The prevalence and significance of genetic abnormalities in older patients with acute myeloid leukemia (AML) are unknown. Polymerase chain reactions and single-stranded conformational polymorphism analyses were used to examine 140 elderly AML patients enrolled in the Southwest Oncology Group study 9031 for FLT3, RAS, and TP53 mutations, which were found in 34%, 19%, and 9% of patients, respectively. All but one of the FLT3 (46 of 47) mutations were internal tandem duplications (ITDs) within exons 11 and 12. In the remaining case, a novel internal tandem triplication was found in exon 11. FLT3 ITDs were associated with higher white blood cell counts, higher peripheral blast percentages, normal cytogenetics, and less disease resistance. All RAS mutations (28 of 28) were missense point mutations in codons 12, 13, or 61. RASmutations were associated with lower peripheral blast and bone marrow blast percentages. Only 2 of 47 patients with FLT3 ITDs also had a RAS mutation, indicating a significant negative association between FLT3 and RAS mutations (P = .0013). Most TP53 mutations (11 of 12) were missense point mutations in exons 5 to 8 and were associated with abnormal cytogenetics, especially abnormalities in both chromosomes 5 and 7. FLT3 and RAS mutations were not associated with inferior clinical outcomes, but TP53mutations were associated with a worse overall survival (median 1 versus 8 months, P = .0007). These results indicate that mutations in FLT3, RAS, or TP53 are common in older patients with AML and are associated with specific AML phenotypes as defined by laboratory values, cytogenetics, and clinical outcomes.
Introduction
The incidence of acute myeloid leukemia (AML) rises dramatically with age,1 yet treatment of elderly AML patients (≥ 55 years) remains unsatisfactory, with reported long-term survival less than 10%.2-5 Why do older patients with AML have such a poor prognosis? Older AML patients may be relatively “undertreated” compared to younger patients,2 or there may be significant differences between the molecular biology of AML in older patients and younger patients. For example, older patients with AML have a higher percentage of unfavorable cytogenetics and a higher rate of multidrug resistance (MDR1) expression compared to younger, de novo AML patients.6 These findings suggest that AML in older patients may frequently arise from prior myelodysplasia,7 and thus, these older patients would be predicted to have inferior clinical outcomes.
RAS, FLT3, and TP53 genes play important roles in the regulatory processes that govern proliferation, differentiation, and apoptosis,8-12 and abnormalities in these 3 genes have been implicated in the pathogenesis of younger adult patients with AML. Point mutations in RAS oncogenes occur in approximately 20% of de novo AML patients.13-15 The prognostic significance of RAS mutations remains unknown, with most large studies finding no clinical significance.14,16 Recently, studies have reported that approximately 20% of younger adult patients with AML have internal tandem duplications (ITDs) within the FLT3 gene, a subclass III tyrosine kinase receptor,17-24 and Kiyoi and coworkers found that the presence of an FLT3 ITD was the strongest predictor for an inferior survival in adult AML patients under the age of 60 years.21 Studies have also found TP53mutations in up to 15% of adult patients with AML,25-28and Wattel and colleagues demonstrated that TP53 mutations were associated with older age and a reduced overall survival.28
Few studies have examined the genetic abnormalities in older patients with AML, and none has examined multiple oncogenes and tumor suppressor genes in a single cohort of elderly patients with AML. BecauseRAS, FLT3, and TP53 have important interactions, examining these genes in the same cohort of patients may provide information about patterns of genetic disruption in AML. Therefore, we evaluated FLT3, RAS, andTP53 mutations in 140 older patients with AML enrolled in Southwest Oncology Group (SWOG) study 9031.
