Abstract
Paclitaxel and carboplatin chemotherapy is reported to be a platelet-sparing drug combination. This study investigated potential mechanisms for this observation by studying the effects of paclitaxel and carboplatin on (1) normal donor and chemotherapy patient-derived erythroid (burst-forming units-erythroid [BFU-E]), myeloid (colony-forming units-granulocyte/macrophage [CFU-GM]), and megakaryocyte (CFU-Meg) progenitor cell growth; (2) P-glycoprotein (P-gp) protein and glutathione S-transferase (GST) messenger RNA (mRNA) expression; (3) serum thrombopoietin (Tpo), stem cell factor (SCF), interleukin-6 (IL-6), IL-11, IL-1β, IL-8, and tumor necrosis factor-α levels in patients treated with paclitaxel and carboplatin; and (4) stromal cell production of Tpo and SCF after paclitaxel and carboplatin exposure. CFU-Meg were more resistant to paclitaxel alone, or in combination with carboplatin, than CFU-GM and BFU-E. Although all progenitors expressed P-gp protein and GST mRNA, verapamil treatment significantly, and selectively, increased the toxicity of paclitaxel and carboplatin to CFU-Meg, suggesting an important role for P-gp in megakaryocyte drug resistance. Compared to normal controls, serum Tpo levels in patients receiving paclitaxel and carboplatin were significantly elevated 5 hours after infusion and remained elevated at day 7 (287% ± 63% increase,P < .001). Marrow stroma was shown to be the likely source of this Tpo. It is concluded here that P-gp–mediated efflux of paclitaxel, and perhaps GST-mediated detoxification of carboplatin, results in relative sparing of CFU-Meg, which may then respond to locally high levels of stromal cell–derived Tpo. The confluence of these events might lead to the platelet-sparing phenomenon observed in patients treated with paclitaxel and carboplatin chemotherapy.
Introduction
Paclitaxel is a novel cancer chemotherapeutic agent that inhibits cell division by targeting the microtuble apparatus.1-4 The drug inhibits mitosis, primarily by its ability to promote the assembly of microtubules from tubulin dimers, and to stabilize these structures by preventing their depolymerization. Carboplatin is a cisplatin analogue that inhibits cell growth in a non–cell cycle-dependent manner by forming interstrand DNA cross-links.2,5 Both drugs suppress the bone marrow in a dose-dependent manner, and marrow toxicity is the major dose-limiting side effect of each. Curiously, although patients receiving combination paclitaxel and carboplatin chemotherapy develop profound, but not unexpected, granulocytopenia, significant thrombocytopenia appears to be uncommon.6-8 The reason for this is unclear, but several possible explanations might be invoked. These include the activity of P-glycoprotein (P-gp), a multidrug resistance-1 (mdr-1) gene–encoded membrane-bound transport protein that plays an important role in resistance to paclitaxel,1-4and intracellular levels of glutathione- or sulfhydryl-conjugating enzymes (GSTM1 and GSTT1)9,10 that are important in detoxifying carboplatin.2 5
To more clearly delineate the mechanisms responsible for the relative platelet-sparing effect of paclitaxel and carboplatin chemotherapy, we focused our attention on the drugs' effects on human hematopoietic progenitor cells and cells of the marrow microenvironment. In these populations, we specifically sought to determine the effects of paclitaxel and carboplatin on (1) in vitro cell growth, (2) expression of P-gp and GST, and (3) production of hematopoietic cytokines, including thrombopoietin (Tpo). Our results suggest that P-gp–mediated efflux of paclitaxel, possibly combined with GST-mediated detoxification of carboplatin, results in relative sparing of megakaryocyte colony-forming units (CFU-Meg) when compared to cells of other lineages. In combination, the drugs also appear to induce Tpo synthesis by marrow stromal cells (MSCs). The physiologic consequence of these findings could well be enhanced growth of surviving megakaryocyte progenitor cells leading to continued platelet production, in the face of otherwise profound suppression of myelopoiesis.
Patients, materials, and methods
Normal marrow cells
Light-density marrow mononuclear cells (MNC) were obtained from consenting healthy donors. Adherent cells were depleted (A−) by overnight culture on plastic dishes. CD34+ cells were isolated from the A− MNC by immunoaffinity selection using anti-CD34 murine monoclonal antibody (anti-HPC1; Becton Dickinson, Mountain View, CA) and magnetic beads coated with goat-antimouse antibodies according to the manufacturer's protocol (Dynal, Oslo, Norway) as described previously.11-13
Patient-derived cells
Colony-forming unit (CFU) assays were performed on CD34+ cells derived from peripheral blood (PB) of patients with solid tumors undergoing treatment with paclitaxel and carboplatin chemotherapy. Paclitaxel was delivered intravenously over 3 hours (175-225 mg/m2) followed by carboplatin (dosed by the Calvert formula to yield an AUC = 6). PB was obtained from consenting patients before therapy, and then at 5 hours and 7 days after infusion. CD34+ cells were isolated from blood samples by using immunomagnetic beads, and CFU assays were done as described below. After 11 days of culture, colonies were scored by use of an inverted microscope.
