Acquired coagulation factor inhibitors include pathologic immunoglobulins that specifically bind to coagulation factors and either neutralize their procoagulant activity, accelerate their clearance from the circulation, or have proteolytic activity to degrade them into inactive polypeptides. Here, an autoantibody against prothrombin is described in a patient with serious hemorrhagic diatheses. The autoantibody exerts its influence by a previously unknown mechanism in which it inhibits coagulation through aberrant activation of the proenzyme in a catalytic manner. The antibody-bound prothrombin formed a stable stoichiometric complex with antithrombin III, consisting of intact prothrombin and an antithrombin III molecule cleaved at the 393Arg-394Ser bond. The antibody dissociated from prothrombin after the complex formation with antithrombin III. Although the bound antibody elicited protease activity from prothrombin, the complex was not able to convert fibrinogen to fibrin or to activate protein C. Thus, this is the first description of an autoantibody that induces protease-like activity from a human proenzyme, permitting subsequent neutralization by its physiological inhibitor.
Introduction
Circulating anticoagulants are usually defined as abnormal substances that directly interfere with mechanisms of blood coagulation.1 They include abnormal immunoglobulins that specifically bind to coagulation factors and either neutralize their procoagulant activity,2 accelerate their clearance from the circulation,3 or have proteolytic activity to degrade them into inactive polypeptides.4
Enzymatic conversion of prothrombin to an active enzyme, thrombin, is essential in the final step of blood coagulation. Patients with hereditary abnormalities of prothrombin generate decreased amounts of thrombin, which typically results in a lifelong bleeding disorder.5 By contrast, acquired inhibitors of thrombin or prothrombin may have variable effects on the blood coagulation system. Nonneutralizing, antiprothrombin antibodies are the most common inhibitors that lead to a prothrombotic state that is associated with the lupus anticoagulant in patients with systemic lupus erythematosus and various other diseases.6 In contrast, neutralizing thrombin antibodies rarely occur. In addition to the antibodies induced by exposure to bovine topical thrombin such as fibrin glue during surgical procedures,7 isolated cases of antithrombin autoantibodies have been reported in patients with collagen diseases, liver cirrhosis, and paraproteinemia, or even without any apparent disease.8-10 In some instances, these inhibitors have been associated with a serious bleeding disorder.3 11-13However, the specificity of these antibodies has never been extensively investigated.
We report a patient in whom a potent human prothrombin inhibitor developed that was associated with recurrent bleedings including retroperitoneal and pulmonary hematoma in the apparent absence of underlying disease. The present observation is the first description of an autoantibody directed toward prothrombin, which induces protease-like activity to facilitate a stable stoichiometric complex formation with its physiological inhibitor antithrombin III without α-thrombin conversion.
Materials and methods
Case history
A 58-year-old man was referred because of retroperitoneal hematoma. He had been well until several days earlier, when a sudden onset of right lower abdominal pain developed. There was no history of trauma or drug exposure. His medical history was unremarkable, and he had no history of easy bruising. Family history was negative for bleeding disorders. Computed tomography of the abdomen showed a large, round nodule (8 × 5 cm in the right retroperitoneal space that disappeared after 3 months. Initial laboratory evaluation revealed a normal blood cell count. Both the prothrombin time (PT) and the activated partial thromboplastin time (APTT) were prolonged at 22.7 seconds (normal range, 10.0-11.7) and 50.3 seconds (normal range, 28.5-40.0), respectively. His prothrombin activity was severely impaired to less than 10% of the normal level, and his antigen level was decreased to 5% of the control level. The antithrombin III level was also decreased to 37% of normal value. Other coagulation factors were within normal range.14 In contrast, the patient's thrombin–antithrombin III (TAT) complex was markedly increased to 7976 ng/mL (normal, less than 2.4) without consumption of fibrinogen. These coagulation disorders persisted for more than 3 months.
Prothrombin inhibitor
Patient plasma was fractionated by gel filtration on a Sephacryl S–200HR column (Pharmacia Biotech, Uppsala, Sweden). Each fraction was incubated with antithrombin III at 37°C for 3 hours, and the generated TAT complex level was measured by Enzygnost-TAT (Behring Diagnostics GmbH, Marburg, Germany).15 Fractions that had activity of TAT complex generation were collected and applied to Sepharose affinity resins containing immobilized Protein A, Protein G, or antihuman immunoglobulin polyclonal antibody (Cappel ICN, Aurora, OH). Total immunoglobulin G (IgG) fraction was isolated from this patient and from normal plasma by chromatography on Protein G–Sepharose and immobilized antihuman IgG antibody (Cappel ICN) Sepharose. The columns were washed extensively with phosphate-buffered saline, pH 7.4, and the IgG was eluted with 0.1 M glycine HCl, pH 3.0, and immediately neutralized with 1 M Tris, pH 8.0, followed by dialysis against phosphate-buffered saline. The IgG fraction was concentrated using a microconcentrator (Centricon 30; Amicon, Beverly, MA).
Characterization of the aberrant thrombin-antithrombin complex
Human prothrombin and IgG were pretreated with 1 mM di-isopropyl fluorophosphate (DFP; Wako Pure Chemical Industries, Tokyo, Japan) at 25°C for 1 hour and dialyzed extensively before additional experiments were conducted. The prothrombin was radiolabeled with sodium iodide I 125 using Iodobeads (Pierce, Rockford, IL) according to the manufacturer's directions. To observe the aberrant thrombin-antithrombin (TAT′) complex formation in vitro with patient IgG, a mixture of 1.0 μM nonlabeled and 10 nM125I-labeled prothrombin was incubated with 750 μg/mL IgG from patient or normal plasma at 4°C for 2 hours, and each mixture was then incubated with 5 μM antithrombin III at 37°C for various intervals (0-24 hours). Samples were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions, and the gel was dried and exposed for autoradiography at 25°C. We purified the TAT′ complex from patient plasma using immobilized antihuman antithrombin III antibody Sepharose followed by chromatography on affinity resins containing antihuman prothrombin antibody (Nordic Immunology, Tilburg, CA). Patient plasma was also fractionated by BaSO4 absorption, and the barium eluate was applied to an affinity column of immobilized anti-TAT monoclonal antibody (JITAT-17).16 Each sample was analyzed by 10% SDS-PAGE under reducing and nonreducing conditions; this was followed by immunoblotting with antihuman antithrombin III and antihuman prothrombin polyclonal antibodies.
3H-DFP incorporation into IgG antibody–prothrombin
Before use, 310.8 kBq/μmol 3H-DFP (NEN Life Science Products, Boston, MA) was diluted 4 times with nonlabeled DFP to a concentration of 1 mM DFP. One micromolar prothrombin was incubated with 750 μg/mL patient IgG at 4°C for 2 hours; this was followed by the addition of 100 μM 3H-DFP and further incubation at 25°C for 2 hours. The sample was fractionated on a Sephacryl S-200HR gel filtration column, and the radioactivity of each fraction was determined with a liquid scintillation counter (LSC-3500; Aloka, Tokyo, Japan).
Prothrombin derivatives and recombinant deletion mutants
Prothrombin was digested with human factor Xa (Calbiochem, La Jolla, CA) into fragment 1, fragment 2, fragment 1 + 2, and α-thrombin. Then the fragments were separated by high-performance liquid chromatography (Waters 996 Alliance system; Milford, MA) using a reverse-phase column (phenyl-5PW RP; Toso, Tokyo, Japan). Meizothrombin was prepared by incubating 3.3 μM Echinus carinatus venom (ECV) with prothrombin (22 μM) and 0.36 mM Dansyl-arginine N, N-(3-ethyl-1,5-pentanediyl)amide (DAPA) (Hematologic Technologies, Essex, VT) in 550 μL Tris-HCl, pH 7.4, for 15 minutes at room temperature. The reaction was quenched by the addition of 1 mL wheat germ agglutinin gel slurry (Vector Laboratories, Burlingame, CA), which binds ECV. Bound ECV was removed by filtration. Immediately before use, the excess DAPA was removed by gel filtration through Sephadex G-50.17 Human cDNA clones for full-length prothrombin were kindly provided by Dr J. E. Sadler (Washington University School of Medicine, St Louis, MO). The recombinant human prothrombin deletion mutants used in this study were constructed by site-directed mutagenesis (Quikchange Site-Directed Mutagenesis Kit; Stratagene, La Jolla, CA) with mutagenic primers 5′-GATGACTCCACGCTCCTAAGGCTCCAGTGTG-3′ (nucleotides 563-593), 5′-CAGACAGGGCCATCTAAGGGCGTACCGCC-3′ (nucleotides 901-929), and 5′-GACGGGCGCATTGTGTAGGGCTCGGATGC-3′ (nucleotides 1062-1090) to produce fragment 1, fragment 1 + 2, and fragment 1 + 2 with the light chain of α-thrombin. Chinese hamster ovary cells, which lacked the enzyme dihydrofolate reductase, were transfected with the expression constructs in pCDNA3 (Invitrogen, Carlsbad, CA). For production of recombinant prothrombin and its deletion mutants, each cell line was grown in serum-free medium containing 10 μg/mL vitamin K2 for 24 hours, as described previously.18
Amino terminal sequence analysis
One micromolar human prothrombin was incubated with 750 μg/mL patient IgG in the presence of 5 μM antithrombin III at 37°C for 12 hours. The mixture was applied to JITAT-17 monoclonal antibody immobilized on Sepharose, and the eluate was separated by 10% SDS-PAGE under reducing and nonreducing conditions. The gel was electroblotted onto a polyvinylidene difluoride membrane at 50 mA for 16 hours. The membrane was stained with 0.25% Coomassie in 50% methanol without acetic acid, and visible bands were cut and processed for NH2-terminal sequencing in an ABI model 496A sequencer (Foster City, CA). The amount of protein sequenced ranged from 4 to 8 pmol, depending on the fragments.
Results and discussion
Mixing patient plasma with normal plasma generates TAT complex
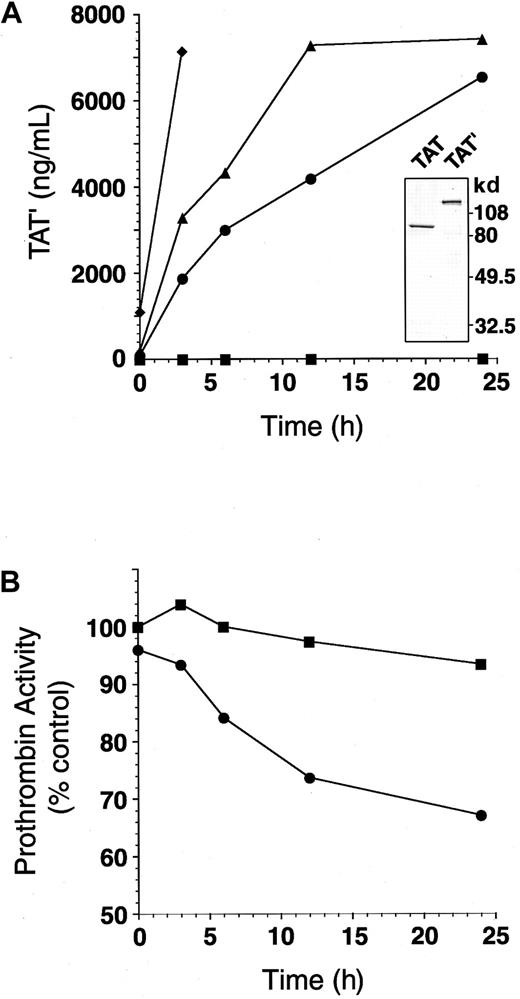
We initially suspected that the markedly prolonged prothrombin and activated partial thromboplastin times in a patient with hemorrhagic diatheses were caused by a coagulation factor(s) deficiency because the prolonged clotting times were corrected after 1-hour incubation at 37°C with normal plasma (Figure 1). However, in further incubation, 25% of the patient plasma led to prolongation of PT and APTT in a time-dependent manner (data not shown). In the mixing study, TAT complex formation markedly increased in a similar manner (Figure 2A). Therefore, we measured residual prothrombin activity in the mixture. Five percent of patient plasma added to normal plasma caused a slow loss of total prothrombin coagulant activity (Figure 2B). Surprisingly, the TAT complex immunopurified from patient plasma showed a higher molecular weight (130 kd) than the standard TAT complex. Thus, we designate this aberrant high-molecular-weight complex as TAT′ complex.
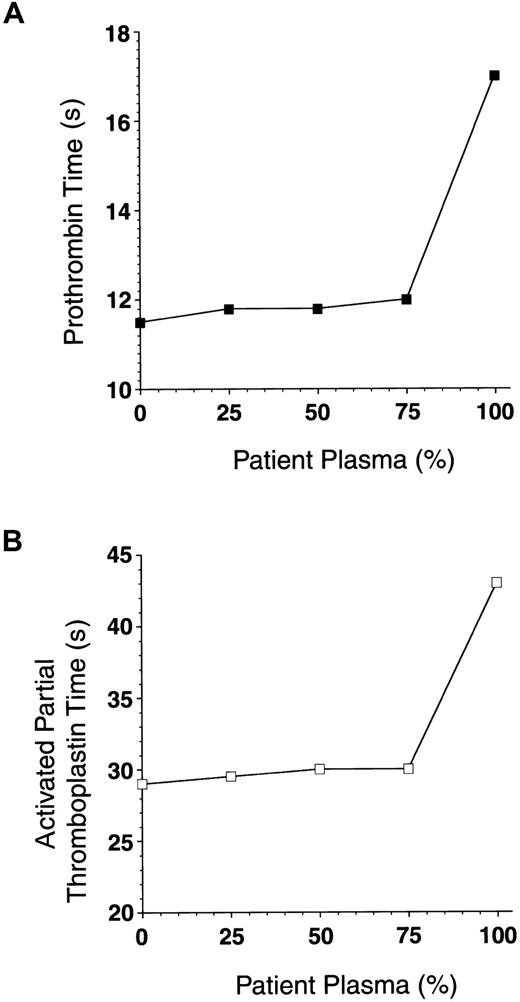
Mixing patient plasma with normal plasma does not prolong clotting times.
Mixtures of patient plasma (0%, 50%, 75%, and 100% final) and normal plasma were incubated at 37°C for 1 hour. Prothrombin time (A) and activated partial thromboplastin time (B) in each sample were measured.
Mixing patient plasma with normal plasma does not prolong clotting times.
Mixtures of patient plasma (0%, 50%, 75%, and 100% final) and normal plasma were incubated at 37°C for 1 hour. Prothrombin time (A) and activated partial thromboplastin time (B) in each sample were measured.
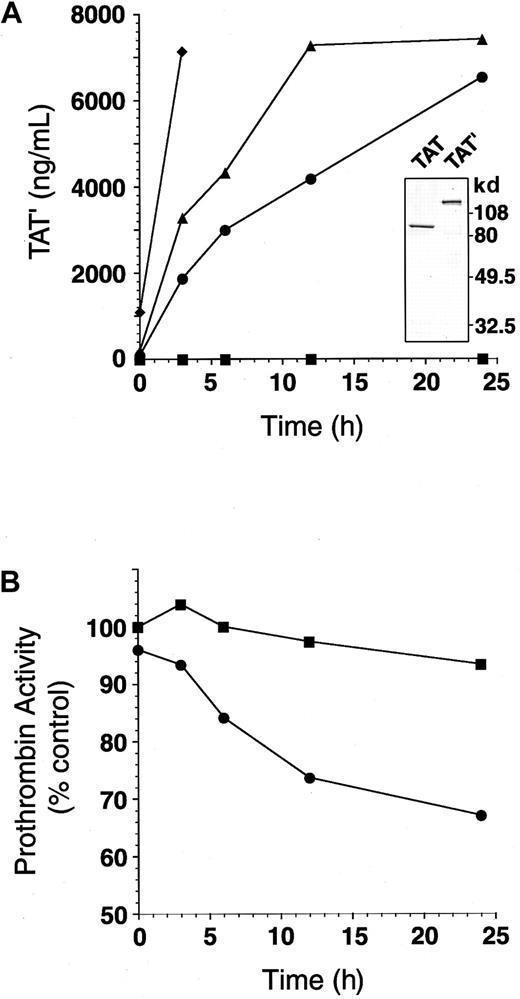
Mixing patient plasma with normal plasma generates TAT′ complex and impairs residual prothrombin activity.
(A) Mixtures of patient plasma (0%, ▪; 5%, ●; 10%, ▴; 50% final, ♦) and normal plasma were incubated at 37°C for 0 to 24 hours. The amount of TAT′ complex generated in each sample was measured by ELISA for normal TAT. (inset) TAT complex and TAT′ complex isolated from patient plasma were subjected to SDS-PAGE (10% separating gels under reducing conditions). (B) Patient plasma (0%, ▪; 5% final, ●) was mixed with normal plasma and incubated at 37°C for 0 to 24 hours. Residual prothrombin activity in each sample was measured.
Mixing patient plasma with normal plasma generates TAT′ complex and impairs residual prothrombin activity.
(A) Mixtures of patient plasma (0%, ▪; 5%, ●; 10%, ▴; 50% final, ♦) and normal plasma were incubated at 37°C for 0 to 24 hours. The amount of TAT′ complex generated in each sample was measured by ELISA for normal TAT. (inset) TAT complex and TAT′ complex isolated from patient plasma were subjected to SDS-PAGE (10% separating gels under reducing conditions). (B) Patient plasma (0%, ▪; 5% final, ●) was mixed with normal plasma and incubated at 37°C for 0 to 24 hours. Residual prothrombin activity in each sample was measured.
Antibody against prothrombin makes a high-molecular-weight complex of prothrombin in the presence of antithrombin III
Gel filtration analysis of the patient's plasma demonstrated that the TAT′ complex generating factor was present in a high-molecular-weight fraction (150 kd). This factor did not bind to a protein A–Sepharose resin but specifically bound to protein G–Sepharose resin (data not shown). Patient and normal IgG were purified using antihuman immunoglobulin polyclonal antibody–Sepharose. Pass-through fractions of patient plasma from the column neither decreased the coagulant activity of prothrombin nor increased the formation of TAT′ in the mixing studies described above. Purified IgGs were incubated with 125I-labeled human prothrombin in the absence or in the presence of a molar excess of antithrombin III (Figure 3, panels A and B, respectively). Incubation of 125I-prothrombin and patient IgG with antithrombin III resulted in a shift of the 72 kd prothrombin band to 130 kd in a time-dependent manner, as judged by SDS-PAGE. The amount of 130-kd complex reached 17% of total prothrombin after 24 hours (Figure3B). In contrast, normal IgG had no effect on the migration of prothrombin or on the complex formation as judged by an enzyme-linked immunosorbent assay (ELISA) in the presence of antithrombin III (Figure3C). The generation of the high-molecular-weight complex was not accelerated by heparin (data not shown). We separated the 130-kd complex from the patient plasma by barium absorption followed by affinity chromatography on antiprothrombin polyclonal IgG–Sepharose resin or immobilized monoclonal antibody against TAT complex (JITAT-17). It has been shown that JITAT-17 recognizes the heavy chain of modified antithrombin III in the TAT complex but that it does not react with free thrombin or intact antithrombin III.15 The 130-kd molecule was present solely in the barium-absorbed fractions and specifically bound to both antibody-affinity columns, indicating the presence of a prothrombin molecule with an intact NH2-terminal γ-carboxyglutamic acid (Gla) domain in a complex with antithrombin III. This prothrombin-antithrombin complex was also stable to treatment with glycine HCl, pH 3.0, 5.0 M urea, or 2.0% SDS.
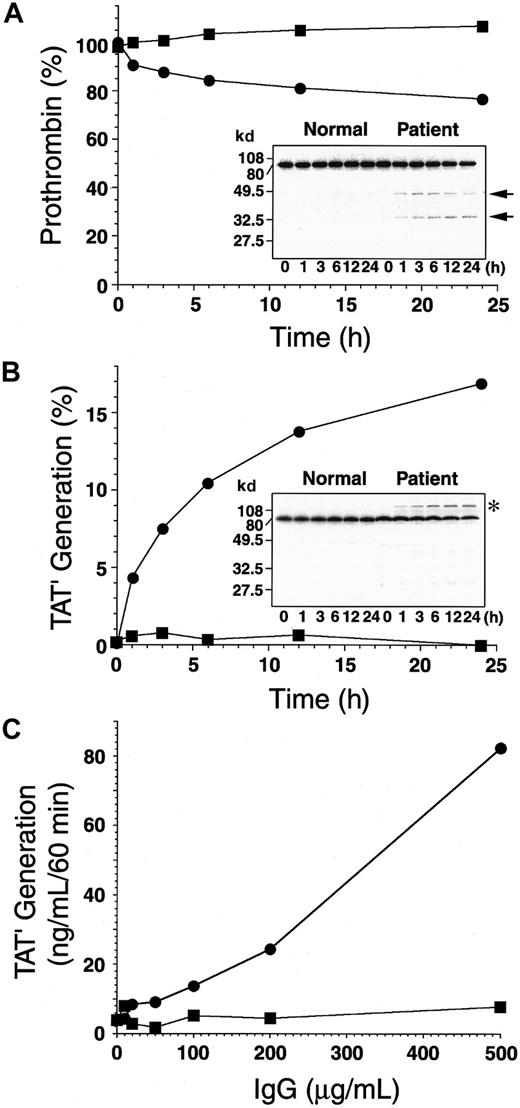
Incubation of 125I-prothrombin with the autoantibody cleaves prothrombin in the absence of antithrombin III or makes a high-molecular-weight complex in the presence of antithrombin III.
(A) Nonlabeled (1 μM) and 125I-labeled prothrombin (10 nM) were incubated with 750 μg/mL normal (▪) or patient IgG (●) in the absence of antithrombin III at 37°C for 0 to 24 hours. Each sample was separated by 10% SDS-PAGE under reducing conditions, followed by autoradiography (inset). Percentage residual prothrombin at each time point was determined by counting the 72-kd band and total radioactivity of each lane. (B) Nonlabeled (1 μM) and125I-labeled prothrombin (10 nM) were incubated with 750 μg/mL normal (▪) or patient IgG (●) in the presence of 5 μM antithrombin III at 37°C for 0 to 24 hours. Each sample was separated by 10% SDS-PAGE under reducing conditions, followed by autoradiography (inset). TAT′ generation (asterisk) was determined by counting radioactivity and was expressed as the percentage of total radioactivity of each lane. (C) Prothrombin (1 μM) was incubated with 0 to 500 μg/mL normal (▪) or patient IgG (●) in the presence of 5 μM antithrombin III at 37°C for 3 hours. The amount of TAT′ complex generated in each sample was measured by ELISA for normal TAT.
Incubation of 125I-prothrombin with the autoantibody cleaves prothrombin in the absence of antithrombin III or makes a high-molecular-weight complex in the presence of antithrombin III.
