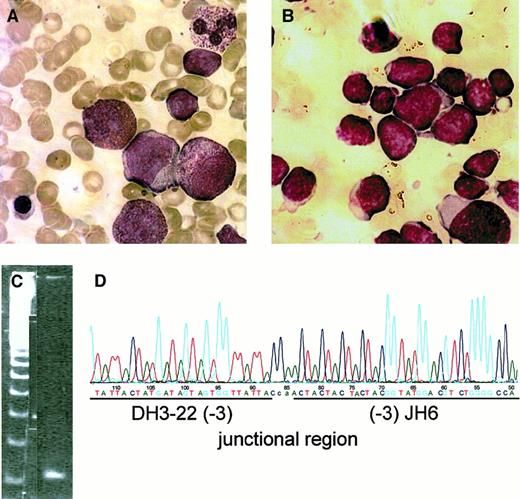
Severe congenital neutropenia (CN) is a group of hematopoietic disorders with variable recessive inheritance characterized by absolute neutropenia due to a maturation arrest of myeloid progenitor cells. Patients with CN carry a predisposition toward the development of myelodysplastic syndrome (MDS)/acute myeloid leukemia (AML) with an incidence of approximately 9%.1,2 Patients with acquired nonsense mutations in the granulocyte colony-stimulating factor (G-CSF) receptor gene leading to the truncation of the membrane-distal region of the receptor have a high risk of leukemic transformation.2,3 So far, none of the known patients with CN have developed a secondary acute lymphocytic leukemia (ALL). Here we report on a 14-year-old girl with CN who developed a secondary pre-B acute lymphoblastic leukemia (pre-B ALL). CN was diagnosed at the age of 4 months, and she was started on r-metHuG-CSF (10 μg/kg/d) at age 9, followed by a prompt increase in neutrophil counts. Yearly bone marrow investigations were consistent with CN (Figure1A). At age 13, she developed an ALL with more than 90% lymphoblasts in the bone marrow (Figure 1B). The immunophenotype of the blasts as judged from flow cytometry was in accordance with a pre-B ALL, coexpressing myeloid markers: CD19 (69%), CD20 (57%), CD45 (68%), CD34 (84%), HLA-DR (91%), CD13 (67%), CD24 (22%), CD33 (29%), CD10 (< 1%). Intracellular staining demonstrated TdT (67%), CD79a (20%), immunoglobulin M (μ chain)(20%), CD22 (10%), and MPO (< 2%). Immunostaining of bone marrow sections confirmed these results and also revealed negative staining for CD15, CD68, and lysozyme. Heteroduplex polymerase chain reaction (PCR) analysis of genomic DNA to identify clonal immunoglobulin and T-cell receptor gene rearrangements revealed an incomplete immunoglobulin heavy-chain recombination reflecting aDH-JH joining that did not involve a VHsegment (Figure 1C). Sequence analysis confirmed monoclonality of the target and allowed identification of a DH3.22-JH6 rearrangement (Figure 1D). Since immature DH-JHrecombinations are preferentially found in early precursor B-ALL malignancies, this result was consistent with the morphological and immunological findings.
Diagnosis of ALL secondary to CN.
Bone marrow smears at age 9 (A; severe congenital neutropenia before start of G-CSF treatment) and at age 13 (B; severe congenital neutropenia with secondary ALL). Heteroduplex PCR analysis (C) and characterization of rearranged immunoglobulin and T-cell receptor gene loci were performed as described earlier.5 Sequence analysis revealed a monoclonal rearrangement of the DH-JHtype (D). Figures in brackets indicate the number of flanking nucleotide deletions. Lowercase letters correspond to N-nucleotides.
Diagnosis of ALL secondary to CN.
Bone marrow smears at age 9 (A; severe congenital neutropenia before start of G-CSF treatment) and at age 13 (B; severe congenital neutropenia with secondary ALL). Heteroduplex PCR analysis (C) and characterization of rearranged immunoglobulin and T-cell receptor gene loci were performed as described earlier.5 Sequence analysis revealed a monoclonal rearrangement of the DH-JHtype (D). Figures in brackets indicate the number of flanking nucleotide deletions. Lowercase letters correspond to N-nucleotides.