Human CFU-Meg assay
The CD34+ MNC (2 × 104) were suspended in 0.4 mL Dulbecco modified Eagle medium (DMEM) and mixed with 0.6 mL heat-inactivated horse serum, 0.2 mL fresh frozen bovine plasma, and 1 mL Iscove modified Dulbecco medium (IMDM) supplemented with 2% bovine serum albumin (BSA), CaCl2, α-thioglycerol, and α-asparagine. CFU-Meg colony growth was suboptimally stimulated with 25 ng/mL Tpo and 10 ng/mL interleukin-3 (IL-3). Cells were plated into 235-mm tissue culture dishes (Corning, Corning, NY). After 11 days of incubation at 37°C in a fully humidified CO2 incubator, colonies were fixed, stained with an anti-IIb/IIIa monoclonal antibody conjugated with fluorescein isothiocyanate (FITC; Immunotech, Marseille, France) and enumerated by immunofluorescence microscopy as described.11-13
Human CFU-granulocyte/macrophage and burst-forming units-erythroidassay
The culture methods used have been previously described.11-14 In brief, 2 × 104CD34+ MNC were cloned in methylcellulose medium in the presence of 5 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF) and 10 ng/mL IL-3 to stimulate the growth of CFU-GM, or in 5 U/mL erythropoietin (Epo) and 5 ng/mL stem cell factor (SCF) to stimulate the growth of burst-forming units-erythroid (BFU-E). Human CFU-GM and BFU-E colonies were scored with an inverted microscope at day 14.
Drug exposure protocol and CFU assays
The CD34+ cells were exposed to paclitaxel (0.1-10.0 μM) and carboplatin (0.1-10.0 μg/mL) alone or in combination for 24 hours. Thereafter, cells were washed in IMDM and assayed for CFU-Meg, CFU-GM, and BFU-E as described above.
Verapamil exposure protocol
The CD34+ cells were incubated for 24 hours in the presence of paclitaxel (0.1 μM), carboplatin (1 μg/mL), and the P-gp inhibitor verapamil (50 μg/mL) (Sigma Chemical, St Louis, MO). The cells were then washed extensively, resuspended in fresh medium, and then cultured as described above to determine effects of CFU-Meg, CFU-GM, and BFU-E on growth.
Expansion of human CD34+ cells
The CD34+ MNC (2 × 104) were cultured in 24-well plates (Corning) in IMDM supplemented with 25% serum substitute containing 1% delipidated, deionized, and charcoal-treated BSA, 270 μg/mL iron-saturated transferrin, insulin (20 μg/mL), and 2 mM glutamine (all from Sigma Chemical). Cell proliferation was either stimulated with the combinations of 5 U/mL Epo plus 5 ng/mL SCF, 25 ng/mL Tpo plus 10 ng/mL IL-3, or 5 ng/mL GM-CSF plus 10 ng/mL IL-3 to drive the expanding cell population along the erythroid, megakaryocytic, or granulocytic/monocytic pathways, respectively.
FACS analysis
Cells (5 × 105) collected after expansion for 11 days were washed twice in phosphate-buffered saline (PBS; free of CaCl2 and MgCl2) supplemented with 0.2% BSA and then labeled for 15 minutes in the dark on a rotating rack with 20 μL murine anti–P-gp monoclonal antibody directly conjugated with phycoerythrin (PE) and directed against an extracellular epitope of the human P-gp (UIC2; Immunotech). Subsequently, cells were washed once in 3 mL PBS containing 0.2% BSA, resuspended in 1 mL PBS plus 0.2% BSA, and immediately subjected to analysis using a FACS Star Plus II (Becton Dickinson).
MSC cultures
The MSCs were obtained by culturing MNC in DMEM supplemented with 12.5% bovine calf serum, 12.5% equine serum, 1%l-glutamine, penicillin, and streptomycin as described.14 After overnight incubation, nonadherent cells were removed and fresh culture medium was added. Once per week, 50% of the culture medium was exchanged. Finally, the confluent cells were trypsinized with 3 mL trypsin-EDTA (GIBCO, Grand Island, NY) for 5 minutes and transferred to 24-well plates (Corning).
On occasion, confluent MSC cultures were exposed to 1.0 μM paclitaxel (Taxol, Bristol-Myers Squibb, Princeton, NJ) and 0.1 μg/mL carboplatin (Paraplatin, Bristol-Myers Squibb). Cells in control wells were maintained in medium alone. After 6, 24, and 48 hours, medium was withdrawn for enzyme-linked immunosorbent assays (ELISA).
ELISA analysis of Tpo and SCF secretion by MSCs
Secretion of Tpo and SCF by normal human bone marrow stroma fibroblasts was evaluated by the Quantikine human Tpo and SCF immunoassays (R&D Systems, Minneapolis, MN) according to the manufacturer's protocol. Normal human bone marrow fibroblasts were grown to confluence and then exposed to paclitaxel and carboplatin for 6, 24, or 48 hours. Control cultures were not exposed to the drugs. Subsequently, the collected media were analyzed by a quantitative sandwich enzyme immunoassay technique for a presence of Tpo and SCF. The sensitivity of ELISA was more than 5 pg/mL and more than 9 pg/mL for Tpo and SCF, respectively.
