Abstract
A new megathrombocytopenic syndrome with giant platelets in peripheral blood and severe thrombocytopenia was diagnosed in a 4-month-old boy. His clinical course included repeated hemorrhagic incidents leading to death at age 37 months. Bone marrow ultrastructural analysis revealed numerous dystrophic megakaryocytes with giant membrane complexes. Although these features were similar to those described for megakaryocytes in mice lacking the gene for transcription factor p45-NF-E2, no abnormalities in thep45-NF-E2 gene could be documented. Platelet membrane analysis showed a reduction in glycoprotein (GP) Ib, but normal content of GPIIb and GPIIIa. Analysis of genes encoding for GPIb α and β, GPV, and GPIX ruled out the possibility that the observed platelet abnormality is a variant of Bernard-Soulier syndrome. A moderate neutropenia was associated with a complete lack of expression of sialyl–Lewis-X on the surface of polymorphonuclear neutrophils. A common defect in posttranslational modification of glycoproteins could account for the diverse cellular abnormalities.
Introduction
Congenital thrombocytopenia has been identified as a heterogeneous group of diseases, some of which are characterized by the presence of giant platelets in circulation, and referred to as macrothrombocytopenia.1-4 Molecular analyses have identified mutations in glycoprotein (GP)Ib α, β, or in GPIX in Bernard-Soulier syndrome in which giant platelets are found in association with thrombocytopenia.4-8 In the present report, we describe a new clinical syndrome in which severe thrombocytopenia with giant platelets occurred in association with neutropenia. Megakaryocytes exhibited striking ultrastructural defects including abnormal demarcation membranes and giant complex membranes. Platelets exhibited a reduced membrane content of GPIb and polymorphonuclear neutrophils (PMNs) were devoid of sialyl–Lewis-X (sialyl Lex). Although we have not been able to identify the specific molecular defect responsible for the observed cellular abnormalities, we suggest that a defect in either the sialylation or fucosylation of glycoproteins is likely to account for the observed phenotype.
Study design
Case history
A 4-month-old boy with a spontaneous massive bleed in the posterior chamber of right eye along with cutaneous hemorrhages was referred to our pediatric department. No increased bleeding tendency was reported during the neonatal period. Blood counts showed marked thrombocytopenia (platelet count ranging from 15 to 27 × 109/L) and neutropenia (0.8-9.2 × 109/L). No circulating or platelet-bound antiplatelet autoantibodies could be documented. The life span of transfused platelets was normal. The prolonged bleeding time was normalized following platelet transfusion. Partial deficiency in von Willebrand factor was noted in the patient and his father. No mutations in the Wiskott-Aldrich syndrome protein gene or in VWF gene sequences encoding the binding domains for platelet GPIb and factor VIII could be documented. Bone marrow karyotype was normal.
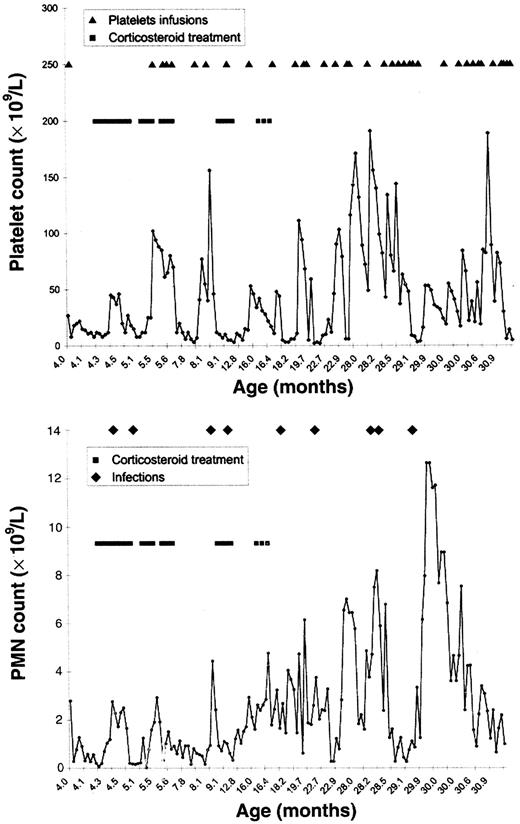
Hemorrhagic complications of varying severity were recorded over the next 30 months, including 6 dramatic episodes of pulmonary hemorrhage resulting in acute respiratory distress syndrome and refractory hypoxemia. Serious infections also occurred including bacterial pneumonia, postvaccinal abscess, and external otitis with cervical cellulitis due to Pseudomonas aeruginosa. The evolution of platelet and PMN counts during this period is summarized in Figure1. Thrombocytopenia progressively worsened with platelet counts reaching values as low as 2 to 6 × 109/L; neutropenia partially resolved at the age of 18 months. Oral steroid therapy (prednisone, 2 mg/kg per day) resulted in a mild and transient increase in the platelet count (from 12 up to 25 × 109/L). Subsequent administration of oral steroids was ineffective. The PMN count was transiently normal during infectious episodes and during steroid treatment (zenith: 2 × 109/L and 6.2 × 109/L, respectively).