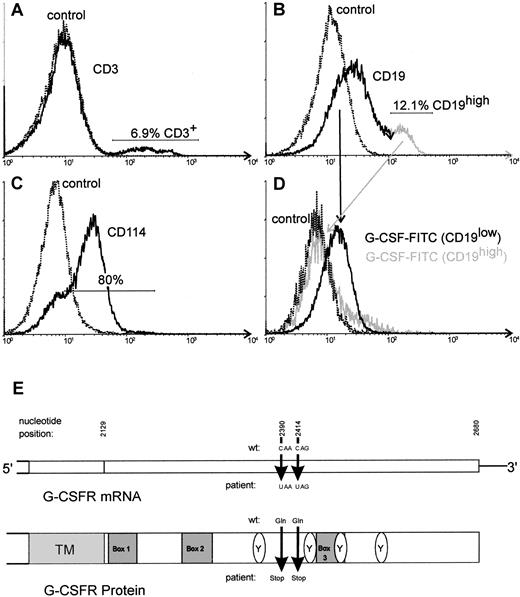
By flow cytometry, we could demonstrate a G-CSF receptor expression on the leukemic blasts (Figure2). Sequencing of G-CSF receptor mRNA of ALL lymphoblasts after subcloning of reverse transcriptase–polymerase chain reaction (RT-PCR) products revealed point mutations similar to those present in patients with CN/AML. In 8 of 15 clones (53%), we detected a mutation at position 2414 of G-CSF receptor mRNA (E726X). One clone (6%) demonstrated a stop mutation at position 2390 (E718X) (Figure 2E). In 6 clones (40%) the wild-type sequence of the G-CSF receptor mRNA was detected.
Analysis of G-CSF receptor in lymphoblasts of the patient.
FACS analysis of the lymphoblasts of the patient. The main population of analyzed cells demonstrated a low-intensity staining for CD19 (B). Minor populations corresponding to nonleukemic T or B cells consisted of CD3+ cells (A) and CD19high cells (B), respectively. Staining of the cells with anti-CD114 (anti–G-CSF receptor) revealed a specific binding on about 80% of the cells (C). Two-color analysis using FITC-labeled G-CSF and CD19-PE revealed a specific binding of FITC–G-CSF on CD19low cells but not on CD19high cells, indicating that the leukemic blast cells of this patient are able to bind G-CSF (D). Schematic structure of the cytoplasmic domain of the G-CSF receptor (G-CSFR) mRNA (E). The nucleotide positions given below indicate the point mutations detected in the patient reported here.
Analysis of G-CSF receptor in lymphoblasts of the patient.
FACS analysis of the lymphoblasts of the patient. The main population of analyzed cells demonstrated a low-intensity staining for CD19 (B). Minor populations corresponding to nonleukemic T or B cells consisted of CD3+ cells (A) and CD19high cells (B), respectively. Staining of the cells with anti-CD114 (anti–G-CSF receptor) revealed a specific binding on about 80% of the cells (C). Two-color analysis using FITC-labeled G-CSF and CD19-PE revealed a specific binding of FITC–G-CSF on CD19low cells but not on CD19high cells, indicating that the leukemic blast cells of this patient are able to bind G-CSF (D). Schematic structure of the cytoplasmic domain of the G-CSF receptor (G-CSFR) mRNA (E). The nucleotide positions given below indicate the point mutations detected in the patient reported here.
We also examined peripheral blood neutrophils and mononuclear cells at a complete remission status after chemotherapy treatment. In both cell groups, the point mutation on nucleotide position 2414 was detected in 2 of 10 clones (20%). None of the clones displayed a mutation on nucleotide position 2390. Eighty percent (16 of 20) of the analyzed clones showed wild-type sequence of the G-CSF receptor mRNA. These data suggest that G-CSF receptor mutations were not restricted to the malignant clone but were also present in a subpopulation of the myeloid lineage. The mutation 2414 probably occured in a common progenitor of the myeloid and B-cell lineage as one of the primary transforming events in leukemogenesis in this patient. In contrast to that, the mutation at position 2390 occurred later in a leukemic subclone, maybe due to a general genetic instability of the G-CSF receptor gene in CN. This would explain why the mutation 2414 is detectable in both ALL-blasts and the neutrophils and mononuclear cells at remission status of the patient.
To date, only myeloid leukemias secondary to CN have been reported. To our knowledge this is the first case of ALL secondary to CN. Interestingly, the G-CSF receptor mRNA of these leukemic cells revealed a point mutation similar to mutations reported from patients suffering from AML secondary to CN. This would support our hypothesis that G-CSF receptor mutations are involved in leukemogenesis.2,3 Indeed, 11 of 12 patients with MDS or AML secondary to CN reveal G-CSF receptor point mutations.2 The fact that there are patients with CN who have not developed leukemia but express a mutated G-CSF receptor mRNA strongly suggest that G-CSF receptor mutation is necessary but not sufficient for the development of leukemia in CN patients.2,4 Other genetic defects that seem to be important steps in leukemogenesis have already been reported in patients with CN/AML-like mutations in the ras gene or monosomy 7.1 4 Indeed, the karyotype of our patient demonstrated an unbalanced t(2;3) translocation and the deletion of 5q. The contribution of these different events to leukemogenesis in CN remains to be investigated.
Supported by grants from the Deutsche Krebshilfe (10-1548-We2), Deutsche Forschungsgemeinschaft (We942/4-3), and Norwegian Cancer Society.