Quantification of growth factor levels
Cytokine levels in patient sera were measured by electrochemiluminescence using an Origen Analyzer and Orios software (Igen International, Gaithersburg, MD). This method uses biotinylated capture and ruthenylated-tagged antibodies and detects cytokine concentrations of 10 pg/mL or less. All experiments were performed according to the manufacturer's protocol. The quantification of Tpo and SCF in patient sera was performed by the Cytokine Reference Laboratory, University of Minnesota, using ELISA kits from R&D Systems.
RNA isolation and reverse transcriptase–polymerase chain reaction
CFU-Meg–, CFU-GM–, and BFU-E–derived cells were assayed for GSTM1 and GSTT1 messenger RNA (mRNA) expression by reverse transcriptase–polymerase chain reaction (RT-PCR). Briefly, cells were lysed in 200 μL RNAzol (Biotecx Labs, Houston, TX) plus 22 μL chloroform as described.11-13 The aqueous phase was collected and mixed with 1 volume of isopropanol (Sigma Chemical). RNA was precipitated overnight at −20°C. The RNA pellet was washed in 75% ethanol and resuspended in 3 × autoclaved H2O. RNA (0.5 μg) was reverse-transcribed with 500 U Moloney murine leukemia virus reverse transcriptase (MoMLV-RT) and 50 pmol of an ODN primer complementary to the 3′ end of GSTM1 (5′-GTC AAG GAC ATC ATA GAC GAG-3′) or GSTT1 (5′-GCG CTG TTT ACA TCT TTG CCA-3′).15,16 The resulting complementary DNA (cDNA) fragments were amplified using 5 U Thermus aquaticus (Taq) polymerase and primers specific for the 5′ end of GSTM1 (5′-CAT GAT ACT GGG GTA STG GGA-3′) or GSTT1 (5′-GTC AAG GAC ATC ATA GAC GAG-3′). The β-actin mRNAs were amplified simultaneously using specific primers as reported previously.13 Amplified products (10 μL) were electrophoresed on a 2% agarose gel and transferred to a nylon filter and further documented photographically. Specificity of the amplified products was subsequently confirmed by Southern blotting (data not shown).
Statisticalanalysis
Arithmetic means and SDs were calculated on a Macintosh computer using Instat 1.14 (GraphPad, San Diego, CA) software. Data were analyzed using the Student t test for unpaired samples. Statistical significance was defined as P < .0.5.
Results
Effect of paclitaxel and carboplatin on growth of PB CFUs
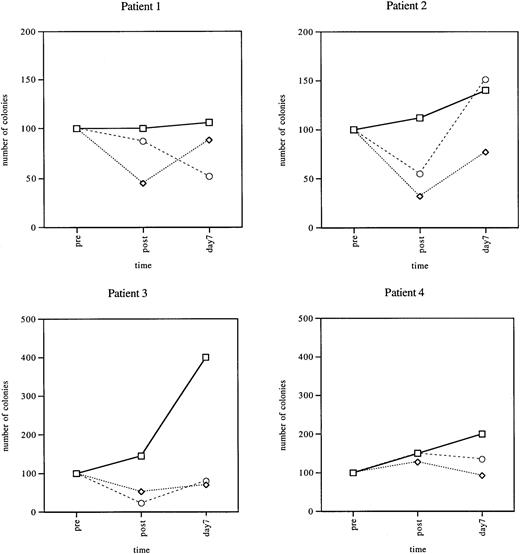
To determine potential mechanisms of platelet sparing by paclitaxel and carboplatin chemotherapy, we initially examined the effect of these drugs on CFU cloning efficiency in 4 patients receiving these drugs. To carry out the studies, PB was obtained just prior to infusion of these agents, 5 hours after infusion, and then at 7 days after infusion. Assays for CFU-GM, BFU-E, and CFU-Meg were carried out on CD34+ cells isolated from the samples as described in “Patients, materials, and methods.” Results were compared to the patient's preinfusion CFU cloning efficiency, which was arbitrarily defined as 100% growth (Figure 1). Colony formation varied with the individual being studied, but some trends were indentified. In all 4 patients, CFU-Meg colonies showed either no decline or increased (from about 10%-50%) in the specimens obtained 5 hours after infusion. In contrast, CFU-GM and BFU-E colony formation declined from about 10% to 80% and approximately 45% to 60% in 3 of 4 samples, respectively (patients 1, 2, and 3). On day 7, CFU-GM and BFU-E cloning efficiency returned to about baseline values in 3 of the 4 patients (patients 2, 3, and 4), whereas CFU-Megs were still elevated in all (10%-400%) when compared to their own controls.
Peripheral blood hematopoietic progenitor cells in patients treated with paclitaxel and carboplatin.
