In addition to its key role in the control of blood loss following injury, fibrin(ogen) has been proposed to play an important role in tissue repair by providing an initial matrix that can stabilize wound fields and support local cell proliferation and migration. To test directly these concepts, the effect of fibrinogen deficiency on cutaneous tissue repair in mice was investigated using incisional and excisional wounds. The time required to overtly heal wounds was similar in fibrinogen-deficient and control mice, but histologic evaluation revealed distinct differences in the repair process, including an altered pattern of epithelial cell migration and increased epithelial hyperplasia. Furthermore, granulation tissue in fibrinogen-deficient mice failed to adequately close the wound gap, resulting in persistent open wounds or partially covered sinus tracts. The tensile strength of these wounds was also reduced compared with control mice. The most profound defect in wound tissue organization was observed in fibrinogen-deficient mice following the subcutaneous implantation of a porous tubing chamber. Cells migrated into the wall of the implants at a similar rate as control mice, but cells from fibrinogen-deficient animals were unable to efficiently organize and migrate into wound fluid-filled dead space within the center of the implants. These studies show that re-epithelialization, granulation tissue formation, including the establishment of neovasculature, and the formation of fibrotic scar tissue can proceed in the absence of fibrin(ogen) and all of its proteolytic derivatives. However, fibrin (ogen) is important for appropriate cellular migration and organization within wound fields and in initially establishing wound strength and stability.
Introduction
Cutaneous wound healing is a process involving the formation of a new extracellular matrix, cellular infiltration and organization of the wound field, tissue remodeling, and ultimately, mature scar formation. Immediately after injury, wound fields fill with blood components leading to the exposure of plasma coagulation factors to the VIIa binding protein, tissue factor. Subsequent thrombin generation and thrombin-mediated cleavage of both soluble fibrinogen and platelet-associated protease activated receptors (eg, PAR-1, -3, and -4) results in thrombus formation and the deposition of a provisional fibrin-rich matrix. Several hours after injury, epithelial migration begins to restore epithelial integrity while leukocytes invade the fibrin clot. After several days, the provisional matrix becomes infiltrated with new capillary endothelium and activated fibroblasts that serve to produce a new collagen-rich matrix. Eventually, the provisional matrix is degraded as the wound contracts, leaving a collagen-rich scar.
The conversion of soluble fibrinogen to an insoluble fibrin polymer is of fundamental importance in the control of blood loss following vascular injury. However, a broader role for fibrin matrices in tissue repair has long been suspected. In particular, fibrin is thought to be an essential component of the provisional matrix that protects the underlying tissue and supports the migration and proliferation of inflammatory cells, endothelial cells, and stromal cells participating in tissue repair. Fibrin may also provide a reservoir for cytokines and growth factors released from platelets and inflammatory cells (reviewed in Martin1). Notably, in addition to supporting cell adhesion and proliferation through integrin (eg, αvβ3, αvβ5, α5β1, αMβ2, αIIbβ3) and nonintegrin (eg, I-CAM) receptors, fibrin(ogen) has recently been shown to bind with high affinity to several angiogenic growth factors, including FGF-2 and VEGF.2-5 Fibrin, as a constituent of granulation tissue, may also play a role in supporting cell-cell and cell-matrix interactions occurring during tissue remodeling.6
Cutaneous wound healing can occur by primary or secondary intention. Primary intention involves the closure of a clean, noninfected surgical incision with sutures joining the wound edges and involves a relatively small amount of granulation tissue formation and wound contraction. Instead, this type of healing relies primarily upon rejoining of the connective tissue matrix. Healing by secondary intention, however, involves the closure of a large open defect, where wound edges are not juxtaposed and significant granulation tissue must be produced to fill the wound area. This process is more complicated, and resolution of the defect relies heavily upon the production of significant amounts of granulation tissue and substantial wound contraction.
In order to directly determine the role of provisional fibrin matrices in tissue repair, the resolution of wounds by both primary and secondary intention was investigated in mice with a complete lack of circulating fibrinogen. The data reveal an abnormal pattern of tissue repair in fibrinogen-deficient mice, including misguided and hypertrophied epithelium, delayed wound closure, reduced wound tensile strength, and a diminished ability to organize a dead space. However, wound repair is ultimately completed in a time frame that is comparable to that in control mice, and the final outcome of repair is remarkably similar in mice with and without fibrinogen.
Materials and methods
Creation of wounds
Fibrinogen-deficient (Aα chain−/−) and littermate hemizygous control mice with a C57BL/6 genetic background were generated and genotyped by ear punch biopsy as previously described.7 Because Aα chain does not appear to be the limiting chain for hepatic fibrinogen assembly and secretion, the hemizygous (Aα+/−) animals used as controls carry circulating fibrinogen levels that are approximately 75% of wildtype (Aα+/+) mice.7 All mice used in these studies were 8-week-old to 12-week-old females and they were housed individually for the duration of the study. Mice were anesthetized with 2% isoflurane during surgical procedures. Dorsal and ventral skin was shaved and cleaned with alcohol. Full skin thickness incisions, 15 mm in length, were created on the dorsal and ventral surfaces lateral to the midline with a scalpel, and were left gaping and not dressed. Incisions to be examined for healing by primary intention were sutured aseptically but not dressed. Sutures were removed one day after the wounds were created, and wounds were examined daily, without manipulation, for evidence of successful closure. Excisional wounds were generated by marking a 1 cm2 area on the midline of the dorsum with a marker pen then excising full thickness skin with a fine scissor. All mice were assessed daily for progression of healing of wounds. Wound areas were assessed by computer-assisted planimetry from photographs taken of excision wounds at 5, 8, 14, and 21 days after wounds were generated. Both incisional and excisional wounds were prepared on separate cohorts of mice, and tissues were collected after predetermined times for tensile strength measurements and histologic analysis.