Patients, materials, and methods
Patientsand samples
Bone marrow and peripheral blood samples from patients were obtained prior to therapy in study SWOG 9031, a randomized, double blind, placebo-controlled trial of standard dose daunomycin and cytosine arabinoside with or without recombinant granulocyte colony-stimulating factor for patients over 55 years of age with previously untreated AML.29 Patients with secondary AML, defined by a history of myelodysplasia or leukemogenic therapy prior to the diagnosis of AML, were not excluded. Details of sample acquisition and risk stratification into cytogenetic groups (Table1) have been previously described.6
Polymerase chain reaction of NRAS,KRAS, FLT3, andTP53 fragments
The polymerase chain reaction (PCR) amplified the DNA sequence of interest: exons 1 and 2 of NRAS and KRAS; exons 5-8 of TP53; and exon 11, intron 11, and exon 12 ofFLT3.21,30,31 For the RAS andTP53 exons, an initial denaturing step at 95°C for 5 minutes was performed, followed by 45 cycles of denaturing at 95°C for 15 seconds, annealing for 20 seconds, and extension at 72°C for 15 seconds. A final extension step at 72°C for 5 minutes was performed. The annealing temperatures were as follows: NRASexon 1 (58°C); KRAS exon 1 (58°C); KRAS exon 2 (65°C); TP53 exon 5 (65°C); TP53 exon 6 (65°C); TP53 exon 7 (55°C); and TP53 exon 8 (55°C). The primers and amplification conditions for NRASexon 2 and FLT3 have been previously described.21 32 The primers for TP53 exons 5 to 8 were purchased from Clontech (Catalog no. 6397-1, Clontech Laboratories, Palo Alto, CA). The sequences for the remaining primers were: NRAS exon 1: 5′CTG GTG TGA AAT GAC 3′ and 5′ GGT GGG ATC ATA TTC ATC TA 3′; KRAS exon 1: 5′ TGT TGG ATC ATA TTC GTC CA 3′ and 5′ CCT GCT GAA AAT GAC TGA AT 3′; and KRASexon 2: 5′AAA GCC CTC CCC AGT CCT C 3′ and 5′ GGA GAA ACC TGT CTC TTG GAT ATT CTC 3′. PCR products were run on 2% agarose gels to verify amplification. The FLT3 products were also run on a 5% polyacrylamide gel.
Single-strand conformation polymorphisms
Single-strand conformation polymorphism (SSCP) analyses were performed to detect mutations as previously described.31,33 34 All gels were stained with SYBR green II RNA stain (Molecular Probes, Eugene, OR) at least 45 minutes without prewashing or destaining. Gels were examined for single-strand DNA (ssDNA) shifts on an Eagle Eye II (Stratagene, La Jolla, CA).
Direct nucleotide sequencing of the amplified fragments
The SSCP bands were cut from gels, and ssDNA was extracted with diethyl pyrocarbonate (DEPC) water.31 The ssDNA was reamplified to generate a sequencing template using the conditions described for the PCR methods. The amplified product was electrophoresed through a 2% agarose gel. The double-strand DNA (dsDNA) PCR product was extracted and sequenced as previously described.31 In the case of the FLT3 gene, reamplified PCR product was also electrophoresed through a 5% polyacrylamide gel. High-molecular-weight bands were excised, and dsDNA was extracted with DEPC water. The dsDNA then was directly sequenced using the Thermo Sequence Dye Terminator Sequencing Reaction.31
Statisticalmethods