CFU assays were carried out as described before (pre), 5 hours after infusion (post), and on day +7 after treatment. CFU assays were set up. Pretreatment numbers were arbitrarily assigned a value of 100%. ■, CFU-Meg; ⋄, BFU-E; ○, CFU-GM. Data from 4 different patients are shown.
Peripheral blood hematopoietic progenitor cells in patients treated with paclitaxel and carboplatin.
CFU assays were carried out as described before (pre), 5 hours after infusion (post), and on day +7 after treatment. CFU assays were set up. Pretreatment numbers were arbitrarily assigned a value of 100%. ■, CFU-Meg; ⋄, BFU-E; ○, CFU-GM. Data from 4 different patients are shown.
Sensitivity of CFU-Meg, BFU-E, and CFU-GM to paclitaxel and carboplatin
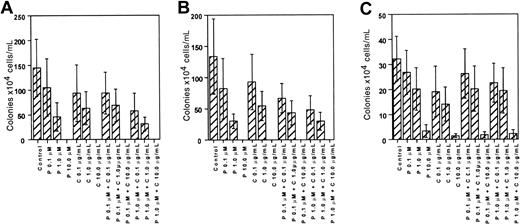
The patient studies suggested that paclitaxel and carboplatin might be less toxic to CFU-Meg than the CFU of other lineages. We sought to substantiate this impression by studying a larger numbers of individuals. For this aspect of the investigation, normal marrow-derived CD34+ cells were exposed to increasing doses of paclitaxel and carboplatin, either alone or in combination (Figure 2). Growth of BFU-E (Figure 2A) and CFU-GM (Figure 2B) was inhibited in a dose-dependent and statistically significant manner (P < .001 at each concentration in comparison to control cell growth) when exposed to either drug alone or both drugs in combination. CFU-Meg growth was also inhibited when cells were exposed to the individual agents; however, the megakaryocyte progenitors appeared to be somewhat more resistant than the BFU-E and CFU-GM (Figure 2C). For example, when cells were exposed to 1 μM paclitaxel alone, erythroid colony growth declined about 66% (Figure 2A) and CFU-GM colony formation declined about 75% (Figure 2B) in comparison to their respective controls (P < .001). In contrast, CFU-Meg decreased only about 33% in comparison to control cell growth (P > .001). At a 10 × higher paclitaxel or carboplatin exposure, erythroid and myeloid colony formation was totally suppressed, whereas some residual megakaryocytic colonies could still be found. This difference was more pronounced when cells were exposed to a fixed dose of paclitaxel (0.1 μM or 1 μM) and varying doses of carboplatin (Figure 2C). For example, at paclitaxel and carboplatin doses of 1.0 μM and 1μg/mL, respectively, CFU-Meg colonies declined about 33% (P > .001 compared to control) whereas CFU-GM and BFU-E colonies decreased about 75% (P < .001). At the highest doses, no residual erythroid or granulocytic colony formation was observed, whereas megakaryocytes, though greatly diminished, still persisted. Accordingly, these data demonstrate that at therapeutically relevant drug concentrations megakaryocytic progenitor cells are relatively more resistant to the effects of these agents than the progenitor cells of the other myeloid lineages.
Effect of paclitaxel and carboplatin on normal hematopoietic progenitor cell growth in vitro.
Marrow CD34+ cells were exposed to paclitaxel (P) and carboplatin (C) alone or in combination and then cultured in the appropriate cytokines to yield BFU-E (A), CFU-GM (B), or CFU-Meg (C) colonies. Data derived from growth of 4 to 5 different donors, each cultured in quadruplicate, are shown.
Effect of paclitaxel and carboplatin on normal hematopoietic progenitor cell growth in vitro.
Marrow CD34+ cells were exposed to paclitaxel (P) and carboplatin (C) alone or in combination and then cultured in the appropriate cytokines to yield BFU-E (A), CFU-GM (B), or CFU-Meg (C) colonies. Data derived from growth of 4 to 5 different donors, each cultured in quadruplicate, are shown.
Flow cytometric analysis of P-gp expression on hematopoietic cells
Resistance to paclitaxel has been shown to be at least partially dependent on the function of an active membrane transporter, P-gp. This transporter, the product of the multidrug resistance (mdr-1) gene, exports toxic taxanes from the cell's interior. With this relationship in mind, we hypothesized that the relative resistance of CFU-Meg to paclitaxel might be explained by the differences in P-gp protein expression, or the activity of this transporter, in cells of the megakaryocyte lineage.