Tensile strength
Six to 10 mice of each genotype were killed at 5, 14, and 28 days after generation of unsutured incisional wounds. A square of skin around the wound was excised and immediately frozen between gauze soaked in saline. In addition, nonwounded skin was collected from both genotypes of mice for analysis and was stored in the same manner. Tensile strength was determined with a tensiometer (Instron, Canton, MA) as a function of force per cross-sectional area of tissue.
Histologic and immunohistochemical analysis
Incisional wound tissue was harvested at days 3-8, 12, and 21. Excisional wound tissue was collected at days 4, 8, 12, and 21. Wound tissues were removed by cutting a square of skin around the wound area, without disturbing the wound. Tissues were fixed in 10% neutral-buffered formalin, processed into paraffin, and 4 micron sections were prepared perpendicular to the original incision. Sections were stained with hematoxylin and eosin. Immunohistochemistry was performed on sections with the following primary antibodies: rabbit antimouse fibrinogen,8 rabbit antihuman fibronectin (Dako, Carpinteria, CA), goat antihuman procollagen α1(I) (Santa Cruz Biotechnology, Santa Cruz, CA), goat antihuman procollagen α(III) (Santa Cruz Biotechnology). Antibodies were detected with biotinylated second antibodies (Vector Laboratories, Burlingame, CA), Vectastain ABC Elite Kit (Vector) and 3,3′-diaminobenzidine substrate (Sigma Chemical, St Louis, MO) or Fast Red substrate (Sigma).
Hydroxyproline content
To establish a defined wound field for quantitative analysis of hydroxyproline (collagen) deposition, short segments (4 mm) of open-ended (3.17 mm internal diameter) polyethylene tubing (Intramedic; Becton Dickinson, Sparks, MD) were placed subcutaneously under wounded skin. After 7 or 14 days, animals were killed and the new tissue inside the tubing was collected, weighed, and stored at –80°C. Although the area inside the tubing provided a geometrically defined wound field for tissue ingrowth measurements, its short length and open-ended configuration immediately adjacent to wound tissue did not restrict tissue invasion which would be required for an authentic dead space model of tissue repair, as did wound chambers (see below). Acid hydrolysis was performed on tissues with 6N hydrochloric acid at 110°C for 24 hours. Hydroxyproline was assessed as previously described.9
Dead space implants
High porosity PTFE tubing (internal diameter: 1.2 ± 0.02 mm, wall thickness: 0.63 ± 0.08 mm, pore size: 60-90 μm; International Polymer Engineering, Tempe, AZ) was cut at lengths of 6 mm to create wound chambers. The ends of the tube sections were closed by pressure from a small toothed clamp, thus all cellular infiltration required directed migration through the porous walls of the tubing. Prior to closure, tubes were filled with normal saline. Tubes were sterilized and implanted subcutaneously in the dorsum of mice. Tubes were left in place for 7, 14, and 21 days, then removed and placed in 10% neutral buffered formalin fixative. Tubes were routinely processed and embedded for cross-sectional histochemical analysis.
Results
Healing by primary intention
Longitudinal incisional wounds (1.5 cm) were generated laterally in the dorsum of 10 mice of each genotype, and the wounds were closed with interrupted sutures. All sutures remained in place overnight and were gently removed the following day to assess the competency of the union of the wound edges. In fibrinogen-expressing animals, all wounds remained closed when examined on the following day (day 2: 10 of 10 wounds closed [100%]). However, in most fibrinogen-deficient (Fib−/−) mice examined at the same time, contact between the wound edges was not maintained (1 of 10 wounds closed [10%],P < .005, chi-square test). Rather, the wounds in Fib−/− mice gaped in a manner similar to that of unsutured wounds. When examined at day 6, wounds of control mice appeared overtly healed (loss of eschar and re-epithelialization). Fib−/− mice re-established juxtaposed wound edges by day 6 and were resurfaced by days 10 and 11. Thus, the absence of fibrin(ogen) greatly influenced the ability of sutured wound edges to remain juxtaposed after removal of sutures, indicating that fibrinogen deficiency reduces initial wound stability.
Unsutured incisional wounds
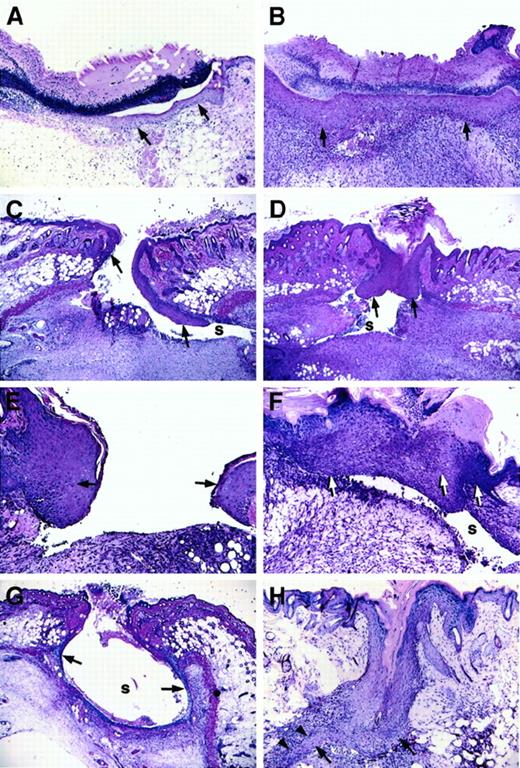
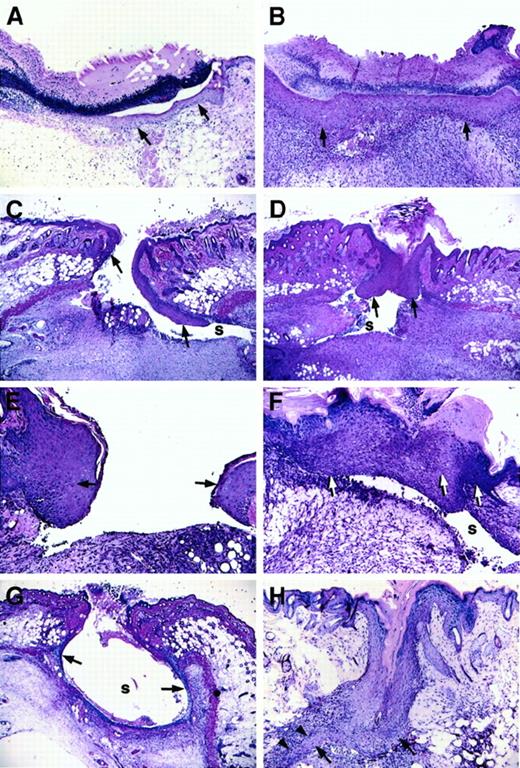
Longitudinal wounds (1.5 cm) were generated in the dorsum of mice, and wounds were left unsutured. Daily monitoring indicated that wounds of both control and Fib−/− mice remained open initially but gradually formed an eschar by day 2 or day 3. No gross differences were seen between healing wounds of control mice and Fib−/− mice, with the exception of occasional wounds of Fib−/− mice that maintained persistent but minor bleeding for up to a week. However, histologic analysis revealed that the pattern of wound healing was markedly different in Fib−/−mice as compared with control animals. Whereas epithelial proliferation and migration occurred rapidly in both genotypes, the direction of migration was dysregulated in Fib−/− mice, and epithelial hyperplasia was common (Figure 1C-H). In Fib−/− mice, the migration of keratinocytes did not follow the typical pattern immediately below the eschar and above the early granulation tissue. Rather, keratinocytes extending from the original wound edge proceeded down the inner dermal edge, moving well under the wound area and usually away from the center of the wound field, during days 3-4 (Figure 1C). In control mice, keratinocytes migrating away from the center of the wound occurred in only a small fraction of control mice (3/24 wound edges [12.5%]), whereas in Fib−/− mice the vast majority of keratinocyte migration was turned away from the center of the wound field (25/28 wound edges [89.3%]; P < .0001, chi-square test; Figure 1B). Despite the altered path of initial epithelial migration, wound re-epithelialization was delayed only briefly, if at all, in Fib−/− mice relative to control animals (assessed histologically, the median day for complete re-epithelialization was day 5 for control mice and day 6 for Fib−/− animals; Figure 1B,F). Completion of re-epithelialization in Fib−/− mice occurred by 3 distinct and aberrant pathways: (1) lining the entire inside of the wound area (Figure 1G); (2) unification of epithelium at the surface and abandonment of the migrating edges leaving an internal cavity, or sinus (see section below and Figure 1D); or (3) epithelial reduplication, which resulted in a spur which successfully rejoined with the other side (Figure 1H). Eschars, which were more clearly discernible by microscopic analysis, generally were lost more quickly in Fib−/− mice than in control mice (controls: median day 6; Fib−/− mice: median day 4) despite the delay in re-epithelialization. Fibrin(ogen) appears to play a role in retention of the protective eschar, but loss of the eschar did not retard healing, consistent with previous reports.10 11
Microscopic appearance of incision wounds, shown perpendicular to the longitudinal direction of the wound, of control and Fib−/− mice.
(A) Normal epithelial cell migration below the eschar in a control mouse 3 days after wounding. (B) Complete re-epithelialization of a control mouse at 6 days. (C) Altered pattern of epithelial cell ingrowth observed in Fib−/− mice on day 3 with the leading edge of epithelial cells proceeding down the dermal wound edge into a sinus (s) formed at the edge of the wound bed, and away from the center of the wound. (D) Re-epithelialization in a Fib−/−mouse 6 days after injury by unification of epithelium that had previously followed the dermal face into a prominent sinus (s). (E) Epithelial hyperplasia commonly observed at the leading edge of 5-day-old wounds of Fib−/− mice. (F) Fusion of an abnormally hyperplastic endothelium in a Fib−/− mouse (day 7) leaving a persistent sinus below the epithelium. (G) Partial re-epithelialization in a Fib−/− mouse (day 4) by “paving” the entire inner surface of a wound sinus. (H) Re-epithelialization in a Fib−/− mouse (day 7) with the formation of a spur (arrowheads). Epithelium is indicated with arrows; gaps, or sinuses, in the wound field are indicated with an “s.” Panels A-D, G, and H: original magnification 40×. Panels E and F, original magnification 100×. Hematoxylin and eosin stain.
Microscopic appearance of incision wounds, shown perpendicular to the longitudinal direction of the wound, of control and Fib−/− mice.
(A) Normal epithelial cell migration below the eschar in a control mouse 3 days after wounding. (B) Complete re-epithelialization of a control mouse at 6 days. (C) Altered pattern of epithelial cell ingrowth observed in Fib−/− mice on day 3 with the leading edge of epithelial cells proceeding down the dermal wound edge into a sinus (s) formed at the edge of the wound bed, and away from the center of the wound. (D) Re-epithelialization in a Fib−/−mouse 6 days after injury by unification of epithelium that had previously followed the dermal face into a prominent sinus (s). (E) Epithelial hyperplasia commonly observed at the leading edge of 5-day-old wounds of Fib−/− mice. (F) Fusion of an abnormally hyperplastic endothelium in a Fib−/− mouse (day 7) leaving a persistent sinus below the epithelium. (G) Partial re-epithelialization in a Fib−/− mouse (day 4) by “paving” the entire inner surface of a wound sinus. (H) Re-epithelialization in a Fib−/− mouse (day 7) with the formation of a spur (arrowheads). Epithelium is indicated with arrows; gaps, or sinuses, in the wound field are indicated with an “s.” Panels A-D, G, and H: original magnification 40×. Panels E and F, original magnification 100×. Hematoxylin and eosin stain.
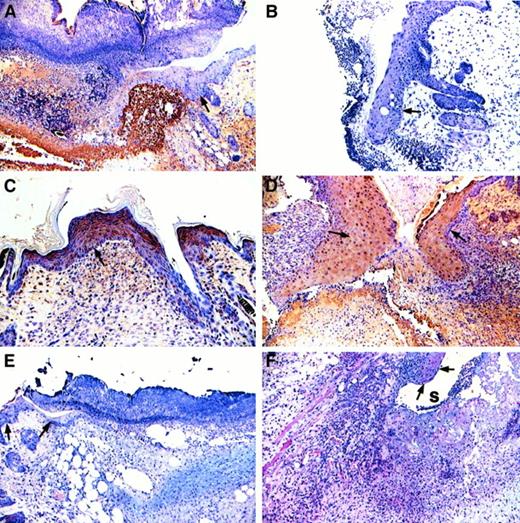
Formation of a cohesive provisional matrix did not occur in the wound area of most Fib−/− mice. Abundant granulation tissue was produced in the wound area of these mice; however, this tissue did not succeed in closing the wound gap (Figure 1C,D,F,G). Instead fissures, or sinuses, formed at the edges of the incision, which persisted on some occasions even after re-epithelialization was complete (Figure1D,F). Fissures within wound fields, at days 3 to 4, occurred in only 2 of 12 (16.7%) control mice, but in 14 of 14 (100%) Fib−/− mice (P < .0001). The wound beds of both control and Fib−/− mice became filled with a highly vascularized matrix containing inflammatory cells and matrix proteins including fibronectin, procollagen, and collagen (Figure2 and below). Predictably, fibrin(ogen) was prominent in early wound fields of control mice (Figure 2A) but not Fib−/− mice (Figure 2B). Immunohistochemistry demonstrated that fibronectin was abundant in the wound bed and particularly at the migrating edge of the epithelium in wounds of both genotypes of mice (Figure 2C,D). Expression of procollagen types I and III was also apparent in wounds of both control and Fib−/− mice after day 4. Interestingly, despite substantial fibroblast infiltration in both groups, expression of procollagen type I was prominent at day 3 in Fib−/− mice, but not until day 4 or day 5 in control mice (Figure 2E,F).
Immunohistochemical analysis of fibrin- (ogen), fibronectin, and procollagen I in wounds of control and Fib−/− mice.
Fibrin(ogen) immunodetection (brown reaction product) in the wound beds of control (A) but not in Fib−/− (B) mice on day 4. Note that the epithelium and the granulation tissue of the wounds contain abundant fibronectin (brown reaction product) in both control (C) and Fib−/− (D) mice. Procollagen I accumulation (pink reaction product) was more prominent in Fib−/− mice (F) than in control mice (E). Original magnification, 100×.
Immunohistochemical analysis of fibrin- (ogen), fibronectin, and procollagen I in wounds of control and Fib−/− mice.
Fibrin(ogen) immunodetection (brown reaction product) in the wound beds of control (A) but not in Fib−/− (B) mice on day 4. Note that the epithelium and the granulation tissue of the wounds contain abundant fibronectin (brown reaction product) in both control (C) and Fib−/− (D) mice. Procollagen I accumulation (pink reaction product) was more prominent in Fib−/− mice (F) than in control mice (E). Original magnification, 100×.
Hydroxyproline content
Analysis of wound tissue for hydroxyproline content, as a measure of collagen accumulation in the wound, indicated that collagen was more abundant in the wounds of Fib−/− mice than in control mice at both days 7 and 14 (P < .0161; Figure3A). No increase in total hydroxyproline was seen between days 7 and 14, consistent with other reports.12 To directly compare the wound granulation tissue formed in control and Fib−/− mice, tissues similar to those collected for hydroxyproline analysis were processed for routine microscopic analysis (Figure 3B,C). The granulation tissue was qualitatively similar in both control and Fib−/− mice, with a prominent neovascularization and similar overall cell densities, indicating that this was unlikely to account for the differences in collagen content of the wounds (Figure 3B,C).
Hydroxyproline content and granulation tissue appearance of incisional wound tissue after 7 and 14 days.
(A) Hydroxyproline content of control mice (░) and Fib−/− mice (■). Histologic analyses indicating similar cell density and qualitative appearance of granulation tissue in wound tissue collected at day 14 from control (B) and Fib−/−mice (C). Hematoxylin and eosin stain; original magnification, 200×.
Hydroxyproline content and granulation tissue appearance of incisional wound tissue after 7 and 14 days.
(A) Hydroxyproline content of control mice (░) and Fib−/− mice (■). Histologic analyses indicating similar cell density and qualitative appearance of granulation tissue in wound tissue collected at day 14 from control (B) and Fib−/−mice (C). Hematoxylin and eosin stain; original magnification, 200×.
