The earliest stages of lymphoid commitment from human pluripotent hematopoietic stem cells have not been defined. A clonogenic subpopulation of CD34+CD38− cord blood cells were identified that expressed high levels of the CD7 antigen and possessed only lymphoid potential. CD34+CD38−CD7+ (CD7+) cells uniformly coexpressed CD45RA and HLA-DR;c-kit and Thy-1 expression was absent to low. Clonal analysis demonstrated that single CD7+ cells could generate B cells, natural killer cells, and dendritic cells but were devoid of myeloid or erythroid potential. In contrast, control CD34+CD38−CD7−(CD7−) cells generated both lymphoid and myelo-erythroid cells. The lymphoid potential (generation of lymphoid progeny in bulk and single cell cultures) of CD7+ cells was equivalent to that of the pluripotent CD7− cells. RNA expression studies showed that CD7+ cells expressed PU.1 and GATA-3, but did not express Pax-5, terminal deoxynucleotide transferase, or CD3ε. In contrast to the previously described murine common lymphoid progenitor, the α chain of the receptor for interleukin-7 was not detected by fluorescence-activated cell sorting analysis or RNA polymerase chain reaction in CD7+cells. These studies identify a clonogenic lymphoid progenitor with both B-cell and natural killer cell lineage potential with a molecular profile that suggests a developmental stage more primitive than previously identified lymphoid progenitors. The CD7+phenotype distinguishes primitive human lymphoid progenitors from pluripotent stem cells, thus allowing the study of regulation of early human lymphopoiesis and providing an alternative to pluripotent stem cells for genetic manipulation and transplantation.
Introduction
The lineage commitment pathways that lead from human, pluripotent hematopoietic stem cells (PHSCs) and culminate in the production of lymphoid and myelo-erythroid progeny have long been the subject of speculation. Assumptions about these pathways have been based largely on data from murine studies. The traditional model of lymphohematopoietic differentiation from pluripotent stem cells proposes that all lymphopoiesis is derived from a common lymphoid progenitor (CLP) that generates a differentiation pathway mutually exclusive from the hematopoietic (myeloid, erythroid, and megakaryocytic) pathway. The CLP would therefore be, by definition, a progenitor with full lymphoid potential (T, B, and natural killer [NK] cell) but no capacity for myeloid, erythroid, or megakaryocytic differentiation.
Against the theory of dichotomous paths toward myeloid and lymphoid differentiation is the demonstration of common B lymphoid and macrophage progenitors in murine fetal liver1 and in adult murine bone marrow.2 However, support for the traditional model has come from direct evidence for the existence of murine CLP3 and common myeloid progenitors (CMPs)4in bone marrow. Murine CLP can be distinguished immunophenotypically from PHSCs by expression of the α chain of the receptor for interleukin-7Rα (IL-7Rα) and by low or absent expression ofc-kit and Thy-1.
Galy et al5 described a CD34+lin−CD38+CD10+progenitor cell population in human adult and fetal bone marrow that possessed lymphoid potential but was severely depleted of myelo-erythroid potential. Limiting dilution analysis was used to study the B and NK potential of these cells, and T-cell potential of the whole population was assessed in vivo. IL-7Rα expression was not reported in these studies.
Identification of primitive, human, lymphoid-restricted progenitors has been hampered by the technical inability to measure the myeloid and lymphoid lineage potential of single human progenitors and pluripotent stem cells. Although in vivo xenogeneic assays can be used to measure the lineage potential of whole populations, the lineage potential (and restriction) of individual cells cannot be assessed without the use of gene marking, a methodology that remains relatively inefficient. In addition, variations in engraftment of certain progenitor populations can lead to difficulties in interpreting the absence of specific lineages within each in vivo model system.
In vitro assays that allow the measurement of both myeloid and lymphoid lineage potential from single human cells have been developed.6-8 The most primitive fraction of the CD34+ population in cord blood and bone marrow has been identified as the subpopulation lacking expression of CD38 antigen, ie, CD34+CD38−.9,10 Using single-cell in vitro assays, researchers have identified pluripotent cells in the CD34+CD38− population of cord blood at a frequency of 1% to 20%.6,8,11 Although the CD34+CD38− population is significantly enriched in pluripotent cells, it is nonetheless functionally heterogeneous.6 12 These functionally disparate subpopulations have as yet defied prospective identification and isolation.
In this report we have identified a CD34+CD38−subpopulation in cord blood that expresses high levels of CD7. The CD34+CD38−CD7+ population is devoid of myeloid or erythroid potential but is highly clonogenic in lymphoid cultures. Single CD34+CD38−CD7+ cells generate B-lymphoid, NK, and dendritic progeny. Molecular characterization places the CD7+ progenitor at an earlier stage than any previously described human B- or T-lymphoid progenitors.
Materials and methods
Cell isolation
Human umbilical cord blood was collected into centrifuge tubes containing citrate-phosphate-dextrose (Sigma, St Louis, MO) according to a protocol approved by the Institutional Review Board (the Committee on Clinical Investigations) at Childrens Hospital Los Angeles. Fresh mononuclear cells were then isolated by density centrifugation using Ficoll-Hypaque (Amersham Pharmacia Biotech, Piscataway, NJ) and incubated with antibodies for CD34+ cell enrichment using the Minimacs magnetic separation column (Miltenyi Biotec, Auburn, CA). Populations enriched in CD34+ cells (40%-95% purity) were then incubated with fluorescent-labeled antibodies for fluorescence-activated cell sorting (FACS) analysis and isolation on either FACSCalibur or FACSVantage flow cytometers (Becton Dickinson Immunocytometry Systems [BDIS], San Jose, CA) using HeNe and argon lasers.
T-cell populations were isolated from postnatal thymic fragments obtained from pediatric patients undergoing cardiac surgery under a protocol approved by the Committee on Clinical Investigations at Children's Hospital Los Angeles. Mononuclear cells were obtained from fresh thymus and incubated with CD3-allophycocyanin (APC), CD8-phycoerythrin (PE), CD4–fluorescein isothiocyanate (FITC) (all BDIS). Two populations were isolated for polymerase chain reaction (PCR) analysis of gene expression: CD3+CD4+CD8+, ie, early T-cell precursors, and CD3+CD4+CD8−, ie, mature T cells.
Immunophenotypic analysis and isolation
Isotype control antibodies were used as follows: For 106 cells in 100 μL phosphate buffered saline (PBS), 20 μL immunoglobulin G1 (IgG1)–PE, 20 μL IgG1-FITC, 5 μL IgG1-APC (all BDIS), and 10 μL IgG1-ECD (Phycoerythrin-Texas red) (Coulter/Immunotech, Miami, FL). Quadrants were set to include at least 97% of the isotype-negative cells. Four-color FACS analysis used 10 μL CD34-ECD (clone 581; Coulter/Immunotech), 5 μL CD38-APC (leu-17; BDIS), and 20 μL CD7-PE (clone 8H8.1; Coulter/Immunotech) combined with one of the following FITC-labeled antibodies: CD45RA, CD2, CD3, CD4, CD8, CD10 (W8E7), CD19, CD56, CD14 (all from BDIS), glycophorin (Coulter/Immunotech), and CD20 (Pharmingen, San Diego, CA) each used at 20 μL for 106 cells in a total of 100 μL PBS. PE-labeled antibodies CD11b (BDIS), HLA-DR (BDIS), Thy-1 5E10 (Pharmingen), c-kit (YB5.B8) (Pharmingen), IL-7Rα (CD127; Coulter/Immunotech), IL-2Rα (CD25; BDIS), and IL-3Rα (CDw 123, clone 9F5; Pharmingen) were combined instead with CD34-ECD, CD38-APC, and CD7-FITC (Coulter/Immunotech).
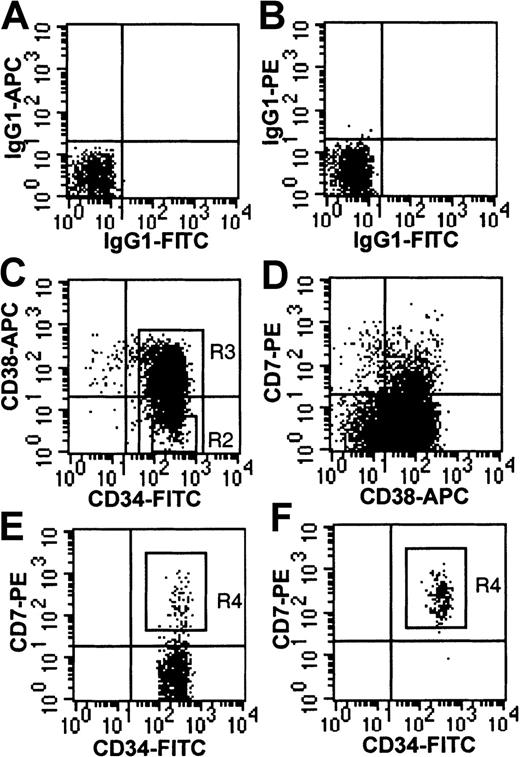
For FACS isolation of CD7+ cells, CD34+-enriched cells (more than 90% purity) were incubated either with CD34-FITC (BDIS), CD38-APC, and CD7-PE or with CD34-ECD (Immunotech), CD38-APC, and CD7-PE and the following lineage FITC-labeled antibodies: CD3, CD19, CD56, CD14, and glycophorin. In some experiments, CD34+lin− cells were first isolated by FACS and then stained with CD38 and CD7 for a second round of isolation. Isolation of CD34+CD38−CD7+ cells was accomplished by gating on those cells with CD34brightexpression, CD38− expression (below isotype control), and CD7bright expression (Figure1).
Immunophenotype of CD34+-enriched cord blood cells.
(A,B) Isotype controls on CD34+ column-enriched cells from lymphoid region defined by forward and side scatter (R1 not shown). (C) CD34+ column-enriched cells gated on R1. Shown are R2 gate (CD34+CD38− cells) and R3 gate (CD34+ cells). (D) CD38 and CD7 expression on CD34+ cells gated from R3. (E) CD34+CD38− cells gated from R2. Shown is the CD7+ sort gate (R4). (F) Re-analysis of CD34+CD38−CD7+ cells isolated from R4 (98.9% purity).
Immunophenotype of CD34+-enriched cord blood cells.
(A,B) Isotype controls on CD34+ column-enriched cells from lymphoid region defined by forward and side scatter (R1 not shown). (C) CD34+ column-enriched cells gated on R1. Shown are R2 gate (CD34+CD38− cells) and R3 gate (CD34+ cells). (D) CD38 and CD7 expression on CD34+ cells gated from R3. (E) CD34+CD38− cells gated from R2. Shown is the CD7+ sort gate (R4). (F) Re-analysis of CD34+CD38−CD7+ cells isolated from R4 (98.9% purity).
Lymphoid cultures
Isolated cells were plated in bulk or single (by automated cell deposition unit [ACDU] of FACSVantage) cell culture in 96-well plates on established S17 stroma (a generous gift from Dr Kenneth Dorshkind, University of California Los Angeles) in lymphoid medium (RPMI 1640 [Irvine Scientific, Santa Ana, CA], 5% fetal calf serum [FCS; Biowhittaker, Walkersville, MD; screened for B-cell cultures], 50 μM 2-mercaptoethanol [2-ME; Sigma], penicillin/streptomycin [Gemini Bio Products, Calabasas, CA], and glutamine [Gemini Bio Products]). During the first 3 days of culture, cells were stimulated on S17 stroma in either interleukin-3 [IL-3; 10 ng/mL; R&D, Minneapolis, MN], IL-6 [10 ng/mL; R&D], and c-kit ligand [KL; 50 ng/mL; R&D] or in IL-3 and Flt 3 ligand (FL; 50 ng/mL; Immunex, Seattle, WA). At day 3, and every 4 to 7 days thereafter, half the lymphoid medium was changed to contain only FL. In some experiments, to stimulate NK-cell growth, IL-15 (50 ng/mL; Endogen, Cambridge, MA) was added to established S17 cocultures. Lineage-specific differentiation in lymphoid cultures was assessed using CD19-APC for B-lymphoid cells, CD56-PE/FITC for NK cells, and CD1a-FITC/PE (all BDIS) for dendritic cells. CD1a+dendritic cells were further analyzed for expression of HLA-DR–PE, CD11c-PE (BDIS), CD14-PE (BDIS), and CD86-FITC (Pharmingen).
NK function assay
Cytotoxicity of CD56+ cells generated in culture was analyzed using a 4-hour Cr51 release assay. The NK-sensitive cell line K562 (American Tissue Type Collection [ATCC], Rockville, MD) was used as target cells after labeling with Cr51 for 60 minutes. CD56+ cells were isolated by FACS from 2-week-old lymphoid cultures (S17 coculture in IL-2 or IL-15); the medium in the final 6 days of culture was changed to 5% human AB serum and 15% FCS. Isolated CD56+ cells were incubated for 4 hours with labeled K562 cells at effector-to-target (E/T) ratios of 2.5:1, 5:1, and 10:1. As a positive control, fresh, unsorted peripheral mononuclear cells from healthy volunteers were tested for NK-mediated cytotoxicity at E/T ratios of 12:1, 25:1, and 50:1. Analysis was performed on triplicate samples. Percentage lysis was calculated, using the formula (experimental mean cpm) − (spontaneous release mean cpm)/(total release mean cpm) − (spontaneous release mean cpm) × 100%.
Myelo-erythroid cultures
To optimize production and differentiation of myeloid and erythroid cells, sorted populations were cultured on irradiated allogeneic human bone marrow stroma in long-term bone marrow culture medium (30% FCS, 1% bovine serum albumin [Sigma], Iscoves modified Dulbecco medium [Gibco BRL, Bethesda, MD], 2-ME, 10−6 M hydrocortisone [Sigma], penicillin/streptomycin, and glutamine). The combination of 10 ng/mL IL-3, 10 ng/mL IL-6, 50 ng/mL KL, and 2 U/mL erythropoietin (EPO) was used in these myelo-erythroid cultures. The establishment of stromal layers has been previously described.10 Every 3 to 4 weeks, nonadherent cells were removed from the cultures and counted, and aliquots were replated in methylcellulose medium to measure progenitor content. Methylcellulose medium contained 1.3% methylcellulose in long-term bone marrow culture medium with IL-3, IL-6, KL, 50 ng/mL granulocyte-macrophage colony stimulating factor (GM-CSF), and EPO. Colony-forming unit–cells (CFU-C) were counted after 14 days in methylcellulose culture. In some cases, cells from lymphoid culture (on S17 stroma) were also switched to methylcellulose medium as above to measure the presence of myeloid and erythroid progenitors.
PCR analysis of RNA expression
Cell populations (400 to 20 000 cells) from 2 to 4 pooled cord blood samples were deposited by FACSVantage into 0.5 cc PBS, and RNA was extracted using RNA Stat-60 (Tel-test, Friendswood, TX). RNA was reverse-transcribed using the Superscript RT kit (Gibco BRL) and random nonamers, in the presence of RNAguard (Pharmacia Biotech, Piscataway, NJ). Complementary DNA (cDNA) thus generated was then split into aliquots for separate PCR assays to detect specific sequences as below.
All cDNA samples were subjected to PCR to detect a 330-base pair (bp) fragment of human β2-microglobulin as a positive control for cDNA integrity using the primers: sense -CTC GCG CTA CTC TCT CTT TC and antisense -CAT GTC TCG ATC CCA CTT AAC under the following conditions: 94°C for 1 minute, 58°C for 1 minute, 72°C for 2 minutes (28 to 32 cycles), and 72°C for 10 minutes (1 cycle).
A 122-bp fragment of PU.1 cDNA was detected using the primers sense -G GAA GGG TTT CCC CTC GTC and antisense -G GTC GCT ATG GCT CTC CCC13 under the following conditions: 94°C for 1 minute, 60°C for 1.5 minutes, 72°C for 2 minutes (30 to 35 cycles), and 72°C for 10 minutes (1 cycle). A 116-bp fragment of IL-7Rα cDNA was detected using the primers sense -CA GGG GAG ATG GAT CCT ATC and antisense -CC ATA CGA TAG GCT TAA TCC14 under the following conditions: 94°C for 15 minutes (1 cycle), 94°C for 1 minute, 53°C for 1.5 minutes, 72°C for 2 minutes (32 to 34 cycles), and 72°C for 10 minutes (1 cycle) with Hot Start taq (Qiagen, Valencia, CA). A 174-bp fragment of Pax-5 cDNA was detected using the primers sense -CCA GTC CCA GCT TCC AGT CAC AG and antisense -GG AGA CTC CTG AAT A CC TTC GTC TC15 under the following conditions: 94°C for 1 minute, 60°C for 1.5 minutes, 72°C for 2 minutes (32 to 34 cycles), and 72°C for 10 minutes (1 cycle). A 600-bp fragment of terminal deoxynucleotide transferase (TdT) cDNA was detected using the primers sense -C AGA ACT CTG AGT AAA GTA AG and antisense -CAT C TT CCG CTC ATG TGT GGC16 under the following conditions: 94°C for 15 minutes (1 cycle), 94°C for 1 minutes, 61°C for 1.5 minutes, 72°C for 1 minutes (32 to 34 cycles), and 72°C for 10 minutes (1 cycle) using Hot Start taq. A 115-bp fragment of GATA-3 was detected using the primers sense -CAC TCC TAC ATG GAC GCG GCG C and antisense GGC CCT GAC CGA GTT TCC G17 under the following conditions: 94°C for 1 minute, 58°C for 1.5 minutes, 72°C for 2 minutes (32 to 35 cycles), and 72°C for 10 minutes (1 cycle). A 640-bp fragment of CD3ε was detected using the primers sense AGT TGG CGT TTG GGG GCA AGA TGG TAA TGA AGA AA and antisense CCC AGG AAA CAG GGA GTC GCA GGG GGA CTG GAG AG18 under the following conditions: 95°C for 15 minutes (1 cycle), 94°C for 1 minute, 55°C for 1 minute, 72°C for 2 minutes (35 cycles), and 72°C for 10 minutes (1 cycle) using Hot Start taq. A 350-bp fragment of pre-T-cell receptor α (pTα) was detected using the primers sense TCC AGC CCT ACC CAC AGG TG and antisense TAG AAG CCT CTC CTG ACA GAT GCA T19 under the following conditions: 95°C for 15 minutes (1 cycle), 94°C for 1 minute, 65°C for 1 minute, 72°C for 2 minutes (35 cycles), and 72°C for 10 minutes (1 cycle) using Hot Start taq. Nalm-6 cells were used as positive controls for β2-microglobulin, Pax-5, IL-7Rα, and TdT expression. Jurkat cells were used as a positive control for GATA-3 expression and HL-60 cells for PU.1 expression.
The risk of detecting contaminating genomic DNA in the above reactions was avoided by designing primer pairs that span introns using known genomic sequence data when available. In addition, all primer pairs were tested on human genomic DNA (extracted DNA, no reverse transcriptase added) to ensure genomic DNA would not be confused with cDNA amplification. In these cases, β actin was used as a positive control for integrity and loading of genomic DNA. All results were confirmed by at least 3 separate experiments for each gene using different pooled samples.
Results
Immunophenotypic analysis and isolation of CD34+CD38−CD7+ cells
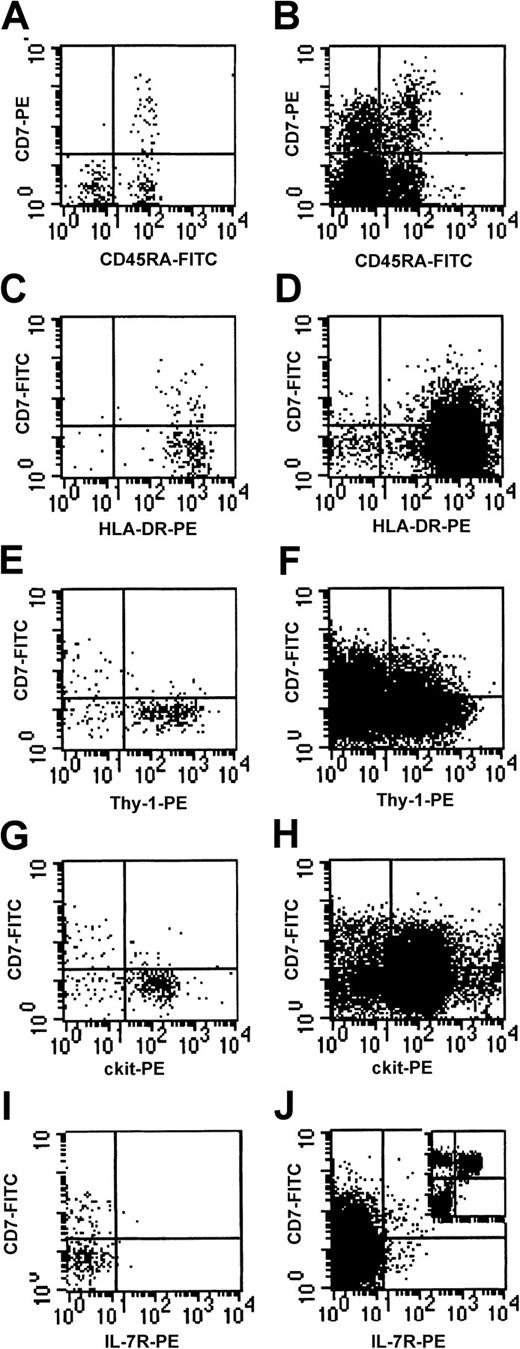
FACS analysis of cord blood mononuclear cells revealed that a subpopulation (8.2% ± 1.6%, n = 10) of CD34+CD38− cells expressed high levels of CD7 (CD7+ cells) (Figure 1). Antigens associated with B-lymphoid (CD19, CD20), NK cell (CD56), T-lymphoid (CD2, CD3, CD4, CD5, CD8), dendritic (CD1a), monocytic (CD11b, CD14), and erythroid (glycophorin) differentiation were not expressed on CD7+cells (data not shown). Four-color analysis demonstrated that the CD7+ population uniformly coexpressed the antigens CD45RA and HLA-DR (Figure 2A,C). Thy-1 andc-kit expression were absent to low, and IL-7Rα was undetectable on CD7+ cells (Figure 2E,G,I). The common γ chain (γc, also known as IL-2Rγ) was detectable at low levels on CD7+ cells, and CD25 (IL-2Rα) and IL-3Rα were undetectable (data not shown). In summary, the CD7+population was immunophenotypically CD34+, CD38−, HLA-DR+, CD45RA+, thy-1neg/lo, c-kitneg/lo, and IL-7Rα−.
Coexpression of cell surface antigens on CD7+ fresh cord blood cells.
(A,C,E,G,I) Cells from CD34+CD38− gate (R2 in Figure 1); (B,D,F,H,J) cells from CD34+ gate for comparison. Insert in (J) shows CD7 and IL-7Rα expression in CD34− cells.
Coexpression of cell surface antigens on CD7+ fresh cord blood cells.
(A,C,E,G,I) Cells from CD34+CD38− gate (R2 in Figure 1); (B,D,F,H,J) cells from CD34+ gate for comparison. Insert in (J) shows CD7 and IL-7Rα expression in CD34− cells.
To generate a highly purified population for functional analysis, CD34+CD38− cells expressing high levels of CD7 (Figure 1E) were isolated by 4-color FACS using counter mode for maximal accuracy. This resulted in 99.5% to 100% purity when checked using test beads and 97% to 99% purity by reanalysis of isolated CD7+ cells (Figure 1F).