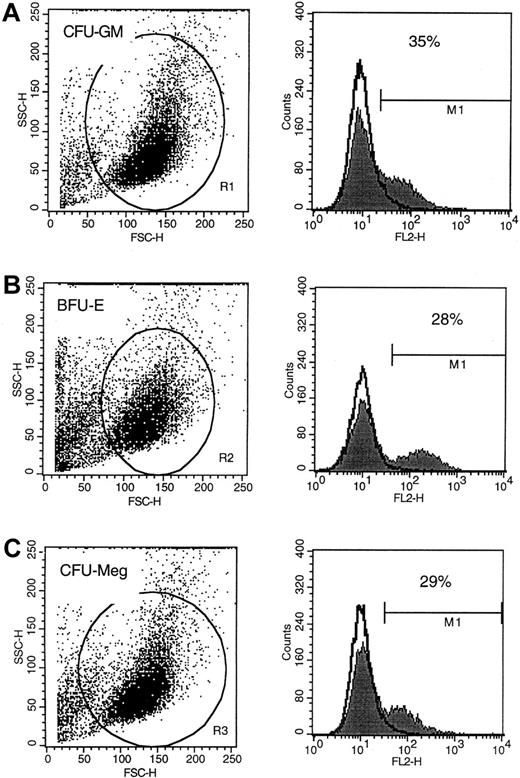
To examine expression of P-gp on cells of various lineages, MNC were expanded ex vivo under serum-free conditions and subsequently subjected to FACS analysis. Using this approach, we found that P-gp was expressed on the surface of about 30% of human erythroid (glycophorin A+), granulomonocytic (CD33+), and megakaryocytic (CD41+) cells (Figure3). The level of expression did not appear to be significantly different between cells of these lineages either (Figure 3A-C). Of interest, the protein was not detectable on the surface of PB platelets or erythrocytes (data not shown). Accordingly, it did not appear that quantitative differences in P-gp expression was a likely explanation for the differential sensitivity that we observed in vitro or in vivo.
P-gp expression on human CFU-GM–, BFU-E–, and CFU-Meg–derived cells.
Flow cytometric analysis of P-gp protein expression on human CFU-GM–derived (A), BFU-E–derived (B), and CFU-Meg–derived (C) cells. Analysis was repeated 3 times with similar results. Representative FACS analysis is shown.
P-gp expression on human CFU-GM–, BFU-E–, and CFU-Meg–derived cells.
Flow cytometric analysis of P-gp protein expression on human CFU-GM–derived (A), BFU-E–derived (B), and CFU-Meg–derived (C) cells. Analysis was repeated 3 times with similar results. Representative FACS analysis is shown.
Influence of verapamil on paclitaxel and carboplatin toxicity
Although absolute levels of P-gp expression on cells of the various hematopoietic lineages did not appear to differ significantly, the possibility still existed that the P-gp function played an important role in CFU-Meg resistance to this compound. We addressed this possibility by blocking the transporter's activity in CD34+ cells with verapamil, an inhibitor of P-gp.17
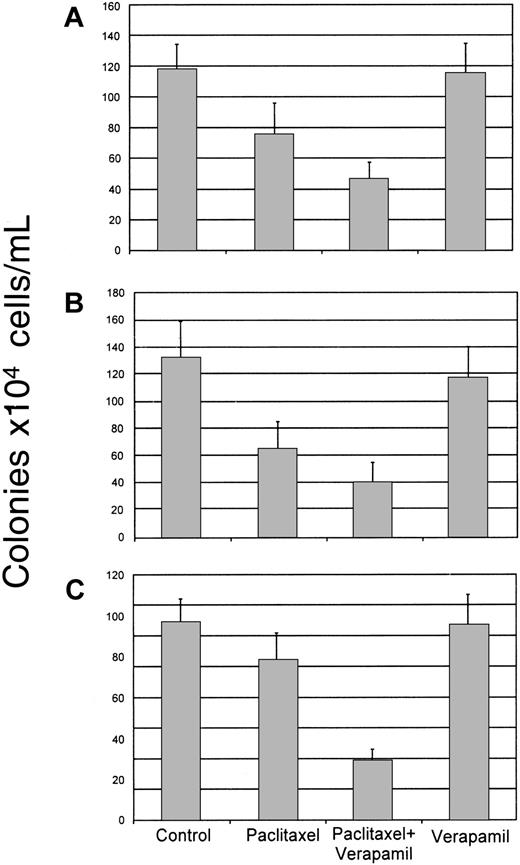
Human CD34+ cells were isolated from the bone marrow and exposed for 24 hours to paclitaxel (0.1 μM) in the absence or presence of verapamil (50 μg/mL). Cells were then washed and cultured in methylcellulose containing growth factors necessary to stimulate growth of the lineage of interest. As noted above, paclitaxel alone significantly inhibited BFU-E and CFU-GM colony formation (P < .0001) when compared to untreated control cultures (Figure 4A,B). CFU-Meg colony growth was again only slightly diminished (Figure 4C). In the presence of verapamil, toxicity to all the lineages was increased but was most marked in the cultures formulated to grow megakaryocyte progenitors where growth inhibition increased from about 20% to 70% of the untreated control (P < .0001) (Figure 4C). Verapamil alone was nontoxic to the hematopoietic progenitors evaluated in the cultures (Figure 4).
Effect of verapamil on paclitaxel toxicity to marrow progenitor cells.
Normal human CD34+ marrow cells were exposed to paclitaxel (0.1 μM) alone or after pretreatment with verapamil (50 μg/mL). Cells were then cultured in methylcellulose in the presence of cytokines designed to stimulate the growth of CFU-GM (A), BFU-E (B), or CFU-Meg (C) colonies. Growth of untreated (control) cells, as well as cells exposed to verapamil alone, is shown for comparison purposes. Data from 4 different donors performed in quadruplicate are shown.
