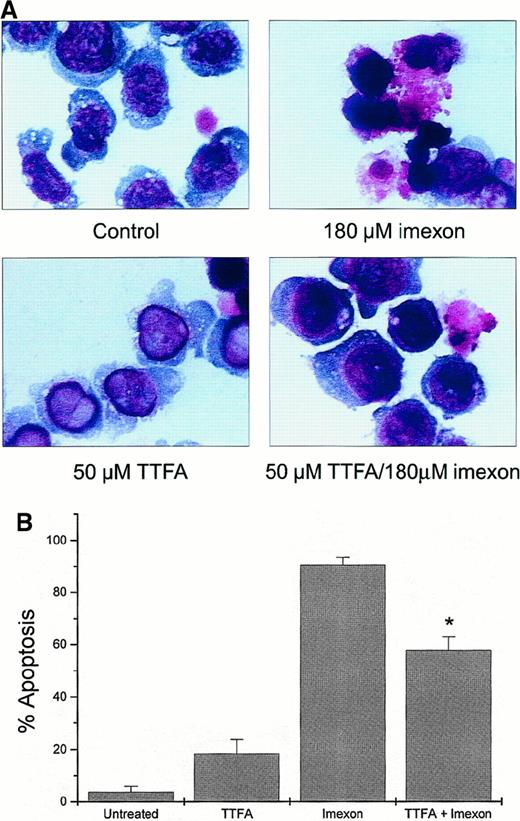
Inhibition of imexon-induced cytotoxicity by the mitochondrial inhibitor TTFA
Theonyltrifluoroacetone inhibits superoxide production in the mitochondrial complex II of the electron transport chain. This compound (50 μM) was not toxic in myeloma cells as measured by eosin Y staining (data not shown). Myeloma cells pretreated overnight with 50 μM TTFA and then simultaneously treated with 180 μM imexon and 50 μM TTFA for 48 hours showed reduction in imexon-induced cytotoxicity. Morphologic changes observed in control cells, imexon-treated cells, cells exposed to TTFA only, and cells treated with imexon and TTFA are shown in Figure 6A. Two hundred cells per slide in each treatment group were evaluated for characteristic features of apoptosis by bright-field microscopy (100 × oil immersion). The majority of cells (90.5% ± 2.9%) exposed to 180 μM imexon for 48 hours exhibit typical features of apoptosis, including chromatin condensation, cell shrinkage, and cytoplasmic blebbing. In contrast, only 57.8% ± 5.2% of the RPMI 8226 cells pretreated overnight with 50 μM TTFA and then treated with 180 μM imexon and 50 μM TTFA simultaneously for 48 hours display characteristic apoptotic features (P < .05). In the untreated RPMI 8226 cells and cells treated with 50 μM TTFA, we found 3.7% ± 2.3% and 18.4% ± 5.3% apoptotic cells, respectively.
Protection of RPMI 8226 myeloma cells against imexon-induced toxicity by treatment with TTFA.
The cells were treated with 180 μM imexon or 50 μM TTFA or both and evaluated for morphologic changes by bright-field microscopy after DiffQuick staining (A, × 100 oil immersion). The untreated cells were included as control. Two hundred cells per slide were evaluated for apoptotic changes. The graph represents the fraction of apoptotic cells in the different treatment groups (B). The star indicates a statistically significant difference as compared to the imexon-treated cells (P < .05).
Protection of RPMI 8226 myeloma cells against imexon-induced toxicity by treatment with TTFA.
The cells were treated with 180 μM imexon or 50 μM TTFA or both and evaluated for morphologic changes by bright-field microscopy after DiffQuick staining (A, × 100 oil immersion). The untreated cells were included as control. Two hundred cells per slide were evaluated for apoptotic changes. The graph represents the fraction of apoptotic cells in the different treatment groups (B). The star indicates a statistically significant difference as compared to the imexon-treated cells (P < .05).
Mitochondrial DNA damage
A semiquantitative PCR assay was used to detect imexon-induced DNA damage in mitochondria or in the nucleus. DNA lesions such as strand breaks, base modification, or apurinic sites will block DNA polymerase activity. Thus, the amount of amplified product will be decreased in PCRs using such damaged templates. The data show that imexon exposure, at low concentrations for 48 hours, induced a loss of 8.9-kb amplified product of the mitochondrial genome. However, amplification of a nuclear 10.4-kb fragment of thehprt gene was not affected (Figure7). These results indicate that imexon damages mitochondrial DNA but not nuclear DNA. Higher concentrations of imexon (180 μM exposure for 48 hours) induced changes in both mitochondrial and nuclear DNA. A bifunctional aziridine containing the DNA alkylator, AZQ, was used as a positive control in this study. As expected, AZQ treatment (2.7 μM) for 24 hours or 48 hours induced a loss of both mitochondrial and nuclear PCR products in the same proportion (Figure 7). Thus, there was not preferential damage of mitochondrial DNA with a bifunctional aziridine alkylator.