Clinical, demographic, cytogenetic, and outcome data for patients in this study were collected and evaluated using standard SWOG procedures. The crude prevalence of one or more mutations in a group of patients was defined by expressing as a percentage the number of patients (Mi) bearing the mutation(s) among those who were genotyped (Ni), that is, Pi = (Mi/Ni) × 100%, where i is an index of patient groups. The selection of genotyped patients was known to be biased in favor of those with high white blood cell (WBC) counts, which might be associated with mutation prevalence. Therefore, adjusted prevalence estimates were calculated as the weighted average P* = Σ (wi × Pi), where wiis the proportion of all patients in study SWOG 9031 in the i-th WBC category. The WBC categories were defined arbitrarily by cut-points at 5000, 20 000, 50 000, and 100 000 cells/μL. The strength of association between mutations in pairs of genes was represented by the cross-product ratio, CPR = (Mpp × Maa)/(Mpa × Map), where Mij is the number of patients with mutation present (p) or absent (a) in the first (i) or second (j) gene. CPR = 1 indicates the absence of association, and CPR approaches 0 for increasingly strong negative associations. Complete response (CR) and resistant disease (RD) were defined as in the original clinical trial.29 Overall survival (OS) was measured from the date of entry into the trial until death from any cause, with observation censored for patients last known to be alive. For patients achieving CR, relapse-free survival (RFS) was measured from the date CR was established until AML relapse or death from any cause, with observation censored for patients last known to be alive without report of relapse. The Fisher exact test, Pearson chi-square test for independence, Wilcoxon and Kruskal-Wallis tests, and the log-rank test were used to test the heterogeneity of variables among groups defined by the presence of mutations. Logistic regression models were used to examine associations of mutation frequencies with patient characteristics and the prognostic effects of mutations and other factors on CR and RD. Proportional hazards regression models were used to examine the prognostic effects of mutations and other factors on OS and RFS. Statistical significance was reported in terms of 2-tailed Pvalues, and confidence intervals (CIs) were all calculated at the 95% confidence level. The analysis was based on data available as of December 16, 1999.
Results
Primary analyses of RAS,FLT3, and TP53mutations
Characteristics of the study population.
Pretreatment specimens were available for 140 of the 234 elderly patients in study SWOG 9031. Genotyped patients had higher peripheral blast counts, higher percentages of marrow blasts, higher WBC counts, lower CD34+ blast percentages, different distribution of French-American-British (FAB) classification, higher proportions of normal cytogenetic samples, and lower proportions of unfavorable cytogenetic samples, compared to the 94 patients unavailable for evaluation (Table 2). No significant differences between the genotyped and unavailable patients were found with respect to age, sex, race, MDR1 status, CR, RD, RFS, or OS.
Characteristics, frequency, and clinical significance ofRAS mutations.
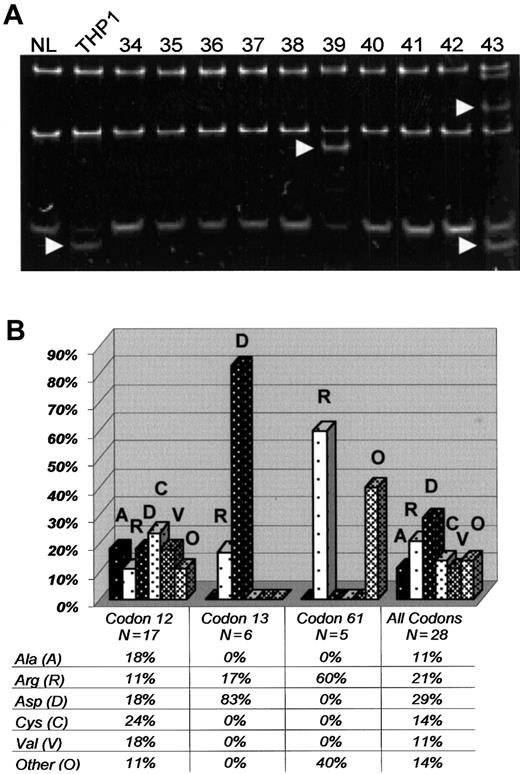
We found 28 RAS mutations in the 140 genotyped patients (Figure 1A). A total of 26 patients (19%, CI 13%-26%) had a mutation in either NRASor KRAS, with 2 patients (1%) having mutations in bothNRAS and KRAS. Accounting for the elevated WBC counts of the genotyped patients, the adjusted estimate forRAS mutations was 16%. The mutational frequencies for individual codons were as follows: NRAS codon 12 (7%, 10 of 140); NRAS codon 13 (2%, 3 of 140); NRAS codon 61 (3%, 4 of 140); KRAS codon 12 (5%, 7 of 140);KRAS codon 13 (2%, 3 of 140); and KRAScodon 61 (1%, 1 of 140). All RAS mutations were missense point mutations, changing the wild-type amino acid to a different amino acid. Overall, a glycine to aspartic acid (G→D) (single-letter amino acid codes) change was the most frequent amino acid substitution (29%, 8 of 28 RAS mutations) and was particularly common in codon 13 (Figure 1B).