CD7+ cells can differentiate into B-lymphoid, NK, and dendritic cells
B-lymphoid potential of CD7+ cells was assessed using conditions previously found to be optimal for B-cell production from primitive pluripotent CD34+CD38−cells.20 Three distinct cell populations were identified in bulk cultures from both the CD7+ and CD7−subpopulations of CD34+CD38− cells (Figure3). B-lymphoid progenitors (CD34−CD19+CD10+CD38+CD56−CD1a−were rapidly generated from CD7+ cells, appearing by day 14 and comprising 26% ± 5% of the cultures (Figure 3D,E). These cells were characterized by their small size by FACS, typical of B-lymphoid progenitors generated from CD34+ and CD34+CD38− cells on S17 coculture as previously described (Figure 3F).21,22 Consistent with previous reports of the S17 coculture system,21 most B-lineage cells were not fully differentiated, lacking expression of CD20 and sIgM (data not shown).
FACS analysis of lymphoid cultures generated from CD34+CD38−lin−CD7+cells.
Three hundred cells were cultured in B-lymphoid conditions for 20 days, then switched to NK conditions (in IL-15) and cultured for another 14 days. (A) Total culture showing R1 gate. (B-E) Analysis of cells from R1 gate. Size (FSC) and side scatter (SSC) of (F) CD19+CD56− B cells, (G) CD19−CD56+ NK cells, and (H) CD19−CD1a+ dendritic cells gated from quadrants shown in (D) and (E).
FACS analysis of lymphoid cultures generated from CD34+CD38−lin−CD7+cells.
Three hundred cells were cultured in B-lymphoid conditions for 20 days, then switched to NK conditions (in IL-15) and cultured for another 14 days. (A) Total culture showing R1 gate. (B-E) Analysis of cells from R1 gate. Size (FSC) and side scatter (SSC) of (F) CD19+CD56− B cells, (G) CD19−CD56+ NK cells, and (H) CD19−CD1a+ dendritic cells gated from quadrants shown in (D) and (E).
NK (CD56+CD19−CD1a−) cells were generated from CD7+ cells by week 3 and comprised approximately 4% of the cultures. The addition of IL-15 to established S17 cocultures increased the proportion of NK cells detectable in culture to 30% of total cells (Figure 3D). As expected of NK cells, CD56+ cells were larger than CD19+ B-lymphoid cells (Figure 3G). Specificity of the CD56+ immunophenotype for identification of NK cells was confirmed functionally by the demonstration that CD56+ cells isolated from culture lysed K562 cells (Table 1).
CD1a+ cells were also present in cultures generated from CD7+ cells cultured in lymphoid conditions (Figure 3E). The CD1a+ cells were larger than either the CD19+or CD56+ population (Figure 3H). Cytospin preparations of cells isolated from lymphoid culture revealed a mixed population with morphology typical of lymphoid and dendritic cells (Figure4A). Cells with morphology of granulocytes, monocytes, or erythroid cells were not detected. CD1a+ cells coexpressed high levels of HLA-DR typical of dendritic cells5 23 but did not express the monocyte marker CD14 (Figure 4B). CD11c and CD86 were coexpressed on most CD1a+ cells.
CD1a+ cells are dendritic cells.
(A) Cytospin preparations from lymphoid cultures showing small B-lymphoid cells with scanty cytoplasm and large cells with typical dendritic morphology. (B) FACS analysis of large cells generated in lymphoid culture (gated using FSC as in Figure 3H). CD1a+cells uniformly express high levels of HLA-DR but do not express the monocyte marker CD14. Wright-Giemsa stain, original magnification × 40.
CD1a+ cells are dendritic cells.
(A) Cytospin preparations from lymphoid cultures showing small B-lymphoid cells with scanty cytoplasm and large cells with typical dendritic morphology. (B) FACS analysis of large cells generated in lymphoid culture (gated using FSC as in Figure 3H). CD1a+cells uniformly express high levels of HLA-DR but do not express the monocyte marker CD14. Wright-Giemsa stain, original magnification × 40.
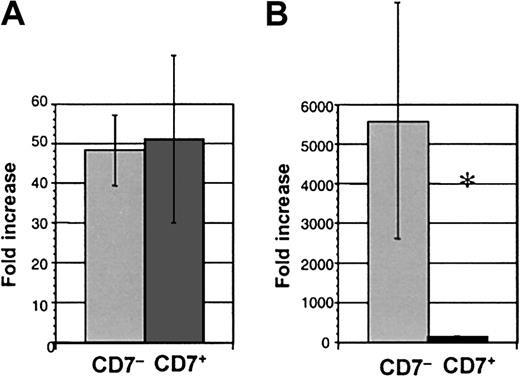
CD7+ cells cultured in lymphoid conditions generated similar numbers of progeny to CD34+CD38−CD7−(CD7−) cells (51.0 ± 21.2 compared with 48.2 ± 8.9-fold increase in cell number, respectively, n = 8) (Figure 5A). The proportion of B and NK cells was also similar in cultures from the CD7+ and CD7− subpopulations. Thus, the capacity to generate lymphoid progeny was similar for CD7+ and CD7−populations.
Cell production from CD34+CD38−CD7+ and CD34+CD38−CD7− cells.
Fold increase of cell numbers over day 0 input in (A) lymphoid culture, n = 8 experiments, and (B) myeloid culture, n = 7 (*P = .016).
Cell production from CD34+CD38−CD7+ and CD34+CD38−CD7− cells.
Fold increase of cell numbers over day 0 input in (A) lymphoid culture, n = 8 experiments, and (B) myeloid culture, n = 7 (*P = .016).
CD7+ cells lack myeloid and erythroid potential
The capacity for myeloid and erythroid differentiation from the bulk CD34+CD38−CD7+ population was tested in 2 different stromal systems, the S17 switch culture assay and the myeloid long-term culture.
We have previously shown that cocultivation of human CD34+CD38− cells on S17 stroma maintains myelo-erythroid progenitor cells that become detectable when S17 cocultures are switched into myelo-erythroid conditions (IL-3, IL-6, KL, GM-CSF, and EPO [3/6/K/GM/E]).8 Therefore, to determine whether myelo-erythroid progenitors could be similarly generated from CD7+ cells, lymphoid cultures were switched after 1 to 3 weeks on S17 stroma into methylcellulose medium containing 3/6/K/GM/E and cultured for a further 2 weeks. Although S17 cocultures of control (CD7− cells) consistently contained both myeloid and erythroid progenitors at a high frequency (4.10% ± 0.85%), cultures from CD7+ cells were devoid of myeloid or erythroid progenitors (n = 4). The same lymphoid cultures of both CD7+ cells and control cells, analyzed before switching to myeloid conditions, consistently generated B-lymphoid, NK, and dendritic cell growth as described above (Figures 3 and 5A).
The CD34+CD38− population in cord blood and bone marrow is highly enriched for primitive progenitors with myeloid and erythroid potential, best revealed during myeloid-specific long-term stromal culture.10 12 As a second test of myeloid and erythroid potential, freshly isolated CD7+cells were cultured from day 0 in conditions optimal for myeloid and erythroid differentiation (ie, on irradiated human stroma in myeloid medium with IL-3, IL-6, and KL). Growth of CD7+ cells was again compared with CD7− cells. Minimal cell proliferation was seen from CD7+ cells cultured in bulk under myelo-erythroid conditions in marked contrast to control cells that exhibited a mean 5577-fold expansion (P = .016, n = 7) (Figure 5B). The progenitor content of CD7+ cells cultured in myeloid conditions was assessed by replating cells from myeloid stromal cultures into methylcellulose medium. Colony-forming unit–granulocyte-macrophage (CFU-GM); colony-forming unit–granulocyte, erythrocyte, megakaryocyte, macrophage (CFU-GEMM); and burst-forming unit, erythroid (BFU-E) were almost undetectable from CD7+-initiated cultures (0.02% ± 0.01% of cells plated from stromal cultures produced CFU) but were readily generated from CD7− cells (4.05% ± 0.93%) (P = .005, n = 7) (Table 2).
Although typical CFU-GM, BFU-E, or CFU-GEMM were not generated in long-term cultures from CD7+ cells, occasionally, loose clusters of cells with a distinctive morphology and pattern of growth could be detected when CD7+ cells were replated in methylcellulose medium. These clusters occurred at a cloning efficiency of 0.06% ± 0.05% of CD7+ cells. Such clusters were always small (less than 100 cells) and when harvested and pooled were found to consist of cells that were typical of dendritic cells, ie, they were CD1a+ by FACS analysis, and cytospin preparations exhibited the same dendritic morphology as the CD1a+ cells previously analyzed from S17 bulk culture. Thus, clonogenic dendritic precursors were generated at low frequency from CD7+ cells, similar to those described in the CFU–dendritic cell assay.24
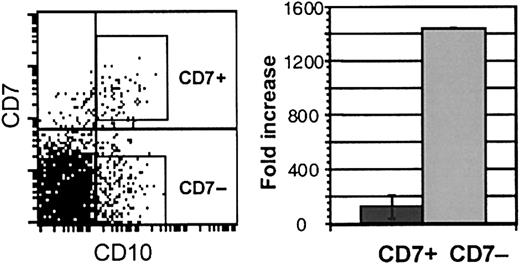
CD34+CD38− cells that express CD10 have myelo-erythroid potential
Galy et al5 have described a CD34+lin− CD10+ population in bone marrow that contains progenitors with B, NK, T, and dendritic potential and is significantly depleted of myeloid progenitors. We, therefore, explored whether CD10 expression is similarly useful in identifying lymphoid-restricted progenitors in cord blood. CD10 was expressed on approximately 5% of CD34+CD38− cord blood cells. More than 70% of CD7hi cells in the CD34+CD38− population coexpressed CD10 (Figure6). However, most (60% to 70%) CD10 cells did not coexpress CD7. CD34+CD38−CD10+ cells that either expressed high levels of CD7 (CD10+ CD7+) or did not express CD7 (CD10+ CD7−) were isolated and studied in myeloid and lymphoid culture. Although both populations generated B-lymphoid and NK cells in lymphoid culture, myeloid potential was detected only in the CD10+ CD7−population. Cell expansion in myeloid stromal cultures, barely detectable in CD10+ CD7+ cultures, was more than 10-fold higher from the CD10+ CD7−cells (Figure 6). Myeloid and erythroid CFU-C were undetectable in cultures from CD10+ CD7+ cells and were consistently generated from CD10+ CD7− cells (Table 3). Thus, CD10 expression alone does not discriminate between cord blood progenitors with lymphoid and myeloid potential.
Immunophenotype and growth of CD10+CD7+ and CD10+CD7− cells.
Left panel: FACS analysis of fresh CD34+CD38−cells showing CD10 and CD7 expression. Right panel: fold increase of cells over input number in myelo-erythroid cultures of CD10+CD7+ and CD10+CD7− cells (n = 3).
Immunophenotype and growth of CD10+CD7+ and CD10+CD7− cells.
Left panel: FACS analysis of fresh CD34+CD38−cells showing CD10 and CD7 expression. Right panel: fold increase of cells over input number in myelo-erythroid cultures of CD10+CD7+ and CD10+CD7− cells (n = 3).
Clonal analysis of single CD7+ cells
The above experiments demonstrated that the CD7+population as a whole can differentiate into B, NK, and dendritic cells. However, clonal analysis was necessary to establish whether multiple lymphoid lineages could be generated from a single common CD7+ progenitor. Single CD7+ cells were, therefore, isolated and deposited by FACS into individual wells and cultured on S17 stroma in lymphoid conditions. In each experiment, 192 CD7+ cells were studied and compared with identical numbers of control CD7− cells. Lymphoid potential (defined as B cells with or without NK cells) was detected at a similar frequency in CD7+ cells (68.1% ± 12.1% of clones) and CD7− cells (50.0% ± 19.7% of clones) (Table4). Simultaneous generation of B-lymphoid and NK cells was detected in 38.1% ± 7.6% of clones from individual CD7+ cells. All lymphoid clones also contained CD1a+ dendritic cells. Thus B, NK, and dendritic cells were generated from a common CD7+ progenitor.
Clones from single cells were also switched from the above S17 stroma (lymphoid) conditions into methylcellulose (myeloid) conditions to again test for myeloid potential. Most clones from control CD7− cells contained myeloid ± erythroid progenitors (59.4% ± 4.8%, n = 5). Both lymphoid and myelo-erythroid potential was detected in 47.1% ± 11.0% of control clones.
In contrast, only 2 of 53 total clones (2.4% ± 1.5%) from single CD7+ cells generated myelo-erythroid progenitors when switched from lymphoid to myeloid conditions. Both of these clones also generated lymphoid cells and thus represented pluripotent progenitors. In view of the level of purity of cell sorting it is possible that these 2 clones resulted from contamination with CD7− cells during sorting.
The above studies demonstrate that, under switch culture conditions in which single clonogenic CD7− cells generated lymphoid and myelo-erythroid potential, clonogenic CD7+ cells generated equivalent numbers of lymphoid clones but were profoundly depleted of myeloid and erythroid potential (P = .0005).
Analysis of RNA expression of lineage-specific genes
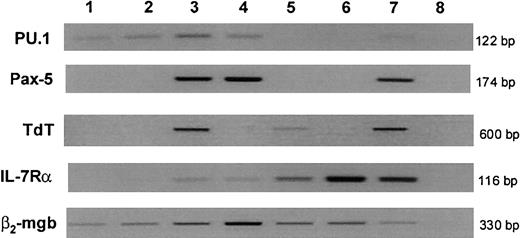
We next determined whether the gene expression profile of the fresh CD7+ population demonstrated early commitment to T and/or B lymphopoiesis on a molecular level. The transcription factor PU.1 was identified in both CD7− (PHSC) and CD7+ cells and also in committed B-lymphoid progenitor (CD34+CD19+) populations but was down-regulated in thymocytes (Figure 7). The B-lineage transcription factor Pax-5 was not expressed in CD7− or in CD7+ cells and first became detectable at the committed B-cell progenitor stage.
Gene expression in cord blood progenitor subpopulations.
RNA was extracted and reverse-transcribed from FACS-isolated populations from pooled cord blood (lanes 1-4) or thymus (lanes 5 and 6), and cDNA from equal cell numbers of each population (600 cells for PU.1, Pax-5, and TdT; 1200 cells for IL-7Rα; 300 cells for β2-mgb) were subjected to PCR to amplify genes shown. Lane 1: CD34+CD38−CD7−, Lane 2: CD34+CD38−CD7+, Lane 3: B-lymphoid progenitors (CD34+CD19+), Lane 4: B cells (CD34−CD19+), Lane 5: CD3+CD4+CD8+ thymocytes, Lane 6: CD3+CD4+CD8− thymocytes, Lane 7: positive control cell lines (see “Materials and methods”), and Lane 8: negative control (no cDNA). Results are representative of at least 3 independent experiments. β2-mgb indicates β2-microglobulin.
Gene expression in cord blood progenitor subpopulations.
RNA was extracted and reverse-transcribed from FACS-isolated populations from pooled cord blood (lanes 1-4) or thymus (lanes 5 and 6), and cDNA from equal cell numbers of each population (600 cells for PU.1, Pax-5, and TdT; 1200 cells for IL-7Rα; 300 cells for β2-mgb) were subjected to PCR to amplify genes shown. Lane 1: CD34+CD38−CD7−, Lane 2: CD34+CD38−CD7+, Lane 3: B-lymphoid progenitors (CD34+CD19+), Lane 4: B cells (CD34−CD19+), Lane 5: CD3+CD4+CD8+ thymocytes, Lane 6: CD3+CD4+CD8− thymocytes, Lane 7: positive control cell lines (see “Materials and methods”), and Lane 8: negative control (no cDNA). Results are representative of at least 3 independent experiments. β2-mgb indicates β2-microglobulin.
TdT expression has been described as one of the earliest events in B- and T-lymphoid differentiation.25 26 TdT messenger RNA (mRNA) was identified in CD34+CD19+ B lineage and CD4+CD8+ (double positive) thymocytes as expected but was absent at both the CD7− and CD7+ stages.
Consistent with mouse studies,4 the T-cell–associated transcription factor GATA-3 was expressed at low levels in CD7− PHSC and CD7+ cells and was up-regulated in thymocytes. Absent expression of CD3ε and pre-TCRα confirmed the lack of T-cell commitment in both CD7+ and CD7− cells (data not shown).27
IL-7Rα is expressed on murine CLP, providing one of the key immunophenotypic differences between murine CLPs and PHSCs.3 In contrast to murine data, but consistent with our FACS analysis, mRNA for IL-7Rα was at or below the level of detection on CD7+ cells. IL-7Rα was undetectable in 2 experiments and only faintly detectable in one experiment, suggesting that, at most, IL-7Rα may be expressed at low levels in a small proportion of CD7+ cells.
Discussion
The above experiments have identified the CD7+subpopulation of CD34+CD38− cord blood cells as a primitive, clonogenic, progenitor population with the ability to generate multiple lymphoid lineages but with no potential for myeloid or erythroid differentiation under the conditions tested. Single cell studies showed that B-lymphoid, NK, and dendritic cells could be generated from individual CD7+ cells. Despite the B-lymphoid and NK-cell lymphoid potential of the CD7+population, RNA expression of the B-lymphoid–specific transcription factor Pax-5 and the T/NK-specific CD3ε gene was absent. TdT expression, described as one of the earliest events in lymphoid development,25 26 was also absent, demonstrating that the CD34+CD38−CD7+ immunophenotype defines a more primitive stage of human lymphoid commitment than the earliest human B-lymphoid or T-lymphoid progenitors previously described.