Semiquantitative PCR of DNA fragments from untreated RPMI 8226 cells and cells treated with various imexon concentrations.
Panel A represents mitochondrial DNA fragments from control cells (0) and cells treated with 45, 90, and 180 μM imexon for 48 hours. AZQ was included as positive control (2.7 μM for 24 or 48 hours; lanes 5 and 6). Panel B represents nuclear DNA fragments from cells treated as above. The corresponding graphs represent relative densities of individual bands analyzed by the Eagle Eye II Video Still System (Stratagene).
Semiquantitative PCR of DNA fragments from untreated RPMI 8226 cells and cells treated with various imexon concentrations.
Panel A represents mitochondrial DNA fragments from control cells (0) and cells treated with 45, 90, and 180 μM imexon for 48 hours. AZQ was included as positive control (2.7 μM for 24 or 48 hours; lanes 5 and 6). Panel B represents nuclear DNA fragments from cells treated as above. The corresponding graphs represent relative densities of individual bands analyzed by the Eagle Eye II Video Still System (Stratagene).
Discussion
Imexon is a monoaziridine compound originally studied for immune-enhancing effects on lymphocytes.35,36 Several studies clearly demonstrated imexon activity against a variety of fresh human tumors and tumor cell lines in culture.3,37 The antitumor effect of imexon was also shown in vivo with inhibition of large cell lymphoma development in severe combined immunodeficient mice.38 However, the precise mechanism of imexon action was unknown. In previous studies, we have shown that imexon induces oxidative stress and apoptosis in RPMI 8226 myeloma cells.4 Data presented here demonstrate that in RPMI 8226 myeloma cells imexon causes mitochondrial alterations associated with the apoptotic cell death pathway. These changes include mitochondrial enlargement, the loss of ΔΨm, and cytochrome c release from the mitochondria into the cytosol. In addition, we investigated the activity of imexon in several other myeloma cells, acute promyelocytic leukemia cells, and peripheral blood lymphocytes. Interestingly, the results in different cell lines show that imexon sensitivity correlates with the extent of mitochondrial changes after imexon treatment.
Mitochondria have been shown to play a major role in programmed cell death. Moreover, these organelles have a central position in the control of cell survival because they are necessary for the generation of energy required for cell function. Mitochondria consume large amounts of molecular oxygen for generating the energy required for the synthesis of ATP from adenosine diphosphate (ADP). However, continued consumption of oxygen by mitochondria routinely leads to the generation of ROS such as superoxide anion, organic peroxides, hydrogen peroxide, or hydroxyl radical, depending on the number of electrons transferred to molecular oxygen.39 Such oxidants can cause cell damage if not detoxified by antioxidant systems. It has been suggested in a number of studies that formation of ROS is a common scheme in some pathways of apoptosis.40-42 Oxidants and compounds that are capable of depleting GSH or damaging the cellular antioxidant defense system can directly induce or potentiate apoptosis.29,43,44 On the other hand, antioxidants such as N-acetyl-l-cysteine, Trolox, or butylated hydroxyanisole can inhibit apoptosis.40 45 Due to high cellular GSH levels, the GSH redox system represents one of the most important cellular defense systems against oxidative stress, particularly in mitochondria. GSH is synthesized solely in the cytoplasm from glutamine, glycine, and cysteine and can be transported into the mitochondria and the nucleus. Importantly, mitochondria from most mammalian cells do not contain catalase, an enzyme that plays a crucial role in the detoxification of hydrogen peroxide in extramitochondrial compartments.