RAS mutations in elderly AML.
(A) SSCP of PCR products fromNRAS exon 1. Banding patterns from the following samples are demonstrated: normal bone marrow (NL, left lane), THP1 (a positive control with known mutation in exon 1 of RAS), and elderly AML samples 34 to 43. Arrows point to shifted bands in THP1, 39, and 43. Direct nucleotide sequencing confirmed mutations in NRASexon 1 for THP1, 39, and 43. (B) Chart revealing the frequency of specific amino acid substitutions (vertical axis) in NRASand KRAS as separated by individual codons (horizontal axis). Alanine (A), arginine (R), aspartic acid (D), cysteine (C), valine (V), and all other amino acids (O).
RAS mutations in elderly AML.
(A) SSCP of PCR products fromNRAS exon 1. Banding patterns from the following samples are demonstrated: normal bone marrow (NL, left lane), THP1 (a positive control with known mutation in exon 1 of RAS), and elderly AML samples 34 to 43. Arrows point to shifted bands in THP1, 39, and 43. Direct nucleotide sequencing confirmed mutations in NRASexon 1 for THP1, 39, and 43. (B) Chart revealing the frequency of specific amino acid substitutions (vertical axis) in NRASand KRAS as separated by individual codons (horizontal axis). Alanine (A), arginine (R), aspartic acid (D), cysteine (C), valine (V), and all other amino acids (O).
Primary analyses revealed that patients with a RAS mutation had significantly lower percentages of peripheral (8% versus 43%,P = .004) and marrow blasts (61% versus 71%,P = .03) compared to those without a RASmutation. However, no significant differences were found between the 2 groups with respect to cytogenetics, age, WBC counts, CD34+blast percentage, or clinical outcomes (Table3).
Characteristics, frequency, and clinical significance ofFLT3 mutations.
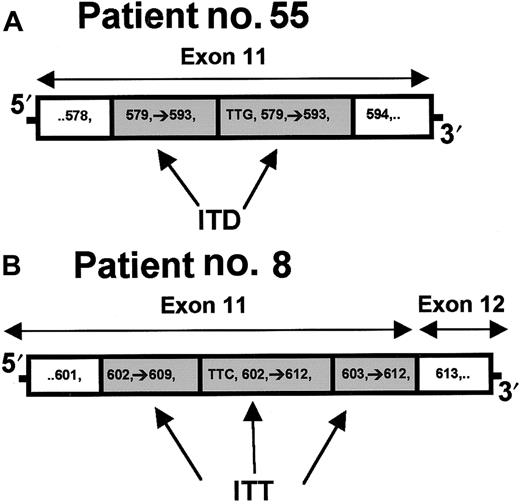
The FLT3 ITDs were detected in 41 of 140 (29%, CI 22%-38%) samples using agarose electrophoresis of PCR product (Figure2A). SSCP analyses found 6 additional ITDs (Figure 2B), increasing the frequency to 47 of 140 (34%, CI 26%-42%). The 6 patients with FLT3 ITDs detected by SSCP had lower marrow blast percentages (61% versus 78%), peripheral blast percentages (34% versus 72%), and WBC counts (21 500 versus 62 000/μL) compared to the other 41 patients with FLT3ITDs. The WBC-adjusted estimate of prevalence for FLT3 ITDs was 27%. Duplications ranged in size from 24 to 141 nucleotides. Most of the ITDs (72%) had between 3 and 15 nucleotides inserted at the beginning of the duplication (Figure 3A). All 47 FLT3 ITDs, including those with nucleotide insertions, were in-frame. In addition, sample no. 8 had a previously undescribed internal triplication (codons 603-609) (Figure 3B). All but one of the ITDs were localized to exon 11. The remaining case involved a large duplication that started in the distal part of exon 11 and ended in the proximal part of exon 12.
FLT3 mutations (ITDs) in elderly AML patients.
(A) PCR products of the FLT3 gene after electrophoresis through a 2% agarose gel. The PCR products from a normal bone marrow (NL, left lane) and elderly AML samples 10 to 14 are demonstrated. High-molecular-weight bands in samples 11, 12, and 14 (arrows) were confirmed to have mutations by direct nucleotide sequencing. (B) SSCP analysis of PCR product from a normal bone marrow (NL, left lane) followed by elderly AML samples 45, 46, 69, 74, 85, 109, and 112. The 7 elderly AML samples had normal-appearing bands with agarose electrophoresis. Arrows point to shifted bands in patient samples 74, 109, and 112. Direct nucleotide sequencing confirmed ITDs in samples 74, 109, and 112.
FLT3 mutations (ITDs) in elderly AML patients.
(A) PCR products of the FLT3 gene after electrophoresis through a 2% agarose gel. The PCR products from a normal bone marrow (NL, left lane) and elderly AML samples 10 to 14 are demonstrated. High-molecular-weight bands in samples 11, 12, and 14 (arrows) were confirmed to have mutations by direct nucleotide sequencing. (B) SSCP analysis of PCR product from a normal bone marrow (NL, left lane) followed by elderly AML samples 45, 46, 69, 74, 85, 109, and 112. The 7 elderly AML samples had normal-appearing bands with agarose electrophoresis. Arrows point to shifted bands in patient samples 74, 109, and 112. Direct nucleotide sequencing confirmed ITDs in samples 74, 109, and 112.
Structural comparison of ITD and internal tandem triplication in the
FLT3 gene. (A) ITD within exon 11 from patient 55. Three nucleotides (TTG = Leu) are inserted prior to the duplication of codons 579 to 593. (B) A novel internal tandem triplication within exon 11 from patient 8. Three nucleotides (TTC = Phe) are inserted prior to duplication of codons 602 to 612. Codons 603 to 612 are duplicated again, forming a triplication of codons 603 to 609.
Structural comparison of ITD and internal tandem triplication in the
FLT3 gene. (A) ITD within exon 11 from patient 55. Three nucleotides (TTG = Leu) are inserted prior to the duplication of codons 579 to 593. (B) A novel internal tandem triplication within exon 11 from patient 8. Three nucleotides (TTC = Phe) are inserted prior to duplication of codons 602 to 612. Codons 603 to 612 are duplicated again, forming a triplication of codons 603 to 609.
Compared to patients without FLT3 ITDs, those withFLT3 ITDs had significantly higher WBC counts (median 59.4 versus 20.3, P = .0003), higher absolute peripheral blast counts (median 26.6 versus 4.1, P = .0001), and lower percentage of CD34+ blasts (median 13% versus 65%,P = .0039). In addition, FLT3 ITDs were associated with normal cytogenetics (77% versus 40%,P = .0006) and consequently with intermediate cytogenetic risk (P = .0008). Despite the cytogenetic differences,FLT3 ITDs were not associated with significant differences in OS or RFS, although RD was slightly less frequent in the patients with FLT3 ITDs (Table 3).
Characteristics, frequency, and clinical significance ofTP53 mutations.
We found TP53 mutations in 12 of 140 (9%, CI 5%-14%) patients, with a WBC-adjusted prevalence estimate of 10%.TP53 mutations occurred in all 4 exons studied: exon 5 (4 mutations); exon 6 (1 mutation); exon 7 (3 mutations); and exon 8 (4 mutations). Most mutations (11 of 12) were missense point mutations that resulted in a single amino acid substitution. In one case, we found a single nucleotide deletion in codon 241 of exon 7, resulting in a premature stop codon. Mutations clustered near previously reported “hot spots,” particularly around codons 175 and 273, but only 2 mutations were found within these “hot spots.” The missense point mutations caused a variety of amino acid substitutions, with no particular amino acid substitution predominating.
The TP53 mutations were not significantly associated with WBC counts, peripheral blast counts, or blast percentages (Table 3), but were significantly associated with abnormal cytogenetics (91% versus 45%, P = .004), unfavorable cytogenetic risk (91% versus 17%, P < .0001), and worse OS (1 versus 8 months,P = .0007). Additional investigation of the 109 patients with cytogenetic data revealed that 6 (55%) of the 11 patients withTP53 mutations had abnormalities of both chromosomes 5 and 7, whereas none of the 98 patients without a TP53 mutation had abnormalities in both chromosomes 5 and 7.
Secondary analyses of RAS,FLT3, and TP53mutations
Correlation between RAS, FLT3, andTP53 mutations.
Approximately 50% (71 of 140) of older AML patients had mutations inFLT3 or RAS, with only 2 having mutations in both FLT3 and RAS. Consequently, the proportion of samples with a RAS mutation was significantly lower among patients with FLT3 mutations (2 of 47 or 4%) compared with those without FLT3 mutations (24 of 93 or 26%, CPR = 0.13, P = .0013). Similarly, nonsignificant negative associations of lesser magnitude were observed betweenTP53 mutations and mutations in either FLT3(CPR = 0.37, P = .34) or RAS (CPR = 0.37,P = .47), as only 3 patients had a TP53mutation and a mutation in one of the other genes. In total, 57% of the elderly AML samples harbored mutations in one or more of theRAS, FLT3, or TP53 genes. After accounting for the effect of WBC counts, the adjusted estimate of prevalence was 50%.
Correlations of specific mutations with laboratory values, cytogenetics, and clinical response.
We were interested in comparing the characteristics of subgroups of patients with a specific mutation to other subgroups of patients with different mutations, believing that these analyses may provide insight into the heterogeneity of AML. We, therefore, defined 3 groups based on the presence of mutations in a single gene: RAS+(23 patients), FLT3+ (43 patients), andTP53+ (9 patients). The patients with no mutations in any of the 3 genes comprised a fourth group, designated NM, for comparison (60 patients). The 5 patients with mutations in 2 separate genes were omitted from the following analyses. There were significant heterogeneities among the 4 groups with respect to WBC counts (P = .0004), marrow blast percentage (P = .0045), peripheral blast percentage (P = .0002), absolute peripheral blast counts (P = .0003), CD34+ blast percentages (P = .0092), presence of any cytogenetic abnormality (P = .0001), and cytogenetic risk group (P < .0001). There was no significant heterogeneity with respect to age (P = .081), secondary AML (P = .72), or FAB classification (P = .48).
To better elucidate the heterogeneity among the 4 groups, each mutated group was compared separately to the NM group (Table4). All of the associations discovered in the primary analyses retained their clinical significance in these secondary analyses. In addition, previously unrecognized associations were demonstrated. For example, in the primary analysis, the presence of RAS mutations was not associated with significant differences in WBC counts or CD34+ blast percentage (Table 3). However, in the secondary analysis, patients in the RAS+ group tended to have higher WBC counts (median 34.2 versus 18.5, P = 0.08) and a significantly lower CD34+ blast percentage (median 35% versus 70%, P = .048), compared with those in the NM group. As Table 4 demonstrates, this occurred because the median CD34+ blast percentage for the RAS+group lies between the relatively high medians of the NM andTP53+ groups and the relatively low median of the FLT3+ group.