Platelet and PMN counts.
Evolution of platelet counts (top) and PMN counts (bottom) during a 3-year follow-up period.
Platelet and PMN counts.
Evolution of platelet counts (top) and PMN counts (bottom) during a 3-year follow-up period.
In the face of such repeated life-threatening hemorrhagic complications and in the absence of viable treatment options, bone marrow transplantation was performed at the age of 34 months. However, complications that included graft-versus-host disease, pulmonary viral infection, and massive pulmonary hemorrhage with refractory respiratory failure led to death at the age of 37 months.
Ultrastructural studies
Platelet membrane glycoprotein analysis
Platelets from the patient and a normal donor were analyzed using sodium dodecyl sulfate–polyacrylamide gel electrophoresis, periodic acid-Schiff staining, and Western blot analysis as previously described.11 Monoclonal antibodies GS 296, SZ 22, and XII F9 specific for GPIbα, GP IIb, and GP IIIa, respectively, were used in Western blot analysis.
Molecular studies
Polymerase chain reaction (PCR)-amplified NF-E2 complementary DNA (cDNA) and genomic DNA were sequenced using a fluorescent automated DNA sequencer.12 The genomic sequence of GPIb α and [beta], GPV, GPIX, FUT4, andFUT7 genes was determined following PCR amplification using appropriate primers. We also assessed the genomic sequence of 2 sialyltransferase genes (ST3Gal4 and ST3Gal6) and 2 fucosyltransferase genes (FUT 4 and FUT 7).
PMNstudies
Sialyltransferase and fucosyltransferase assays
Global sialyltransferase activity of PMNs and platelets was quantitated as previously described.15 The α-2- and α-3-fucosyltransferases were assayed in plasma and in the extracts prepared from an isolated lymphocyte/monocyte fraction.16
Sequences of the different primer sets used in the molecular studies and details of various experimental protocols are available on request.
Results and discussion
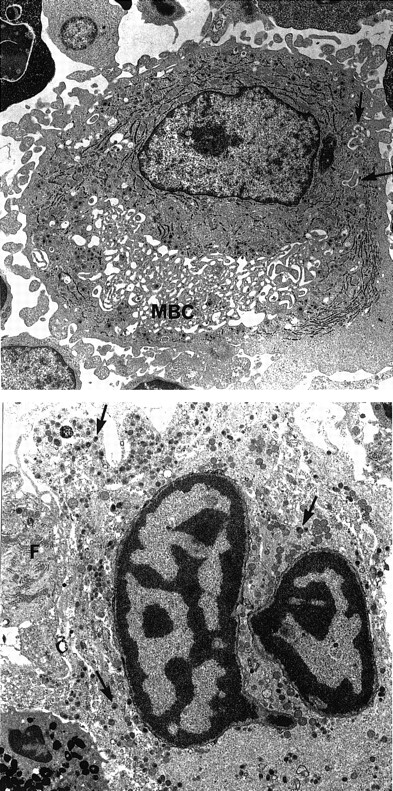
Morphologic examination of peripheral blood smears revealed hypogranular platelets and the presence of some giant platelets (diameter ≥ than that of lymphocytes). Bone marrow aspirates showed normocellular marrow with megakaryocyte hyperplasia. Megakaryocyte morphologic abnormalities included numerous small mononuclear or hyposegmented megakaryocytes, vacuolated cells, and abnormal fragmentation of megakaryocyte cytoplasm into large platelet masses. This abnormal subpopulation coexisted with a population of apparently normal megakaryocytes. Electron microscopic studies showed that alpha granules were produced but appeared to exhibit abnormal cytoplasmic distribution due to the presence of giant membrane complexes with associated smooth endoplasmic reticulum and demarcation membranes (Figure 2). These membrane complexes appeared very early in megakaryocyte maturation and were also observed in cultured megakaryocytes. In addition, numerous large lytic megakaryocytes were seen, some exhibiting apoptotic nuclei (Figure 2). Analysis of platelet membrane proteins showed a markedly reduced amount of GPIb but normal content of GPIIb and GPIIIa. Furthermore, GPIb exhibited a higher apparent molecular weight, suggesting altered glycosylation. Because the ultrastructural features of the patient's megakaryocytes were similar to those described in NF-E2 knockout mice,17 the cDNA and genomic sequence of this gene were analyzed but failed to reveal any mutation. The genomic sequences of GPIb α and β, GPV, and GPIX were also normal.