Effect of verapamil on paclitaxel toxicity to marrow progenitor cells.
Normal human CD34+ marrow cells were exposed to paclitaxel (0.1 μM) alone or after pretreatment with verapamil (50 μg/mL). Cells were then cultured in methylcellulose in the presence of cytokines designed to stimulate the growth of CFU-GM (A), BFU-E (B), or CFU-Meg (C) colonies. Growth of untreated (control) cells, as well as cells exposed to verapamil alone, is shown for comparison purposes. Data from 4 different donors performed in quadruplicate are shown.
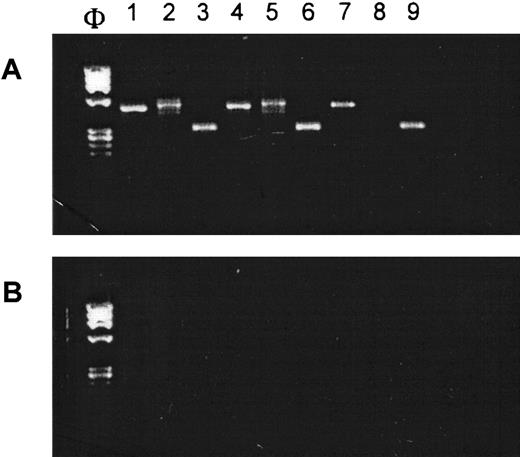
Glutathione transferase enzyme mRNA expression inhematopoietic cells
Resistance to platinum-based agents depends in part on the intracellular levels of glutathione- or sulfhydryl-conjugating enzymes such as GSTT1 or GSTM1.2,5 GSTT1 is known to be expressed in a wide range of tissues, including erythrocytes, but it is apparently not detectable in lymphocytes.9 Because we did not have a protein or functional assay for these enzymes in our laboratory, we used RT-PCR to determine if normal human hematopoietic cells expressed the relevant mRNAs (Figure5). This seemed an acceptable surrogate for protein levels because it has been reported that mRNA and protein distribution of these enzymes are identical.10Accordingly, CD34+ MNC were expanded ex vivo along the megakaryocytic, myelocytic, and erythroid pathways using the appropriate cytokine cocktails (see “Patients, materials, and methods”). RT-PCR carried out on total cellular RNA revealed that GSTM1 and GSTT1 mRNAs were expressed in CFU-Meg– and CFU-GM–derived cells. Interestingly, BFU-E expressed the mRNA for GSTM1 only. These results are consistent with the dose-dependent, lineage indifferent, carboplatin sensitivity patterns noted in Figure 2, and the marked pancytopenia that is often observed in patients receiving carboplatin as a single agent.2 6-8
GST mRNAs are expressed in human CFU-GM–, BFU-E–, and CFU-Meg–derived cells.
(A) RT-PCR analysis on mRNA expression for GSTM1 (lanes 1, 4, and 7), GSTT1 (lanes 2, 5, and 8) and β-actin (lanes 3, 6, and 9) in human CFU-GM–derived (lanes 1-3), CFU-Meg–derived (lanes 4-6), and BFU-E–derived (lanes 7-9) cells. (B) Negative RT-PCR control reactions (H20 in place of mRNA). Experiment was repeated 3 times with similar results.
GST mRNAs are expressed in human CFU-GM–, BFU-E–, and CFU-Meg–derived cells.
(A) RT-PCR analysis on mRNA expression for GSTM1 (lanes 1, 4, and 7), GSTT1 (lanes 2, 5, and 8) and β-actin (lanes 3, 6, and 9) in human CFU-GM–derived (lanes 1-3), CFU-Meg–derived (lanes 4-6), and BFU-E–derived (lanes 7-9) cells. (B) Negative RT-PCR control reactions (H20 in place of mRNA). Experiment was repeated 3 times with similar results.
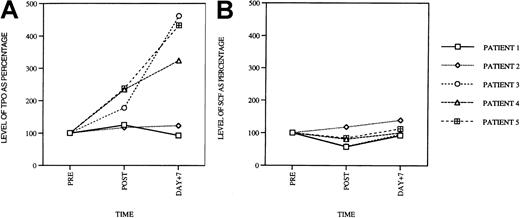
Quantification of cytokine levels in patient sera
Having explored toxicity profiles of paclitaxel and carboplatin on hematopoietic progenitor cells, we turned our attention to the drugs' effects on cytokine production in patients receiving these agents as combination chemotherapy. We were particularly interested in levels of cytokines that could stimulate megakaryocytopoiesis such as Tpo, IL-6, IL-11, and SCF. We were also interested in the levels of inhibitors of megakaryocytopoiesis (IL-1β, IL-8, and tumor necrosis factor-α [TNF-α]) because diminished inhibitor levels might also lead to indirect stimulation of megakaryocyte and platelet production. Therefore, levels of all these cytokines were measured in the patient's serum before drug infusion, 5 hours after infusion, as well as 7 days after treatment.
In 3 of 5 patients, serum Tpo levels were elevated compared to pretreatment control when measured 5 hours after infusion of paclitaxel and carboplatin (Figure 6A). The mean increase was 104% ± 27% and was of statistical significance in comparison to baseline levels (P < .05). On day 7, levels of Tpo were elevated to an even greater degree and were 287% ± 63% in comparison to pretreatment values (P < .0001). Levels of SCF (Figure 6B) and IL-1β, IL-6, IL-8, and TNF-α (data not shown) remained the same as baseline values in all of the patients. These results suggest that combined paclitaxel/carboplatin administration, at least at the doses used in these patients, leads to a dramatic increase in Tpo serum levels.
Serum Tpo levels in patients treated with paclitaxel and carboplatin.
(A) Peripheral blood Tpo levels in patients prior to treatment, 5 hours after infusion (post), and on day +7 after treatment. The serum levels are shown as percentage of the pre-values (PRE = 100%). Serum SCF levels at the same time points, for the same patients, are shown in panel B.
Serum Tpo levels in patients treated with paclitaxel and carboplatin.
(A) Peripheral blood Tpo levels in patients prior to treatment, 5 hours after infusion (post), and on day +7 after treatment. The serum levels are shown as percentage of the pre-values (PRE = 100%). Serum SCF levels at the same time points, for the same patients, are shown in panel B.
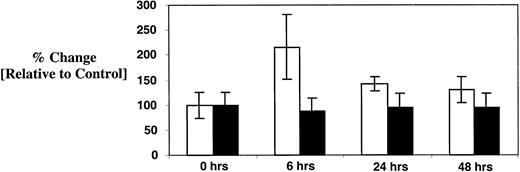
Growth factor production by MSCs exposed to paclitaxel and carboplatin
Because Tpo secretion by the liver and kidney is thought to be constitutive,18 the source of the elevated serum Tpo in patients treated with paclitaxel and carboplatin was uncertain. Possible explanations included an alternative source of synthesis or a drug-induced change in Tpo clearance. Because stromal cell synthesis of Tpo is reportedly inducible,19 20 we investigated this possibility directly. Marrow stromal fibroblasts were grown to confluence in DMEM supplemented with artificial serum. Cells were then exposed to paclitaxel (1.0 μM) and carboplatin (0.1 μg/mL) for 6, 24, and 48 hours. Culture supernatants were harvested at these time points and assayed for Tpo and SCF content using a sensitive ELISA (Figure 7). Tpo concentration in confluent control cultures was determined to be 23 ± 6 pg/mL. At 6 hours, the concentration of Tpo increased by 110% ± 58% in comparison to unexposed cells (50 ± 15 pg/mL;P < .001). Levels returned toward baseline at 24 (33 ± 3 pg/mL) and 48 hours (30 ± 6 pg/mL) but were still slightly elevated in comparison to the untreated control. Of note, SCF levels were unchanged throughout the entire observation period (about 450 pg/mL). Accordingly, the elevated Tpo levels observed in some of the patients might be explained by induction of stromal cell synthesis though concurrent effects on Tpo clearance cannot be excluded.
Effect of paclitaxel and carboplatin on stromal cell elaboration of Tpo and SCF.
Confluent cultures of normal human marrow fibroblasts were exposed to paclitaxel and carboplatin as described in “Patients, materials, and methods” for 6, 24, and 48 hours. Culture supernatants were then harvested and assayed for Tpo (■) and SCF (▪) by ELISA. Results are compared to baseline, which was arbitrarily defined as 100% in the control cultures. Data from 3 independent experiments are pooled together, and plotted as mean ± SD.
Effect of paclitaxel and carboplatin on stromal cell elaboration of Tpo and SCF.
Confluent cultures of normal human marrow fibroblasts were exposed to paclitaxel and carboplatin as described in “Patients, materials, and methods” for 6, 24, and 48 hours. Culture supernatants were then harvested and assayed for Tpo (■) and SCF (▪) by ELISA. Results are compared to baseline, which was arbitrarily defined as 100% in the control cultures. Data from 3 independent experiments are pooled together, and plotted as mean ± SD.
Discussion
A number of reports suggest that combination chemotherapy with paclitaxel and carboplatin is relatively platelet sparing, especially when compared to toxicity to other myeloid elements of the marrow.6-8 Identifying potential biochemical/molecular explanations for this observation was the object of our studies. To focus our work, we formulated the simple hypothesis that megakaryocyte progenitor cells were more resistant to these drugs than the progenitor cells of other lineages. Although of some potential interest, we did not examine the effects of these drugs on mature megakaryocytes because it was very unlikely that such cells were involved in the platelet-sparing phenomenon under investigation. This follows from the fact that mature megakaryocytes live no more than 2 to 3 days in the marrow,21 and the drugs are largely gone from the circulation within this time period as well.7 Accordingly, it is only the progenitor cell compartment, from whence mature megakaryocytes are derived, that can account for the platelet-sparing effect that is observed in these patients over time.