In our previous report,4 it was shown in RPMI 8226 myeloma cells that (1) imexon can bind cysteine and glutathione in vitro, (2) imexon treatment is associated with decreased levels of cellular thiols in myeloma cells, and (3) imexon induces oxidative damage of cytosolic nucleotides and apoptosis. Thus, we speculated that after exposure to imexon, endogenous antioxidant defense systems in myeloma cells are compromised and the cellular ability to scavenge ROS is reduced. This can lead to increased endogenous production of ROS in mitochondria, leading to oxidative stress and the induction of apoptosis. The oxygen radicals produced in mitochondria can escape detoxifying pathways and induce various cellular injuries characterized by protein inactivation, DNA damage, and lipid peroxidation. Although some ROS diffuse from mitochondria to damage more distant cellular components, the half-life of most radicals is short. It is, therefore, conceivable that mitochondria may be affected by ROS to the greatest extent. One of the first consequences induced by imexon treatment in RPMI 8226 myeloma cells involves morphologic alteration of the mitochondria and formation of ROS. The significant enlargement of mitochondria was observed after imexon treatment and may represent an attempt by the cell to dilute the ROS by enlarging the area occupied by the ROS.46 This hypothesis is also supported by the fact that imexon affected mitochondrial DNA (mtDNA), but not nuclear DNA.
Data presented here from MTT and flow cytometric studies also indicate that sensitivity to imexon highly correlates with the loss of mitochondrial membrane potential and formation of ROS. For example, increased levels of ROS and loss of Δψm were detected in myeloma RPMI 8226 and NCI-H929 cells, and NB-4 cells treated with 180 μM imexon (Figure 5). These cell lines are highly sensitive to imexon effects (Table 1). In contrast, no such effects were observed in imexon-resistant U266 myeloma cell line treated with 180 μM imexon. Importantly, the results from MTT experiment indicated that human normal lymphocytes or lymphocytes stimulated with PHA were partially resistant to imexon.
That the generation of ROS comprises a crucial event in imexon action is also supported by our previous finding that antioxidants such as N-acetyl-l-cysteine protect against imexon-induced cytotoxicity.4 In agreement with these previous experiments the data from flow cytometry experiments indicate that NAC treatment inhibits formation of imexon-related ROS and loss of mitochondrial membrane potential. Furthermore, in this paper we have shown that TTFA-induced inhibition of the electron transport from succinate dehydrogenase (complex II) partially reduces imexon-induced apoptosis in RPMI 8226 cells. The sensitivity of imexon in RPMI 8226 myeloma cells and the lack of myelotoxicity could be explained by imexon effects on mitochondria.
The generation of ROS leads to the consequences associated with apoptosis such as release of cytochrome c from mitochondria to cytosol and morphologic features of apoptosis. We have shown that imexon in RPMI 8226 cells induces translocation of cytochrome c from mitochondria into the cytosol that can be detected as early as at 8 hours (Figure2).
However, the current findings do not explain why imexon selectively inhibits growth of RPMI 8226, NCI-H929 myeloma cells, and NB-4 acute promyelocytic leukemia cells, yet U266 myeloma cells are relatively resistant to imexon effects. Interestingly, U266 cells have been shown to be insensitive to the effects of dexamethasone and interferon-α.47 One explanation may be that imexon-sensitive cells are highly sensitive to oxidative stress and changes in thiol levels. For example, it is known that myeloma colony formation requires exogenous sulfhydryl supplementation for proliferation in soft agar.48,49 It is probable that levels of thiols in imexon-sensitive cells are intrinsically lower than in other cell types and that antioxidant defense systems of these cells are relatively less efficient at handling ROS. For example, it is known that levels of manganese superoxide dismutase, a mitochondrial enzyme responsible for detoxification of superoxide produced as a by-product in the electron transport chain, are lower in most cancer cells.50 Clearly, more studies need to be done to explain the unique sensitivity of these cancer cells to imexon. One of the strategies to clarify the differences in sensitivities to imexon in various cell lines could use DNA microarray studies in imexon-sensitive RPMI 8226 cells and imexon-resistant U266 cells.
In summary, this study highlights the mitochondrial effects of imexon in human myeloma cells. These effects are unique among existing anticancer agents. They support previous reports showing imexon effectiveness in various malignancies and low toxicity in limited human phase I/II trials conducted in Europe. Imexon is a promising chemotherapeutic agent and should be investigated further for potential in vivo activity against multiple myeloma.
Supported by grants CA 23074 and 17094 (to R.T.D.) from the National Institutes of Health (NIH), Bethesda, Maryland, and CA7176 (to M.M.B.) from the National Cancer Institute (NCI), NIH. K.D. and M.E.T. were partially supported by grant CA09213 from the NCI.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Robert T. Dorr, Arizona Cancer Center, 1515 N Campbell Ave, Tucson, AZ, 85724; e-mail: bdorr@azcc.arizona.edu.