The finding of both B and NK potential in individual CD7+cells begs the question of whether these progenitors also possess T-cell potential and are thus true CLPs. Attempts to generate unequivocal T cells from either CD7+ or CD7−cells in fetal thymic organ culture yielded inconsistent results, and we are thus unable to draw any conclusions from these studies. Although definitive clonal proof has not been reported, there is strong evidence from murine and human studies that, at least in the fetus, NK and T cells originate from a common progenitor, distal to the split of the B/T cell pathways.19,23 28-30 T and NK cells both express a number of the same differentiation markers (eg, CD7, CD16, CD2, and cytoplasmic CD3ε) and respond to similar cytokine combinations (eg, IL-2, IL-7, KL). It is likely, therefore, as NK potential exists in the CD7+ population, that T-cell potential is also present. The absence of T-cell potential in clones from CD7+ cells would place B- and NK-cell production on a common pathway separated from T lymphopoiesis.
This report adds further evidence for the existence of dendritic cells of lymphoid origin.31 Murine studies have revealed that a lymphoid-restricted precursor population in the thymus generates dendritic cells and T cells.32,33 Björck and Kincade34 demonstrated that dendritic cells can also be generated from murine CD19+ pro-B cells. Galy et al5 found that the CD10+ lymphoid-restricted progenitors in human bone marrow generated dendritic cells. The absence of myeloid progenitor activity in the CD7+ population and the fact that dendritic cells were generated in lymphoid conditions from single cells that also possessed B and NK potential strongly supports the contention that dendritic cell differentiation arose from a common lymphoid pathway.
As previously mentioned, Galy et al5 reported a candidate CLP population in human bone marrow with the immunophenotype CD34+lin−CD10+. The CD10+ bone marrow population had functional similarities to the CD7+ cord blood progenitors described in this report; it was severely depleted of myeloid and erythroid potential and generated B-lymphoid, NK, and dendritic cells in limiting dilution analysis. T-cell potential was demonstrated in vivo from bulk populations of CD34+lin−CD10+cells. Immunophenotypically, the CD10+ bone marrow and the CD7+ cord blood population reported here were similar; both expressed high levels of CD45RA and HLA-DR but had low-to-absent expression of Thy-1 and c-kit. However, in contrast to the CD7+ population described in this report, 97% of the bone marrow CD10+ cells coexpressed CD38. No data on expression of IL-7Rα or lineage-specific transcription factors was given.
Most of the cord blood CD34+CD38−CD7+ cells described here coexpressed CD10. However, CD10 expression per se was not sufficient to identify lymphoid-restricted progenitors in cord blood. Myelo-erythroid potential was readily demonstrated in experiments using CD34+CD38−CD10+ cells that did not express CD7. We have also found that cord blood populations with an identical (CD34+lin−CD10+) immunophenotype to that reported by Galy et al5 also possessed myelo-erythroid potential (data not shown). Thus, expression of the CD10 antigen does not have the same discriminative power for lymphoid restriction in cord blood as reported in bone marrow. We have detected CD7+ cells within the CD34+CD38dim population of bone marrow but at a lower frequency (1%-2%) than those seen in cord blood. Whether this population is also lymphoid-restricted has not yet been established in functional assays.
Human CD7 is a 40-kDa member of the immunoglobulin superfamily. Although its exact function is unknown, cross-linking of CD7 results in tyrosine kinase–mediated up-regulation of β1 and β2 integrin expression on T35 and NK cells,36 thus modulating adhesion to fibronectin.37 The ligand for CD7 has not been identified, although a recent report suggested that galectin-1 induced apoptosis in T cells may involve binding to CD7.38 CD7 is considered to be one of the earliest surface antigens expressed during T-lymphoid and NK development.30,39 A subset of CD19+ cells that coexpressed CD7 was identified in human fetal bone marrow,40 although B-lymphoid potential of CD7+ cells has not been demonstrated using functional assays.
The lymphoid-restricted cord blood progenitors described here were identified specifically in the CD7+ fraction of the CD34+CD38− compartment. Myelo-erythroid potential has been found in fetal liver CD34+lin−CD7+populations41 and in cord blood CD34+CD38loCD7+ populations in our own studies (data not shown), demonstrating that antibody binding to CD7 does not specifically inhibit myelopoiesis.
An important difference between our own studies and those of the murine CLP is the lack of expression of IL-7Rα in the human CD7+lymphoid progenitors. IL-7Rα is detectable by FACS on murine CLP and is the key marker used to discriminate this population from murine PHSCs.3 However, in our own studies, IL-7Rα was not detectable by FACS and was essentially undetectable by PCR in the human CD7+ population. Ryan et al42 reported that CD34+lin− cells of human bone marrow that express IL-7Rα were depleted of myeloid potential but had relatively increased capacity for clonogenic B-cell production; NK and T-cell potential were not assessed in that study.42 The CD34+lin− IL-7Rα population expressed Pax-5 and TdT and thus presumably represented a B-cell differentiation stage later than the CD7+ progenitor described here. The occasional detection of IL-7Rα by PCR at very low levels in the CD7+ population may represent a subset of cells undergoing further lineage differentiation. The absence of expression of IL-7Rα at such an early stage of human lymphopoiesis adds further evidence for critical differences in the role of IL-7 between murine and human lymphopoiesis.43
These studies identify a clonogenic lymphoid progenitor with both B-cell and NK-cell lineage potential with a molecular profile that suggests a developmental stage more primitive than previously identified human lymphoid progenitors. The CD34+CD38−CD7+ phenotype distinguishes primitive human lymphoid progenitors from pluripotent stem cells and more committed lymphoid progenitors, providing a highly purified population for analysis and manipulation. The isolation of functionally distinct populations will facilitate the study of how the earliest events in human lymphoid commitment are regulated and how each of the distal lymphoid pathways are related. Primitive lymphoid progenitors may prove useful as targets for genetic manipulation and transplantation, providing a therapeutic alternative to PHSC in disease states specifically affecting lymphopoiesis.
Supported by grants 5RO1DK54567 and 2P50HL54850 from the National Institutes of Health (G.M.C.). G.M.C. is a Scholar of the Leukemia and Lymphoma Society. K.J.P. is supported by National Research Service Award 1F32DK10101 and a fellowship from the Childrens Hospital Los Angeles Research Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Gay Crooks, Childrens Hospital Los Angeles, MS#62, 4650 Sunset Blvd, Los Angeles, CA 90027; e-mail: gcrooks@chla.usc.edu.