Accordingly, we began by assaying the numbers of PB progenitor cells in patients with solid tumors receiving paclitaxel and carboplatin. In agreement with our primary hypothesis, we found that the numbers of CFU-Meg in the PB of patients receiving this drug combination were preserved, or even increased, in comparison to the progenitors of the other major hematopoietic lineages (CFU-GM and BFU-E) (Figure 1). This finding indicated that CFU-Meg were almost certainly more resistant to the toxic effects of these drugs. It was also possible, though unlikely, that we were merely detecting relatively increased numbers of CFU-Meg mobilized from the marrow by the effects of these drugs.
To directly examine the sensitivity of marrow-derived CFU-Meg, CFU-GM, and BFU-E to paclitaxel and carboplatin, alone and in combination, we cultured normal donor-derived marrow cell progenitors in vitro in the presence of increasing concentrations of each drug alone and then in combination (Figure 2). It is important to note that the doses used in these studies were of physiologic significance in that they represented drug concentrations that might be achieved in vivo in patients receiving these agents. We found, again in support of our initial hypothesis, that CFU-Meg were relatively more resistant to paclitaxel alone or in combination with carboplatin. This observation was in fact reminiscent of similar studies performed in our laboratory in which we demonstrated that normal human CFU-Meg were more resistant to 4-hydroxyperoxycyclophosphamide (4-HC) than CFU-GM and BFU-E.23 These data suggested then that common mechanisms might be responsible for all of these observations and prompted us to identify what these mechanisms might be.
Resistance to paclitaxel depends at least in part on the activity of the P-gp efflux pump, whereas resistance to carboplatin depends partly on increased activity of glutathione S-transferase enzymes. We therefore phenotyped human CFU-Meg–, CFU-GM–, and BFU-E–derived cells for expression of P-gp protein as well as for the GSTT1 and GSTM1 mRNAs. We found that P-gp is detectable on about 30% of human megakaryocyte progenitor cells, but at the same time, P-gp expression was not restricted to cells of the megakaryocyte lineage (Figure 3). Accordingly, differences in P-gp distribution alone could not explain the differential sensitivity we observed among the lineages. However, additional studies, which consisted of blocking the P-gp pump with verapamil, suggested that the P-gp pump was of particular importance for protecting CFU-Meg in that toxicity to cells of this lineage became identical to that observed in CFU-GM and BFU-E in the presence of the pump inhibitor (Figure 4). Finally, we also found that human CFU-Meg–derived cells express the mRNAs for GSTM1 and GSTT1, suggesting that these cells might also synthesize the proteins responsible for detoxifying carboplatin derivatives (Figure 5). A definitive conclusion on this point, however, awaits immunochemical and functional assays that would allow us to examine the amounts and activity of these enzymes in hematopoietic progenitor cells. Other resistance mechanisms, such as those governing drug binding to α- and β-tubulin proteins might also play a role in these phenomena.1-4
In addition to the potential megakaryocyte-sparing mechanisms elucidated above, we also found that serum Tpo levels were elevated in 3 of 5 patients treated with paclitaxel and carboplatin (Figure 6). Although the numbers of patients studied was small, the assay used is both sensitive and reproducible. Accordingly, these data suggested that megakaryocyte and platelet growth–promoting activities might be elaborated by cells exposed to these drugs. Because production of Tpo is constitutive in liver and kidney but inducible in MSCs,19,20,22 we examined the latter for evidence of such an effect. As shown in Figure 7, we indeed found that within 6 hours of exposure, Tpo can be found in serum-free medium conditioned by MSCs exposed to paclitaxel and carboplatin at treatment-relevant doses. Why stromal cell exposure to these drugs would induce the synthesis of Tpo is not certain, but it has been reported that paclitaxel induces AP-1 and NF-κB transcription factors.24 These proteins are postulated to play an important role in the expression of several cytokines and growth factors,24 and one could speculate that Tpo gene expression in stromal cells might also be among these. Studies beyond the scope of this report will be required to investigate this possibility.
In conclusion, we believe that P-gp–mediated efflux of paclitaxel, perhaps in association with GST-mediated detoxification of carboplatin, results in relative sparing of marrow CFU-Meg after exposure to these chemotherapeutic agents. In comparison to CFU-Meg, the P-gp efflux pump does not appear to be as active in CFU-GM and BFU-E leading to higher toxicity among cells of these lineages. Surviving CFU-Meg may in turn be stimulated to mature more rapidly by locally high levels of Tpo elaborated by stromal cells of the marrow microenvironment. In aggregate, these events might result in continued platelet production thereby giving rise to the sparing phenomenon observed in patients treated with this drug combination.
Acknowledgments
The editorial assistance of Elizabeth Bien and Susan Weber is gratefully acknowledged.
Supported in part by grants from Bristol-Myers Squibb Oncology and the Leukemia and Lymphoma Society of America. A.M.G. is a Distinguished Clinical Scientist of the Doris Duke Charitable Foundation.
E.P. and J.R. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Alan M. Gewirtz, Department of Medicine, Division Hematology/ Oncology, University of Pennsylvania, 713 BRB II/III, 421 Curie Blvd, Philadelphia, PA 19104; e-mail:gewirtz@mail.med.upenn.edu.