Wound tensile strength
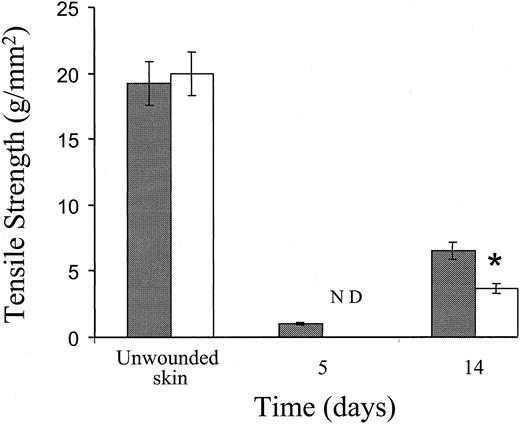
The tensile strength of wounds from control and Fib−/− mice was measured to quantitate the contribution of fibrin(ogen) to the early integrity of incision wounds. The tensile strength of early incision wounds in Fib−/− mice was visibly less than control mice, and so low that tensiometer measurements could not be made in Fib−/− mice at day 5; the extremely fragile wound edges fell apart prior to being placed in the tensiometer. Fourteen days after incision, an advanced stage in wound repair for both genotypes of mice, the tensile strength of wounds in Fib−/− mice remained significantly less than control animals (Figure 4). However, in more-mature wounds collected at day 28, the tensile strengths were equivalent in Fib−/− mice and control mice. Thus, fibrin(ogen) contributes to wound strength in the early stages of wound repair, but the stability of the mature scar is not dependent on the presence of fibrin(ogen).
Tensile strength of unwounded skin and incisional wounds of control and Fib−/− mice.
Tensile strength of control mice (░) and Fib−/− mice (■) mice at various times after injury. ND indicates not determined; measurements could not be taken due to the fragility of wounds in Fib−/− mice at this time.
Tensile strength of unwounded skin and incisional wounds of control and Fib−/− mice.
Tensile strength of control mice (░) and Fib−/− mice (■) mice at various times after injury. ND indicates not determined; measurements could not be taken due to the fragility of wounds in Fib−/− mice at this time.
Healing by secondary intention
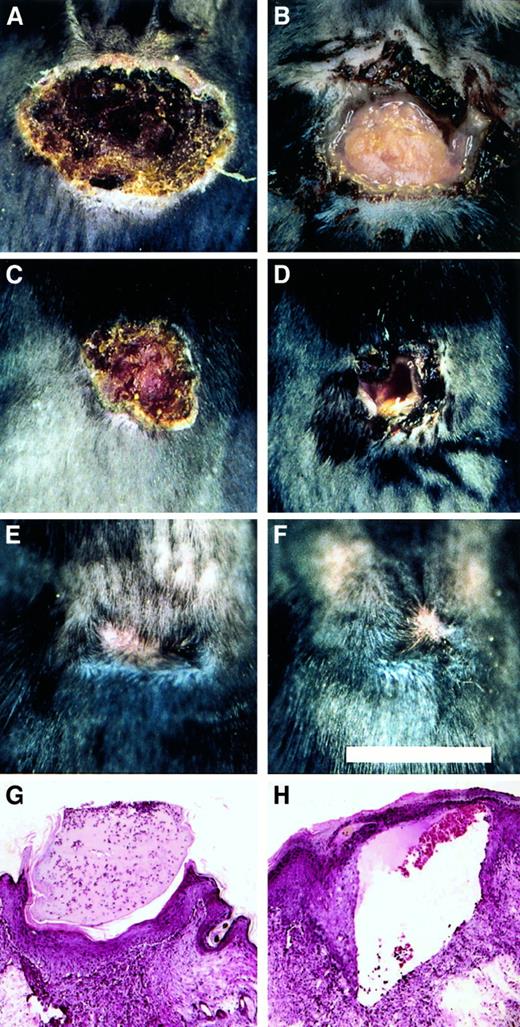
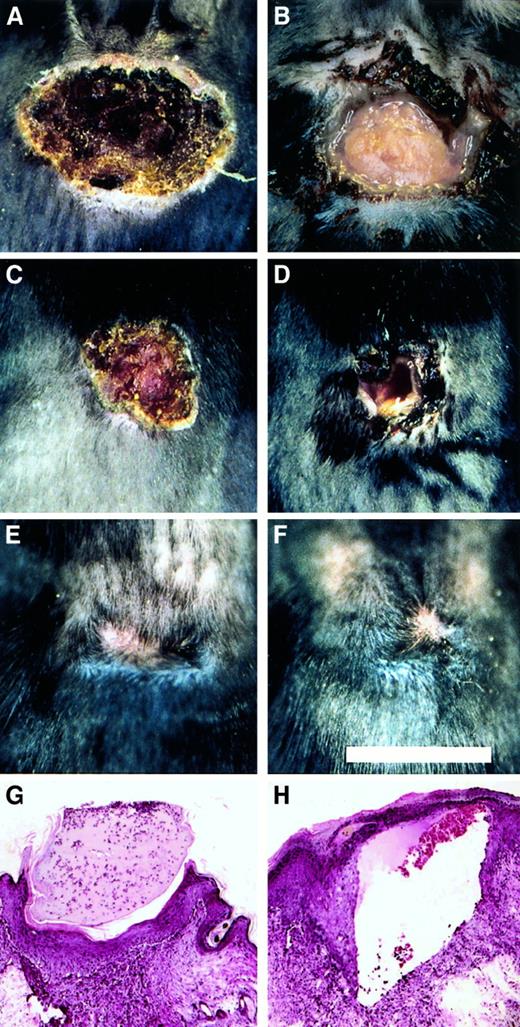
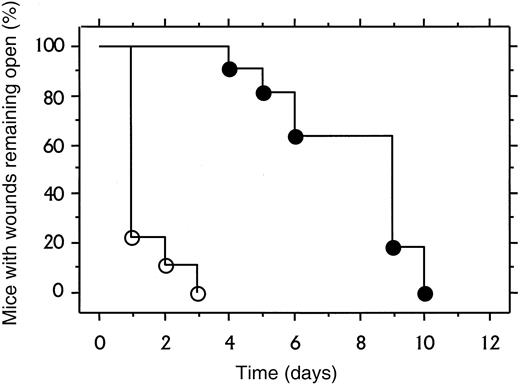
To explore the role of fibrinogen in wound repair in the more challenging context of excisional wounds, a 1 cm square segment of full thickness skin was removed from the dorsum of control (n = 11) and Fib−/− (n = 9) mice. Within 1 to 2 days, control mice developed a dry eschar over the entire wound area (Figure5). In contrast, the wound area of Fib−/− mice remained open for several days, and often filled with blood and wound fluid. The median time required for wound closure (stable eschar development) in control and Fib−/−mice was day 2 and day 7, respectively (Figure6). Generally, control and Fib−/− mice maintained their scabs for a similar time and completed healing between days 12 and 17, despite delayed eschar development. One Fib−/− mouse failed to develop any visible eschar but nevertheless granulation and contraction closed the wound edges rapidly, completing the closure by day 11. Wound area was measured in mice to detect whether tissue ingrowth and contraction of the wound perimeter were occurring at similar rates in control and Fib−/− mice. There was a trend toward an increased rate of contraction in wounds of Fib−/− mice between days 5 and 14 but this did not reach significance (median change in wound area between days 5 and 14, control mice: −0.74 ± 0.07 cm2; Fib−/− mice: −0.87 ± 0.06 cm2;P = .06). A modest enhancement of wound contraction in Fib−/− mice may result from the diminished or absent eschar formation in these animals.10 Thus, despite the wound instability and inability of Fib−/− mice to form an initial blood clot in the wound area, these animals completed the process of skin wound repair in a similar time frame as control mice (Figure 5E,F). Histologically, excisional wounds exaggerated the impaired healing processes observed in incisional wounds of Fib−/− mice. By day 4, keratinocyte migration in Fib−/− mice was frequently directed away from the center of the (empty) wound field, and granulation tissue accumulated only at the wound edges. In contrast, control mice at day 4 made significant progress in re-epithelialization over the wound field, which was filled with early granulation tissue. Nevertheless, exuberant granulation tissue developed in the wounds of both genotypes of mice (see Figure3B,C) by 8 days after wounding, and re-epithelialization was either complete or near completion. Epithelial hyperplasia and sinuses were present in some Fib−/− mice at 8 days but most wounds were healing in a manner analogous to control mice, with the exception of some persistent sinuses (Figure 5H).
Healing pattern of excisional wounds of control and Fib−/− mice.
(A-F) Representational views of the gross appearance of excision wounds after 4 days (A and B), 8 days (C and D), and 21 days (E and F) in control (A, C, and E) and Fib−/− (B, D, and F) mice. Note the open wounds in the Fib−/− mice in B and D. Microscopic appearance of day 8 excisional wounds of control mice (G) and Fib−/− mice (H). Note the presence of a prominent sinus or fissure that is bordered by exuberant granulation tissue and covered by epithelium typical of that encountered in Fib−/− mice (H).
Healing pattern of excisional wounds of control and Fib−/− mice.
(A-F) Representational views of the gross appearance of excision wounds after 4 days (A and B), 8 days (C and D), and 21 days (E and F) in control (A, C, and E) and Fib−/− (B, D, and F) mice. Note the open wounds in the Fib−/− mice in B and D. Microscopic appearance of day 8 excisional wounds of control mice (G) and Fib−/− mice (H). Note the presence of a prominent sinus or fissure that is bordered by exuberant granulation tissue and covered by epithelium typical of that encountered in Fib−/− mice (H).
Time course of excisional wound closure in control and Fib−/− mice.
Cohorts of 11 control mice (open symbols) and 9 Fib−/−mice (closed symbols) were assessed daily for wound closure as defined as stable covering or eschar over the wound field.
Time course of excisional wound closure in control and Fib−/− mice.
Cohorts of 11 control mice (open symbols) and 9 Fib−/−mice (closed symbols) were assessed daily for wound closure as defined as stable covering or eschar over the wound field.
Migration into a dead space
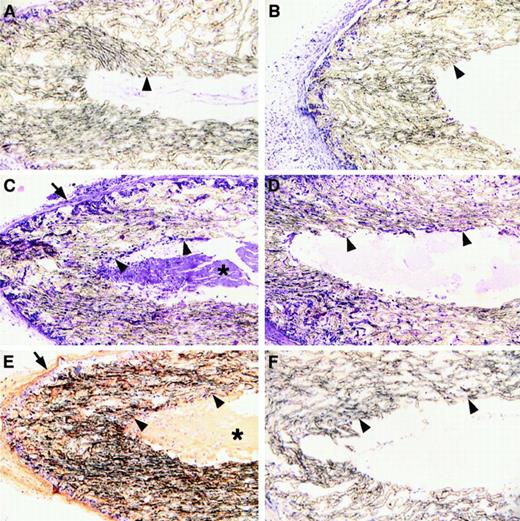
Fibrin in the provisional matrix may support the cellular reorganization of damaged tissues, but the requirement for this matrix component may be most profound in contexts where other existing extracellular matrices are not immediately available, such as in the context of a hematoma or a dead space. The sinuses that formed in the wounds of Fib−/− mice may result from an impediment of these animals to build new tissues in dead space areas. To directly explore the role of fibrin in the organization of wound fluid-filled dead spaces, highly porous, sealed wound chambers were implanted subcutaneously in control and Fib−/− mice. At 7, 14, and 21 days after implantation, the chambers were collected, processed into paraffin, and sectioned for microscopic analysis of cellular organization of the chamber cores. At day 7, cells had successfully infiltrated the porous walls of the chambers in both control and Fib−/− mice (Figure 7A,B). By 14 and 21 days, the lumens of the chambers implanted in control mice contained a fibrin-rich matrix into which cells from the pores had successfully migrated (Figure 7C,E). In contrast, the lumens of chambers of Fib−/− mice contained only amorphous proteinaceous material that was completely devoid of cells, despite abundant cells in the pores of the chamber wall (Figure 7D,F). At 21 days, the luminal aspects of the chambers collected from Fib−/− mice showed only occasional evidence of a layering of cells on the walls. However, cells were not seen infiltrating deep within the lumen even at these later time points. The presence of a provisional fibrin matrix is apparently required for the cellular migration into, and organization of, dead space areas. In the absence of a provisional fibrin matrix, pervasion of the wound field with fibrotic scar tissue appears to be accomplished by gradually building layers of cells inward upon existing cellular layers, analogous to the process seen in wounds healed by secondary intention.
Microscopic analysis of high porosity chambers implanted subcutanteously into control and Fib−/− mice.
Panels A, C, and E show microscopic sections prepared from control mice; panels B, D, and F show sections prepared from Fib−/− mice. Panels A and B show implants left in place for 7 days and panels C-F show implants left in place for 21 days. Panels A-D were stained with hematoxylin and eosin; panels E and F were stained for fibrin(ogen) by immunohistochemistry (brown reaction product). The inside and outside boundaries of the porous tubing are indicated with arrowheads and arrows, respectively. Note that by day 21 the walls of the porous tubing are heavily infiltrated with cells regardless of the presence or absence of fibrin(ogen). Also note that dense fibrillar material (asterisk) containing fibrin(ogen) is present within the lumen of implants of control mice, and this is deeply infiltrated with cells (C and E). In contrast, only amorphous material is present within these spaces in Fib−/− mice and cells are only found proximal to the luminal surface of the implants.
Microscopic analysis of high porosity chambers implanted subcutanteously into control and Fib−/− mice.
Panels A, C, and E show microscopic sections prepared from control mice; panels B, D, and F show sections prepared from Fib−/− mice. Panels A and B show implants left in place for 7 days and panels C-F show implants left in place for 21 days. Panels A-D were stained with hematoxylin and eosin; panels E and F were stained for fibrin(ogen) by immunohistochemistry (brown reaction product). The inside and outside boundaries of the porous tubing are indicated with arrowheads and arrows, respectively. Note that by day 21 the walls of the porous tubing are heavily infiltrated with cells regardless of the presence or absence of fibrin(ogen). Also note that dense fibrillar material (asterisk) containing fibrin(ogen) is present within the lumen of implants of control mice, and this is deeply infiltrated with cells (C and E). In contrast, only amorphous material is present within these spaces in Fib−/− mice and cells are only found proximal to the luminal surface of the implants.
Discussion
Fibrinogen is a key hemostatic factor and mice lacking this fundamental building block of fibrin clots have a life-long risk of spontaneous bleeding events. Nevertheless, Fib−/− mice can tolerate many types of surgical challenges, including full thickness cutaneous wounds, presumably due to the preservation of platelet activation pathways and the availability of multiple ligands for platelet receptors capable of supporting platelet thrombus formation in vivo.13 Therefore, Fib−/− mice provide an opportunity to directly examine the role of fibrin(ogen) in tissue repair processes in vivo. In these studies, we show that provisional fibrin matrices within cutaneous wound fields support the initial wound ‘closure,’ protecting the underlying tissue and providing a structural framework that supports cell migration and organization, including the ingrowth of granulation tissue and re-epithelialization. Although prominent granulation tissue forms in Fib−/− mice, which is microscopically similar to that observed in the control animals, this newly formed tissue is less mechanically stable and fails to appropriately contract the wound, leaving prominent fissures within early wound fields. Furthermore, re-epithelialization does not follow the usual pattern whereby proliferating and migrating keratinocytes project toward the center of the wound field immediately above the newly formed granulation tissue and below the eschar. Rather, epithelial extensions follow paths deep into the sinuses that form in Fib−/− mice, often projecting in a direction away from the center of the wound fields. The result of these reparative failures and cellular detours is inappropriately organized early wound fields with poor tensile strength. Nevertheless, the repair of both incisional and excisional skin wounds in Fib−/− mice is completed in a time frame that is comparable to that of control animals, with a remarkably similar final outcome.
Keratinocyte migration in vitro is stimulated by many extracellular matrix components, including fibrin, fibronectin, and collagen types I and IV, and is inhibited by contact with laminin, a component of the intact basement membrane underlying epithelial cells.14Consistent with the fact that several matrix proteins are supportive of keratinocyte migration, epithelial projections from the wound edge developed rapidly in both fibrinogen-deficient and control mice. Furthermore, fibrin deposition is clearly not essential to the initiation of the process of re-epithelialization. However, the pattern of migration of epithelial sheets at the interface between the eschar and the granulating wound bed was altered in Fib−/− mice, indicating that provisional fibrin-rich matrices are important for effective migration of keratinocytes over the wound field and efficient unification of the 2 wound edges. It is possible that fibrin at the wound edge plays a role in directing keratinocyte migration. However, it seems more likely that vertical gaps in the available matrix and/or adjacent granulation tissue in Fib−/− mice stymie cell migration over the wound field. Therefore, migrating keratinocytes appear to follow the only available path of adhesion: down the blunt surface of the dermis and often proceeding away from the wound center along a wound fissure, or sinus. Wounds from Fib−/− mice eventually became re-epithelialized by either paving the entire inner edge of the wound bed, or by a process of reduplication in which epithelial cells proceeded in several directions, or by the joining of opposite surfaces of epithelium that were not at the leading edge. This abnormal re-epithelialization procedure may be partially compensated for by an “advantage” of not having to proteolytically negotiate fibrin,15,16 increased epithelial hyperplasia which improves the chances of epithelial joining, and increased wound contraction due to early scab loss.10 In this regard, it should be noted that the presence of scab can slow the rate of wound contraction by splinting the wound.11 In addition, the rate of contraction may increase due to excess early collagen/procollagen formation.
Several indirect findings support the concept that fibrin(ogen) might be critical for the formation of granulation tissue in wound fields, including: (1) fibrin(ogen) is a prominent component of early wound fields; (2) endothelial cells, fibroblasts, smooth muscle cells, and inflammatory cells can bind fibrin(ogen) through a variety of integrin and nonintegrin receptors2,3; (3) fibrin matrices prepared in vitro and then implanted into experimental animals support the rapid ingrowth of neovasculature in vivo6,17; (4) specific proteolytic derivatives of fibrin have been shown to be angiogenic18,19; and (5) several angiogenic growth factors (FGF-2 and VEGF) interact with fibrin(ogen) with high affinity.4,5 Nevertheless, the findings presented here show that fibrinogen-deficient mice form copious, highly-vascularized granulation tissue in response to injury which at the level of light microscopy is qualitatively similar to that observed in control mice. Comparable features include abundant fibroblasts, inflammatory cells, neovessels, and neomatrix, indicating no apparent granulation deficit. However, there are significant differences in the collagen content in the granulation tissue of control and Fib−/− mice at early times, and other biochemical or subtle organizational differences in both early and mature wounds may be revealed in more detailed studies. While the newly formed granulation tissue in Fib−/− mice reaches into and closes the wound, it is flawed based on an inability to seamlessly draw together the wound edges without the formation of fissures, or wound sinuses, and to maintain wound stability. Movement of the animal may continually disrupt the granulation tissue, and the gaps that form may be more difficult to bridge in Fib−/− mice. This feature of Fib−/− mice was exaggerated in excisional wounds, which remained open for a week. Less wound stability is consistent with reduced tensile strength in wounds of Fib−/− mice. However, this defect may be eventually overcome as granulation tissue is remodeled and replaced by mature connective tissue containing collagen types I and III, fibronectin, and proteoglycans.20-23
Fibrin(ogen) appears to be most important in tissue repair when cells must infiltrate and organize fluid-filled areas, such as an internal dead space established by a porous chamber. The difficulty of cells in Fib−/− mice to efficiently infiltrate and obliterate a dead space is consistent with the previous observation that spontaneous subcapsular hematomas in the liver and kidney of these mice were not readily organized and populated with cells.7 In both cases, evidence of a gradual, layered ingrowth of cells from the borders appears to be the only means for cells to “fill in” these defects. The data suggest a general model whereby nonfibrin(ogen) matrix components associated with the porous walls of a chamber support initial cell migration into the internal space, but without fibrin to provide a provisional scaffold, further organization of the internal dead space must proceed by a gradual layering assembly of cells and matrix.
Based on the notion that a provisional fibrin matrix may support the development of mature granulation tissue, the elevated levels of collagen in early granulation tissue of Fib−/− relative to control mice was unexpected. The mechanism or mechanisms that account for this distinction have not yet been established, but there is some evidence that increased stress on a wound (such as the instability associated with the inability to efficiently close fissures) may indirectly result in an increase in collagen production.24 It is conceivable that such a compensatory mechanism may account for increased collagen production in wounds of Fib−/− mice. Previous studies have indicated that the presence of fibrin may delay fibroblast procollagen production in an in vitro setting.25 Collagen deposition may be enhanced in early granulation tissue of Fib−/− mice since fibrin matrices need not be cleared as a prelude to the development of more permanent matrices. The increased collagen deposition may partially compensate for the defect in provisional matrix while providing a stronger biomechanical coupling between cells and extracellular matrix. Whatever the mechanisms, this increased collagen deposition in Fib−/− mice did not result in an overall increase in wound tensile strength at early times. This may be in part due to a subtle lack of organization of the new matrices, and electron microscopy and detailed biochemical analyses of wound tissue may prove to be illuminating in this regard. It is important to note that the finding that collagen deposition is prominent within definitive granulation tissue formed at wound borders of incisional wounds in Fib−/− mice does not argue that collagen and other connective tissue will form more rapidly in Fib−/− mice in other settings. For example, in a dead space setting (as illustrated in Figure 7), tissue organization in general, and collagen deposition in particular, will likely be diminished (delayed) in Fib−/− mice relative to control animals deep within the open zone. Studies in several dead space models, including open-ended, long (2 cm), non-porous tubing implants, are presently underway to test this hypothesis.
These studies directly establish that fibrinogen serves a broader physiologic role than merely controlling blood loss. This hemostatic factor contributes significantly to the reparative process following mechanical injury to skin. Although direct analyses of repair in Fib−/− mice following other types of injury (eg, chemical and thermal burns, bacterial infection, metabolic disease) and in other organ systems have not yet been performed, it is likely that fibrinogen contributes to repair in a broad spectrum of contexts. However, given that fibrin(ogen) is not essential for embryonic development, including all aspects of organogenesis, it would not be anticipated that fibrinogen would not be essential for certain types of organ repair (eg, liver regeneration following partial hepatectomy). An intriguing extension to the hypothesis that fibrinogen is important in tissue repair in most organ systems is that this protein may be an important determinant of the progression of a variety of diseases resulting in significant tissue damage. Studies of atherosclerosis, cancer, inflammatory joint disease, and glomerulonephritis in Fib−/− mice support this concept.26-28
We wish to acknowledge the assistance of Alicia Emley with photography and Jill M. Potter with mouse genotyping.
Supported by awards from the American Heart Association, Ohio Valley Affiliate (A.F.D.) and the Department of Veterans Affairs (J.M.D.), and grants from the National Institutes of Health (nos. AG-06528 and AR-41943 to J.M.D.; nos. HL47826 and HL63194 to J.L.D.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jay L. Degen, Children's Hospital Research Foundation, Children's Hospital Medical Center, IDR-NRB Rm 2042, 3333 Burnet Ave, Cincinnati, OH 45229-3039; e-mail: degenjl@chmcc.org.