(A) Nonlabeled (1 μM) and 125I-labeled prothrombin (10 nM) were incubated with 750 μg/mL normal (▪) or patient IgG (●) in the absence of antithrombin III at 37°C for 0 to 24 hours. Each sample was separated by 10% SDS-PAGE under reducing conditions, followed by autoradiography (inset). Percentage residual prothrombin at each time point was determined by counting the 72-kd band and total radioactivity of each lane. (B) Nonlabeled (1 μM) and125I-labeled prothrombin (10 nM) were incubated with 750 μg/mL normal (▪) or patient IgG (●) in the presence of 5 μM antithrombin III at 37°C for 0 to 24 hours. Each sample was separated by 10% SDS-PAGE under reducing conditions, followed by autoradiography (inset). TAT′ generation (asterisk) was determined by counting radioactivity and was expressed as the percentage of total radioactivity of each lane. (C) Prothrombin (1 μM) was incubated with 0 to 500 μg/mL normal (▪) or patient IgG (●) in the presence of 5 μM antithrombin III at 37°C for 3 hours. The amount of TAT′ complex generated in each sample was measured by ELISA for normal TAT.
Amino terminal sequencing of the high-molecular-weight complex
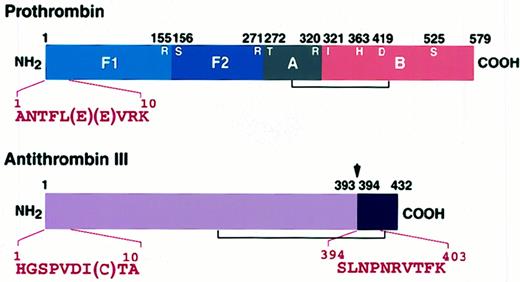
Amino acid sequencing of the first 10 residues of the 130-kd protein revealed that it consisted of an equimolar complex of prothrombin and antithrombin III cleaved between393arginine and 394serine (Table1; Figure4). Autoradiographic analysis of125I-labeled prothrombin, a 72-kd single-chain molecule, incubated with patient IgG in the absence of antithrombin III revealed prothrombin-derived bands at 32.5 and 40 kd (Figure 3A). The result was similar, even if the patient IgG and prothrombin had been pretreated separately with 1 mM DFP and dialyzed extensively (data not shown). The amount of native prothrombin at 24 hours was decreased to less than 80% of initial level. Amino acid sequencing of the first 5 residues of the 32.5- and 40-kd peptides showed that prothrombin was cleaved between 284arginine and 285threonine (Table2). Antithrombin III did not form a complex with either the 32.5-kd or the 40-kd prothrombin derivatives, which were generated by incubation of prothrombin with purified patient IgG in the absence of antithrombin III. Furthermore,125I-antithrombin III was not cleaved, nor did it form a high-molecular-weight complex with patient IgG even after a 24-hour incubation. These results suggest that patient IgG induces a conformational change in the prothrombin molecule sufficient to permit the formation of a complex with antithrombin III.
Amino-terminal sequence analyses of the complex.
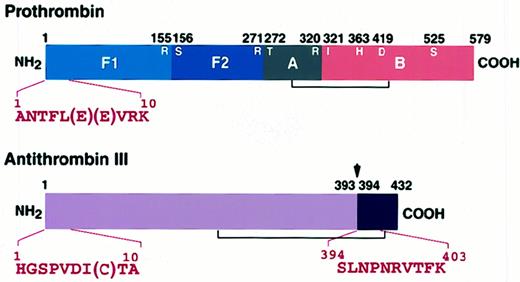
Prothrombin (1 μM) was incubated with 750 μg/mL patient IgG in the presence of 5 μM antithrombin III at 37°C for 12 hours. The mixture was applied to immobilized JITAT-17 monoclonal antibody, and the eluate was separated by 10% SDS-PAGE. The gel was electroblotted onto a polyvinylidene difluoride membrane and stained, and appropriate bands were sequenced directly with an ABI Model 476A protein sequencer. The thrombin cleavage site (155R-156S), the factor Xa cleavage sites (271R-272T and320R-321I), and the catalytic triad (363H, 419D, and 525S) of prothrombin are shown. Arrow shows the reactive center of antithrombin III (39 3R-394S).
Amino-terminal sequence analyses of the complex.
Prothrombin (1 μM) was incubated with 750 μg/mL patient IgG in the presence of 5 μM antithrombin III at 37°C for 12 hours. The mixture was applied to immobilized JITAT-17 monoclonal antibody, and the eluate was separated by 10% SDS-PAGE. The gel was electroblotted onto a polyvinylidene difluoride membrane and stained, and appropriate bands were sequenced directly with an ABI Model 476A protein sequencer. The thrombin cleavage site (155R-156S), the factor Xa cleavage sites (271R-272T and320R-321I), and the catalytic triad (363H, 419D, and 525S) of prothrombin are shown. Arrow shows the reactive center of antithrombin III (39 3R-394S).
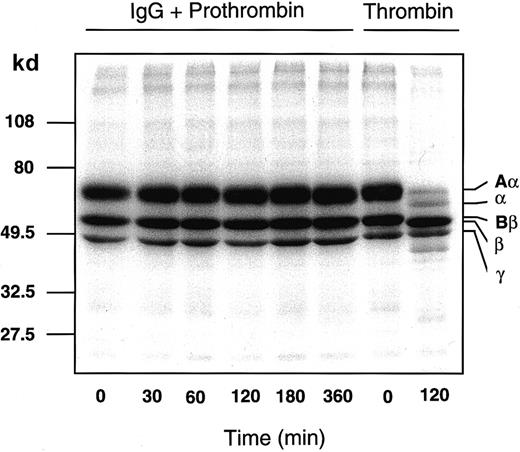
Antibody-bound prothrombin is unable to convert fibrinogen to fibrin or to activate protein C
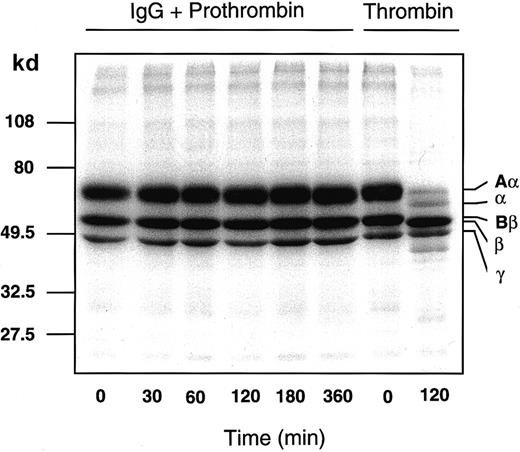
After incubation of 125I-fibrinogen with the patient IgG-prothrombin mixture for various time periods, each sample was analyzed by SDS-PAGE subjected to autoradiography (Figure5). The IgG-prothrombin mixture could not convert fibrinogen to fibrin even after a 6-hour incubation. This result is consistent with the clinical data (“Materials and methods”). Moreover, protein C was not activated by either the patient IgG or an IgG-prothrombin mixture even if soluble thrombomodulin (a kind gift of Mochida Pharma, Tokyo, Japan) was present, as judged by an amidolytic assay using a substrate specific for activated protein C (data not shown).
Patient IgG-prothrombin complex does not convert125I-fibrinogen to 125I-fibrin.
Nonlabeled (30 μM) and 300 nM 125I-fibrinogen were incubated with a mixture of 750 μg/mL patient IgG and 1 μM purified prothrombin that had been preincubated at 4°C for 2 hours or with 10 NIH Us/mL α-thrombin, at 37°C for 0 to 360 minutes. Each sample was analyzed by 10% to 15% gradient SDS-PAGE under reducing conditions, followed by autoradiography.
Patient IgG-prothrombin complex does not convert125I-fibrinogen to 125I-fibrin.
Nonlabeled (30 μM) and 300 nM 125I-fibrinogen were incubated with a mixture of 750 μg/mL patient IgG and 1 μM purified prothrombin that had been preincubated at 4°C for 2 hours or with 10 NIH Us/mL α-thrombin, at 37°C for 0 to 360 minutes. Each sample was analyzed by 10% to 15% gradient SDS-PAGE under reducing conditions, followed by autoradiography.
Serine protease activity appears in antibody-bound prothrombin without its prior conversion to a 2-chain molecule
Pretreatment of patient IgG with a variety of protease inhibitors, such as antipain-dihydrochloride (100 μM), bestatin (150 μM), chymostatin (100 μM), E-64 (25 μM), leupeptin (10 μM), pepstatin (1 μM), phosphoramidon (400 μM), Pefabloc SC (4 mM), EDTANa2 (1 mM), and aprotinin (0.3 μM) for 1 hour did not decrease the TAT′ complex-forming activity of the IgG (data not shown). Patient IgG pretreated with the serine protease inhibitor DFP (final concentration, 1 mM) also generated TAT′ complex on the addition of prothrombin and antithrombin III. In contrast, when the mixture of prothrombin and patient IgG was treated with the same concentration of DFP or 10 mM p-amidino phenylmethylsulfonyl fluoride (pAPMSF; Wako Pure Chemical Industries) before it was added to antithrombin III, the TAT′ complex generation was completely inhibited (data not shown). These results indicate that IgG itself does not have enzymatic activity analogous to the alloantibodies against factor VIII described previously4 and that it appears to elicit protease activity in the prothrombin zymogen without peptide bond cleavage.
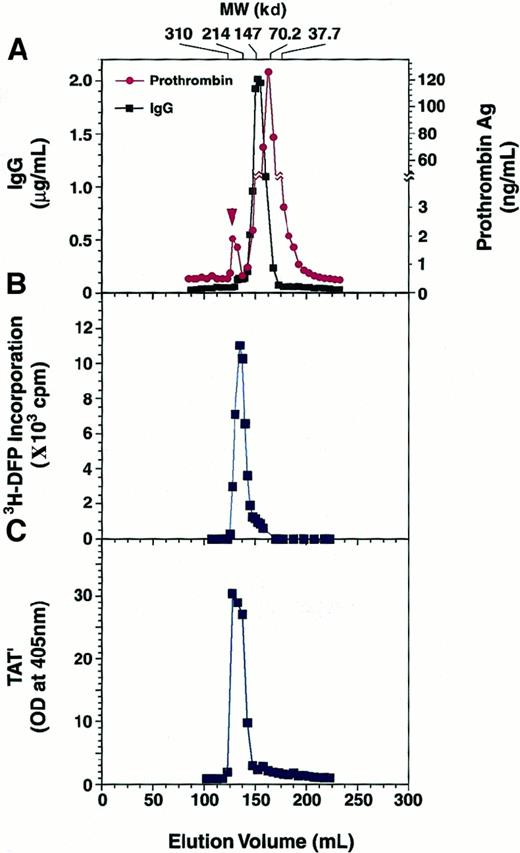
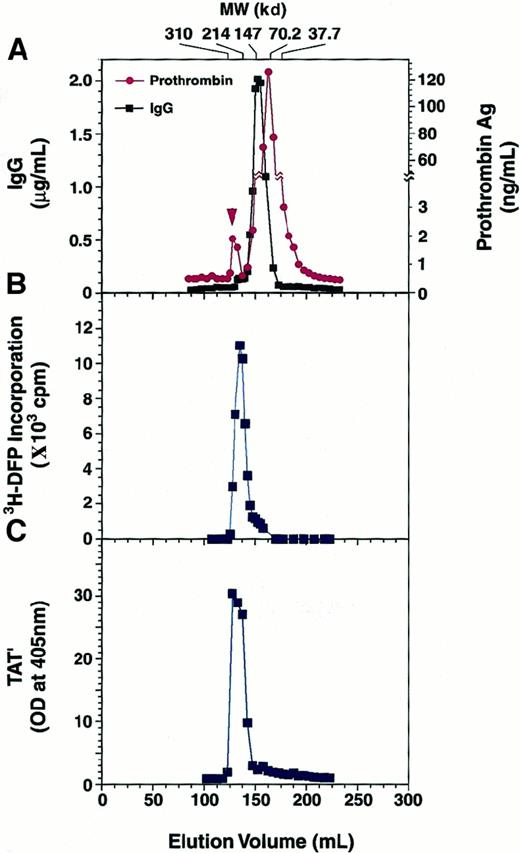
To address this question of aberrant IgG-induced prothrombin activation, we examined whether 3H-DFP could be incorporated into the antibody-bound prothrombin. Prothrombin was mixed at 1 μM with 750 μg/mL normal IgG or patient IgG at 4°C for 2 hours, and the mixture was incubated with either DFP or3H-DFP (37 kBq/μL) at 25°C for 2 hours followed by gel filtration analysis. There was prothrombin antigen in the fractions corresponding not only to 70-kd but also to 220-kd molecules that were supposed to be the complex of prothrombin and the patient IgG (Figure6A). However, prothrombin antigen existed only in the fractions corresponding to 70-kd molecules when we used normal IgG (data not shown). When we mixed prothrombin with patient IgG, 3H-DFP coeluted with fractions corresponding to 220-kd molecules (Figure 6B), and there was no 3H-DFP signal in the 150-kd fractions (IgG) or in the 70-kd fractions (prothrombin). Considering the amount of prothrombin antigen in the fractions corresponding to 220-kd molecules, we estimated less than 1% of total IgG made complex with prothrombin (Figure 6A). In the absence of DFP, we further confirmed that these 220-kd fractions had TAT′ complex generation activity when antithrombin III was added (Figure 6C). Therefore, these results are consistent with the observation that the serine protease activity appears in the IgG-bound prothrombin, without its prior conversion to a 2-chain molecule, and that this active molecule subsequently cleaves antithrombin III at its reactive center to make a stable complex.
Gel filtration profile of a prothrombin and patient IgG mixture.
Prothrombin (1 μM) was incubated with 750 μg/mL patient IgG at 4°C for 2 hours. This was followed by the addition of3H-DFP and a further incubation at 25°C for 2 hours. The sample was separated on a Sephacryl S-200HR gel filtration column, and the IgG and prothrombin concentrations of each fraction were measured (A). Arrowhead shows the prothrombin complexed with IgG. Radioactivity in the fractions was determined by counting in a liquid scintillation counter (B). In the absence of DFP treatment, a prothrombin (1 μM) and IgG (750 μg/mL) mixture was applied to the column at 4°C, and each fraction was separately incubated with 5 μM antithrombin III at 37°C for 3 hours. The generation of TAT′ complex was analyzed by ELISA (C).
Gel filtration profile of a prothrombin and patient IgG mixture.
Prothrombin (1 μM) was incubated with 750 μg/mL patient IgG at 4°C for 2 hours. This was followed by the addition of3H-DFP and a further incubation at 25°C for 2 hours. The sample was separated on a Sephacryl S-200HR gel filtration column, and the IgG and prothrombin concentrations of each fraction were measured (A). Arrowhead shows the prothrombin complexed with IgG. Radioactivity in the fractions was determined by counting in a liquid scintillation counter (B). In the absence of DFP treatment, a prothrombin (1 μM) and IgG (750 μg/mL) mixture was applied to the column at 4°C, and each fraction was separately incubated with 5 μM antithrombin III at 37°C for 3 hours. The generation of TAT′ complex was analyzed by ELISA (C).
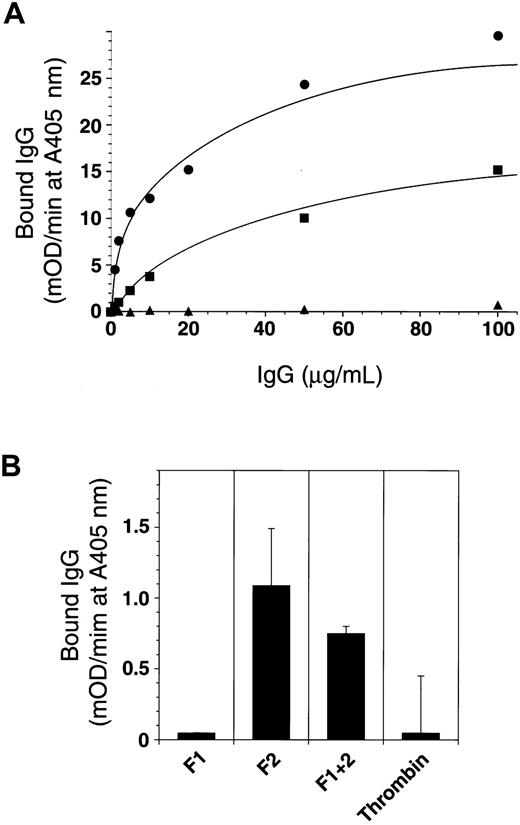
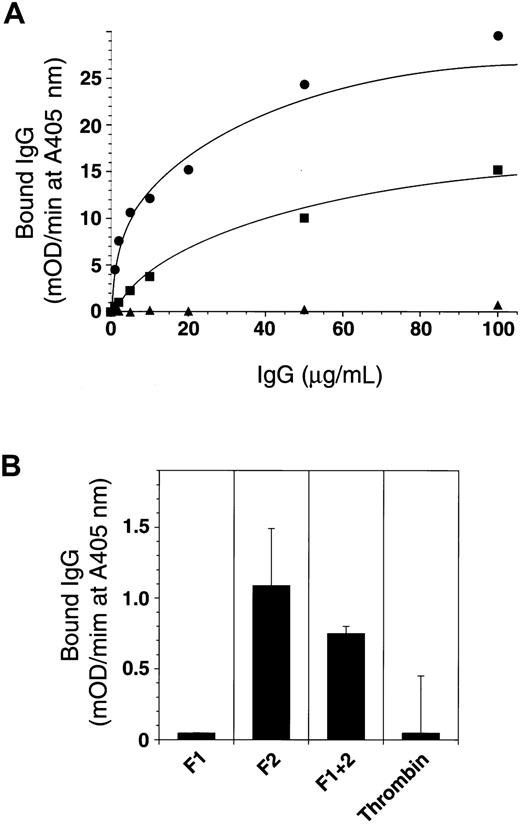
The patient IgG showed specific and saturable binding to immobilized prothrombin, dissociating from prothrombin after its complex formation with antithrombin III (Figure 7A). This result and the observations shown in Figures 2 and 3 suggest that the effect of the prothrombin autoantibody is catalytic. Prothrombin fragment 2 was recognized by the antibody (Figure 7B), but this recognition was far poorer than that of prothrombin. Interestingly, meizothrombin bound to the patient IgG more efficiently than prothrombin did (Figure 7A). This may imply that another conformation-dependent epitope exists near the catalytic domain of prothrombin because the antibody did not bind to the prothrombin complexed with antithrombin III.
Epitope analysis of anti-prothrombin antibody.
(A) A 96-well microtiter plate was coated with 5 μg/mL prothrombin (▪), meizothrombin (●), and the prothrombin–antithrombin III complex (▴) at 4°C for 18 hours. Then the plate was blocked and washed, and increasing concentrations of patient IgG (0-120 μg/mL) were added. Bound IgG was detected using antihuman IgG polyclonal antibody conjugated with horseradish peroxidase (HRPO) and HRPO substrate with absorbance read on a model 3550 Microplate Reader (Bio-Rad). (B) Plates were also coated with prothrombin derivatives and recombinant deletion mutants (fragment 1, F1; fragment 2, F2; fragment 1 + 2, F1 + 2; and α-thrombin), and the ability of the patient IgG to bind was determined in a similar fashion.
Epitope analysis of anti-prothrombin antibody.
(A) A 96-well microtiter plate was coated with 5 μg/mL prothrombin (▪), meizothrombin (●), and the prothrombin–antithrombin III complex (▴) at 4°C for 18 hours. Then the plate was blocked and washed, and increasing concentrations of patient IgG (0-120 μg/mL) were added. Bound IgG was detected using antihuman IgG polyclonal antibody conjugated with horseradish peroxidase (HRPO) and HRPO substrate with absorbance read on a model 3550 Microplate Reader (Bio-Rad). (B) Plates were also coated with prothrombin derivatives and recombinant deletion mutants (fragment 1, F1; fragment 2, F2; fragment 1 + 2, F1 + 2; and α-thrombin), and the ability of the patient IgG to bind was determined in a similar fashion.
Kinetic analysis of antibody-bound prothrombin
Because antibody-bound prothrombin acquires serine protease activity and hydrolyzes an arginine-serine peptide bond in antithrombin III, we evaluated its hydrolytic activity toward various synthetic substrates. Antibody-bound prothrombin was unable to hydrolyze the fluorescent peptide Bz-Arg-methylcoumarylamide (trypsin substrate), Z-Pyr-Gly-Arg-MCA (factor Xa), or Pyr-Gly-Arg-MCA (urokinase and tissue-type plasminogen activator), but it could cleave Boc-Val-Pro-Arg-MCA (substrate for α-thrombin). This hydrolytic activity was completely inhibited in the presence of a molar excess of antithrombin III. α-Thrombin hydrolyzed the substrate with Km = 13.5 ± 2.7 μM and kcat = 26.5 ± 1.5 s−1, which is consistent with previous results.19 Incubation of IgG-prothrombin with increasing concentrations of the Boc-Val-Pro-Arg-MCA peptide led to saturation of the hydrolytic activity toward the substrate. The curves of the reciprocal of the velocity plotted as a function of the reciprocal of the substrate concentration were linear (r = 0.99), indicating that the reaction conformed to simple Michaelis-Menten kinetics (data not shown). The calculated average apparent Km and kcat for the reaction were 42.5 ± 10.1 μM and 0.132 ± 0.065 s−1, respectively. This apparent kcat value was obtained based on the total prothrombin concentration. Because less than 1% of total IgG seemed to be concerned with prothrombin activation as shown (Figure 6), the apparent kcat obtained was likely to be a significant underestimate of the true kcat. However, the IgG-bound prothrombin exhibited quite a different macromolecular specificity toward physiological substrates such as fibrinogen and protein C.
Acquired coagulation factor inhibitors include pathologic immunoglobulins that specifically bind to coagulation factors and either neutralize their procoagulant activity or accelerate their clearance from the circulation.2,3,12 These inhibitors may develop as alloantibodies in patients who have congenital deficiencies and undergo replacement therapy, or as autoantibodies in patients with no known previous factor deficiency.20,21 The prothrombin autoantibody described here is distinct from staphylocoagulase because it causes fibrinogen-fibrin conversion22 and is unable to be neutralized by any in vivo inhibitors.23 Thus, to our knowledge, this is the first description of an acquired inhibitor that induces protease activity from the human zymogen protein, which is then neutralized by its own physiological inhibitor. We found a large amount of TAT′ complex and a decreased level of prothrombin and antithrombin III without any consumption of fibrinogen in patient plasma. It is, however, almost impossible to estimate how much this antibody contributes to the low level of prothrombin antigen in the patient plasma because the IgG-bound prothrombin should be rapidly catabolized from reticuloendothelial systems in vivo. From a structural standpoint, characterization of this antibody-zymogen complex could provide insight into the molecular transition of the active site pocket from the inactive zymogen form to the active enzyme for which there is little data even from crystallographic studies.
We thank Dr Toshiyuki Miyata (National Cardiovascular Center, Osaka, Japan) for his useful comments on prothrombin mutants.
Supported in part by a Grant-in-Aid from the Ministry of Health and Welfare of Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Yoichi Sakata, Division of Cell and Molecular Medicine, Center for Molecular Medicine, Jichi Medical School, Minamikawachi-machi, Tochigi 329-0498, Japan; e-mail:yoisaka@jichi.ac.jp.