Multiple logistic regression analysis was used to examine the impact ofFLT3, RAS, and TP53 mutations on CR, controlling for the 3 factors previously documented by Leith and colleagues to be significantly associated with CR in our cohort of patients: secondary AML, unfavorable cytogenetics, and MDR1 expression.6 Among the 105 genotyped patients with complete cytogenetic and MDR1 data, there was no significant additional prognostic contribution with respect to CR from RAS(P = .54), FLT3 (P = .63), orTP53 (P = .91). In this same cohort of elderly AML patients, Leith and coworkers found that unfavorable cytogenetics was a strong, independent negative prognostic factor for OS (P < .0001), whereas increasing age and increasing WBC count were negative prognostic factors of marginal significance.6 After controlling for these 3 factors, we found that FLT3 (P = .25), RAS(P = .46), or TP53 (P = .53) had no significant independent prognostic value with respect to OS.
Discussion
We examined the prevalence and significance of FLT3, RAS, and TP53 mutations in 140 of 234 older patients with AML in a single SWOG trial. The genotyped group differed in several respects from the patients not included (Table 2). In particular, the genotyped patients had higher WBC and blast percentages. These differences probably reflect the fact that the genotyped patients were selected on the basis of having material available for study. Although genotyped patients had higher WBC counts, there were no significant differences between the genotyped patients and unavailable patients with respect to clinical outcomes. Fifty-seven percent of the older AML patients in our study had a mutation inFLT3, RAS, and/or TP53. This prevalence was reduced slightly to 50% after adjusting for the overrepresentation of patients with higher WBC counts. Individually, the WBC-adjusted prevalences for FLT3, RAS, andTP53 mutations were 27%, 16%, and 10%, respectively. We also demonstrated that specific mutations were associated with specific AML “phenotypes.” For example, FLT3 ITDs were associated with higher WBC counts, higher blast counts, normal cytogenetics, lower percentage of CD34+ blasts, and less resistant disease. Patients with RAS mutations had higher WBC counts, lower percentage of blasts, and lower percentage of CD34+ blasts, compared to patients without a mutation in either FLT3,RAS, or TP53. Mutations of TP53 were associated with unfavorable cytogenetics and inferior OS. Finally, unlike most larger retrospective studies in adult patients with AML,FLT3 ITDs were not associated with inferior clinical outcomes in our population of older patients with AML.21 35-37
The WBC-adjusted prevalences of RAS and TP53mutations (16% and 10%, respectively) in our cohort of older patients with AML were similar to the prevalences that have been reported in younger adults with de novo AML (15%-20% for RAS mutations and 5%-15% for TP53mutations).13,14,21,27,28,38 However, the prevalence ofFLT3 ITDs in our cohort of AML patients (WBC-adjusted prevalence, 27%) was higher than in previous reports (5%-15% in children, 15%-23% in younger adults).19,21,22,35 Taken together, the results suggest that the prevalence of FLT3ITDs increases as the age of the AML patient increases. However, some of the increased prevalence of FLT3 ITDs in our study may have resulted from the use of the more sensitive SSCP assay. We have found that SSCP detects 0.4% to 0.8% of FLT3ITD+ DNA in a background of DNA from a normal bone marrow, whereas agarose electrophoresis only detects 3% to 6% ofFLT3 ITD+ DNA in a background of normal DNA (D.L.S., unpublished data, August 2000). In our study, SSCP identified 6 additional FLT3 ITDs, reiterating the improved sensitivity of SSCP. Why FLT3 ITDs would be present in patients with leukemia at levels below 3% to 6% is unknown. These patients had relatively lower blast counts in the marrow and peripheral blood, and thus, the improved sensitivity of SSCP may have been required to detect the FLT3 ITD. Or, perhaps, only subclones of leukemia cells harbored FLT3 ITDs. Indeed, mutations in other genes such a RAS may occur in small populations of AML cells within a given patient.16
No prior studies have evaluated a single cohort of previously untreated patients with AML for mutations in FLT3, RAS, andTP53. Nakano and coworkers examined 28 relapsed AML patients (median age, 53.5 years) who were enrolled on a variety of different treatment protocols.36 They found 23 mutations in the 28 relapsed AML patients, with 5 patients harboring more than one mutation. A total of 60% (17 of 28) of patients had at least one mutation in either FLT3, RAS, or TP53. Although many of the mutations were present in both the diagnostic and relapsed samples, 8 mutations were found exclusively at relapse, and 5 mutations were found exclusively at diagnosis. This study also analyzed the clinical significance of NRAS, FLT3, andTP53 mutations in their relapsed AML patients. They found that NRAS mutations had no impact on prognosis, whereasFLT3 and TP53 mutations were associated with a shorter overall survival.36
Other retrospective studies have also demonstrated that FLT3ITDs are associated with inferior clinical outcomes, especially in younger AML patients and children,19,21,35,36 and a recent small subgroup analysis of 31 older patients with AML (> 60 years) found that the 7 patients with FLT3 ITDs had a worse prognosis.37 However, all these studies were composed of heterogeneous groups of AML patients who were enrolled in a variety of different treatment protocols.19,21,36 37 In our cohort of 140 older patients with AML enrolled in a single treatment trial,FLT3 ITDs were not associated with inferior clinical outcomes, but rather with less disease resistance. Several reasons could explain the differences in clinical outcomes between our study compared to previous studies. Perhaps the clinical effects ofFLT3 ITDs in older patients with AML are overshadowed by the poor OS in this population. Alternatively, FLT3 ITDs in older patients may have slightly different effects due to intrinsic biologic differences in the myeloid cells of older patients. Or,FLT3 ITDs may differ between younger and older AML patients. Additional analyses comparing the sequences, location, and characteristics of FLT3 ITDs will be required to address this later possibility.
Only 4% (2 of 47) of older, AML patients with FLT3 ITDs also had a RAS mutation. If FLT3 andRAS mutations were independent, a significantly higher percentage of samples would harbor both FLT3 andRAS mutations. Also, we found some phenotypic similarities between patients with FLT3 and RAS mutations. Both groups had higher WBC counts and lower percentages of patients with CD34+ blasts. These findings suggest thatFLT3 and RAS mutations may elicit their effects through similar pathways, and in vitro biologic data link theFLT3 and RAS pathways. Activation of theFLT3 gene phosphorylates phospholipase C-γ1, guanosine triphosphatase-activating protein, Shc, and Vav, some of which have been linked to RAS pathways,11,12,39 and a recent study found that FLT3 ITDs constitutively activate mitogen-activated protein kinase in murine cell lines, a kinase also activated by RAS.40 The rarity of samples with mutations in both genes, the phenotypic similarities, and the biologic data reinforce the hypothesis that FLT3 and RASmay share a common pathway. However, some phenotypic differences between the FLT3 and RAS groups were also found. These differences may be a consequence of other genetic abnormalities that have yet to be identified or suggest that although FLT3and RAS share parts of a common pathway, mutations inFLT3 and RAS may activate additional effectors that are not common to both pathways.
Mutations in the FLT3, RAS, and TP53genes are relatively common in elderly patients with AML, with estimated prevalences of 27%, 16%, and 10%, respectively. The prognostic significance of these mutations remains unknown, and larger, prospective analyses will be needed to clarify the clinical significance of these mutations. Also, additional genetic analyses may discover other prognostic factors that can be used to develop a more informative system of risk stratification for AML. This risk stratification will be invaluable for patient selection in future treatment protocols. Because of the high frequency of theseFLT3 ITDs, these mutations may be useful for monitoring minimal residual disease in AML, and we and others are developing assays to use FLT3 ITDs for this purpose. Also, molecular investigations that interrogate the pathways of these 3 genes may illuminate critical points within these pathways that can be targeted for future treatments. Similar molecular targets have already been developed for chronic myelogenous leukemia.41
Supported by National Institutes of Health grants CA18029, CA 38926, CA32102, and K12-CA76930, and the Friends of Jose Carreras International Leukemia Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Derek L. Stirewalt, Fred Hutchinson Cancer Research Center, D4-100, 1100 Fairview Ave N, Seattle, WA 98109; e-mail: dstirewa@fhcrc.org.