Ultrastructural aspect of megakaryocytes.
(Top) A large mononucleated megakaryocyte with large membrane complex (MBC). Only few demarcation membranes are free (arrows). Magnification × 4750. (Bottom) Lytic mature megakaryocyte. The nucleus has the typical aspect of apoptotic cells with clumps of chromatin. The cytoplasm contains numerous granules (arrows). The cell membrane and intracytoplasmic membrane are lysed. A granulocytic peroxidase is present at the periphery. Note the presence of a fibroblast (F) closely associated to the lytic megakaryocyte. Magnification × 5320.
Ultrastructural aspect of megakaryocytes.
(Top) A large mononucleated megakaryocyte with large membrane complex (MBC). Only few demarcation membranes are free (arrows). Magnification × 4750. (Bottom) Lytic mature megakaryocyte. The nucleus has the typical aspect of apoptotic cells with clumps of chromatin. The cytoplasm contains numerous granules (arrows). The cell membrane and intracytoplasmic membrane are lysed. A granulocytic peroxidase is present at the periphery. Note the presence of a fibroblast (F) closely associated to the lytic megakaryocyte. Magnification × 5320.
Functional studies on PMNs did not reveal any abnormality either in chemotaxis or in the generation of reactive oxygen species. The expression of β2-integrin adhesion molecules (CD11a/CD18, CD11b/CD18, and CD11c/CD18) and of l-selectin was normal on both unstimulated PMNs and following stimulation by formyl peptides. However, expression of sialyl Lex was undetectable on the patient's PMNs (median fluorescence intensity 25 versus 1500-2500 for normal). There was also a 2-fold increase in the expression of CD15 both at baseline and following fMLP stimulation. Extensive studies of erythrocyte membrane antigens failed to reveal any abnormalities. A moderate increase in sialyltransferase activity was found in platelets and in the PMN population, suggesting the absence of a defect in the specific sialyltransferase activity. Fucosyltransferase assays showed no reduction in the activity of plasma enzymes FUT1 and FUT6, but a slight reduction in FUT4 activity was noted in PMNs. Because the patient's PMNs failed to express sialyl Lex, we explored if mutations in fucosyltransferase (FUT4 andFUT7) genes or in sialyltransferase (ST3Gal4 andST3Gal6) genes were responsible for the observed phenotype. Sequencing of these 4 genes in the patient failed to reveal any abnormality. However, we cannot rule out the possibility of mutations in noncoding regions or a chromosomal rearrangement involving these genes.
Our study provides the first description of a new dysmegakaryocytopoietic syndrome that appears to be due to a defect in posttranslational modification of membrane glycoproteins. Although we were unsuccessful in our attempts to delineate the precise molecular defect responsible for the complex clinical phenotype, the heterogeneous features encountered in our patient could all be related to an abnormal defect in fucosylation or sialylation pathways.
Acknowledgments
We would like to thank Dr Philippe Delanoy and Dr Raphael Oriol for helpful discussions regarding the biochemistry of membrane polyosides, Dr Vincent Jallu for help with platelet glycoprotein studies, Dr Marie Dreyfus for reviewing with us the results of hemostasis studies, and the nursing staff of Hopital Bicetre's Pediatric Department for the extraordinary care they provided to the patient.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Narla Mohandas, Lawrence Berkeley National Laboratory, Bldg 74-157, 1, Cyclotron Rd, Berkeley, CA 94720; e-mail:mnarla@lbl.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal