Abstract
Acute graft-versus-host diseases (GVHD) is a major cause of morbidity and mortality in patients undergoing allogeneic bone marrow transplantation (BMT). T helper 1 (Th1)-type cytokines such as interferon-γ or tumor necrosis factor-α have been implicated in the pathogenesis of acute GVHD. TAK-603 is a new quinoline derivative, which is now in clinical trials for use as a disease-modifying antirheumatic drug. In preclinical studies, it inhibited delayed-type hypersensitivity, but not Arthus-type reaction, in mice, and selectively suppressed Th1 cytokine production. Thus, the present study was designed to investigate whether the Th1 inhibitor (TAK-603) ameliorates lethal acute GVHD in a mouse model. Administration of TAK-603 into BALB/c mice given 10 Gy total body irradiation followed by transplantation of bone marrow and spleen cells from C57BL/6 mice markedly reduced the mortality in association with minimal signs of GVHD pathology in the liver, intestine, and skin. TAK-603 reduced not only the production of Th1-type cytokines, but also the proportion of Th1 cells in CD4+ helper T cells in this GVHD mouse model. These results suggest that TAK-603 could be a potent therapeutic agent for acute lethal GVHD.
Introduction
Acute graft-versus-host diseases (GVHD) is a major cause of morbidity and mortality in patients undergoing allogeneic bone marrow transplantation (BMT).1 Mortality attributed to acute GVHD has been reported in up to 50% of cases,2 and levels of cytokines such as interleukin (IL)-2, interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), and IL-12 have been known to increase in human3-7 and animal8-12acute GVHD models, suggesting that T helper 1 (Th1)-type cytokines are implicated in its pathogenesis13 in contrast with chronic GVHD, which was supposed to be mediated by Th2-type cytokines.13
To date, most therapeutic approaches designed to reduce acute GVHD have focused on the ex vivo removal of unfractionated donor T-cell proportions from the marrow graft.14,15 Although this strategy has reportedly reduced GVHD,16,17 there has been a reciprocal increase in the rate of graft rejection, more severe immunosuppression, and higher relapse rate of original malignancies. Another approach was directed toward inhibiting donor T-cell recognition of host alloantigen by interfering with CD28/B7 recognition using monoclonal antibodies (mABs). This approach, however, resulted in incomplete prevention.18 19 Therefore, specific Th1 inhibition may offer an attractive approach to preventing or reducing acute GVHD.
In this context, blocking CD40 by using its antibody on antigen-presenting cells (APC) by stimulation with CD40 ligand on activated T-cell signals to secrete IL-12 was shown to be very effective in preventing acute GVHD. Similarly, using antibodies to deactivate IL-12 itself was reasonably effective in preventing acute GVHD. However, administration of antibodies against Th1 cytokines such as IFN-γ,20 TNF-α,21 and IL-222 has also been reported to prevent acute GVHD in mice. However, none of these methods have completely reduced the mortality rate because without continuous administration of antibodies, the titer will quickly drop to insufficient levels.
Alternatively, Rosario et al23 recently reported that polarization of donor T cells to Th2 response is associated with reduced severity of acute murine GVHD. Th2 polarization was established by tolerance induction by priming mice with injections of allogeneic leukocytes. However, as they themselves mentioned, the safety and ethical concerns of using leukocytes from patients with neoplastic diseases to induce tolerance in bone marrow donors may preclude this approach.24
Recently, rapamycin25 (sirolimus) has been shown to be an effective means of reducing the lethality of GVHD by interfering with the production of Th1 cytokines in a murine model. However, rapamycin-treated mice developed an autoimmune-like syndrome26 consisting of ulcerative dermatitis, bile duct proliferation, and a nondestructive peribronchial pulmonary infiltration; this will prove to be a major prohibiting factor in its clinical application.
TAK-603 was developed on the basis of the structure of justicidin,27,28 which is known to be a potent bone resorption inhibitor,28 and is now in clinical trials for use as an antirheumatic drug.27,28 In preclinical studies, it was suggested that TAK-603 acts on cellular immunity because it attenuated adjuvant arthritis much more clearly than it did collagen-induced arthritis in rats.27 In an in vitro study, TAK-603 inhibited IFN-γ production, but not IL-4 production, in established Th1- and Th2-type T-cell lines, suggesting it may be a potent Th1 inhibitor.29
Therefore, in the present study, we attempted to determine whether TAK-603, a Th1-specific inhibitor, could prevent acute GVHD in a fully allogeneic murine BMT model. The results obtained have clearly shown that oral administration of TAK-603 completely prevented the acute lethal GVHD by reducing the Th1-type cytokine production.
Materials and methods
Mice
Female C57BL/6 (H-2b), BALB/c (H-2d) mice were purchased from Charles River Laboratories (Raleigh, NC). The mice were bred in the Animal House of the Institute of Sapporo Medical University, Sapporo, Japan. The mice were housed 5 to a box and received Milling chow and water ad libitum and were used when they were 8 weeks old. The experimental protocol used in this study was approved by the Sapporo Medical University Animal Experimentation Ethics Committee.
Preparation of the drug
TAK-603 [ethyl 4-(3,4-dimethoxyphenyl)-6,7-dimethoxy-2-(1,2,4,-triazol-l-ylmethyl) quinoline-3-carboxylate, compound 7]27,28 was kindly provided by Takeda Chemical Industries, Ltd (Osaka, Japan). To administer orally to mice, it was emulsified in 0.5% methylcellulose solution at a concentration of 10 mg/mL.29
BMT and induction of lethal acute GVHD
On the day before transplantation, recipient BALB/c mice received 10 Gy total body irradiation (TBI) from Softex M-150 WE (Softex, Tokyo, Japan) 60Co source at a rate of 0.5 Gy/min. On the day of transplantation, donor mice (C57BL/6 for allogeneic transplantation; BALB/c for syngeneic transplantation) were killed by cervical dislocation. Their femora were removed aseptically, and bone marrow (BM) was removed from the femoral shaft by insertion of a 25-gauge needle at the proximal end. The marrow cells were displaced with RPMI 1640 medium with 0.5% fetal calf serum (FCS)/1% penicillin-streptomycin, and single cell suspension was produced. A single cell suspension of donor spleen cells was obtained by passage of minced spleen through a fine wire mesh and resuspended in RPMI 1640 medium. Irradiated BALB/c mice were randomly divided into 6 groups of 15 mice each and received a single injection of 0.25 mL RPMI 1640 medium containing 2 × 107 nucleated BM cells and 1 × 107 (groups 1-3) or 5 × 107 (groups 4-6) nucleated spleen cells through the tail vein (groups 1, 2, 4, and 5 were used for allogeneic transplantation, and groups 3 and 6 for syngeneic transplantation).
Groups 1 and 4 received 50 mg/kg (in 100 μL 0.5% methylcellulose solution) of TAK-603 (Takeda, Japan) orally once a day from the day of transplantation to 45 days after transplantation. Groups 2, 3, 5, and 6 received 100 μL 0.5% methylcellulose solution orally once a day from the day of transplantation. Survival was monitored until day 45. Peripheral blood was serially obtained by retro-orbital venipuncture once every 3 days from the day of the transplantation. On day 6, 3 mice from each of the 6 groups were killed; cytokine concentrations in sera were measured, and histopathologic and flow cytometric analyses were conducted as described below.
Hematologic and histopathologic analysis
The total number of white blood cells (WBC) was counted by an automated cell counter (Toa Medical Electronics, Tokyo, Japan). Hemogram was assessed by May-Giemsa staining. The ear skin, liver, and small intestine were excised immediately, fixed in 10% buffered formalin and embedded in paraffin. Sections were stained with hematoxylin and eosin and examined under microscopy.
Immunohistochemistry for murine CD4 and CD8 antigen expression in tissue-infiltrating lymphocytes
Liver, small intestine, and ear skin sections obtained on day 6 after transplantation from GVHD mice with or without TAK-603 and syngeneic transplant mice were perfused in 99% methanol containing 1% H2O2 for 20 minutes at room temperature. After washing 3 times with 6% phosphate-buffered saline (PBS), the sections were incubated with 3% bovine serum albumin (BSA) for 15 minutes, followed by another incubation with antimouse CD4 or CD8 mAb (Caltag Laboratories, Burlingame, CA) or anti-IgG control mAb for 1 hour at room temperature. The sections were again washed 3 times with PBS, incubated with fluorescein isothiocyanate (FITC)-conjugated antirat IgG for 10 minutes at room temperature and visualized on a Laser Sharp MRC-1024 Confocal Analysis System (Bio-rad Laboratories, Hercules, CA).
Flow cytometric analysis
Three-color flow cytometric analysis was used to identify and enumerate Th1-like and Th2-like cell populations of peripheral blood mononuclear cells (PBMNC) or spleen mononuclear cells (SMNC). PBMNC or SMNC from mice were separated from heparinized venous blood by Gey balanced salt lysing solution containing 0.7% NH4CI for removal of red blood cells (RBC). PBMNC and SMNC (1 × 106) were washed with FACS buffer (5% FCS, 0.1% NaN3 in PBS), incubated on ice for 30 minutes, and were stained with peridium chlorophyll (Per-CP)–conjugated anti-CD4 (Leu-3a; Becton Dickinson Immunocytometry, San Jose, CA), FITC-conjugated anti-CD45RO (UCHL1; Dako Japan, Kyoto, Japan), and phycoerythrin (PE)-conjugated anti-CD62L (TQ-1; Counter Immunology, Hialeah, FL) mAbs. Acquisition and data analysis for the 3-color immunofluorescence procedures were performed using a FACScan flow cytometer (Becton Dickinson) and Lysis II software (Becton Dickinson), respectively. The CD4+ cells expressing CD45RO and CD62L antigens were recorded as Th2-like cells, and those expressing the CD45RO antigen as Th1-like cells.30 The percentage of CD8+ cells was identified by flow cytometry using Per-CP–conjugated anti-CD8 (5F2; Southern Biotechnology Associates, Birmingham, AL) mAb.
Enzyme-linked immunosorbent assay
Mice were anesthetized and exsanguinated; their blood was clotted on ice for 5 to 10 minutes, and serum was separated by centrifugation at 4°C and stored at −80°C for further analysis of cytokine levels, which were measured in triplicate wells. The murine IL-4, IL-12, INF-γ, and TNF-α were measured using enzyme-linked immunosorbent assay (ELISA) kits purchased from Endogen (Woburn, MA) following the instructions of the provided manufacturer's manuals as previously described.25 31Sensitivities of ELISA kits were as follows: IL-4 less than 5 pg/mL; IL-12 less than 5 pg/mL; IFN-γ less than 15 pg/mL; TNF-α less than 10 pg/mL.
Statistical analyses
Statistical analyses were performed using the StatView computer program (Abacus Concepts, Berkeley, CA). Group comparisons of continuous data were made by the Mann-Whitney test. P values less than .05 were considered significant, and P values less than .1 and more than .05 were considered to be a statistical trend.
Results
TAK-603 treatment confers protection from lethal acute GVHD
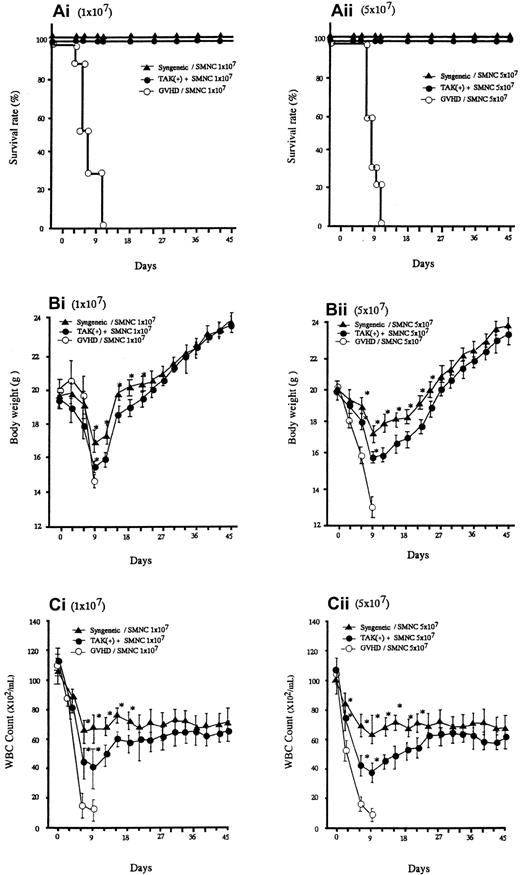
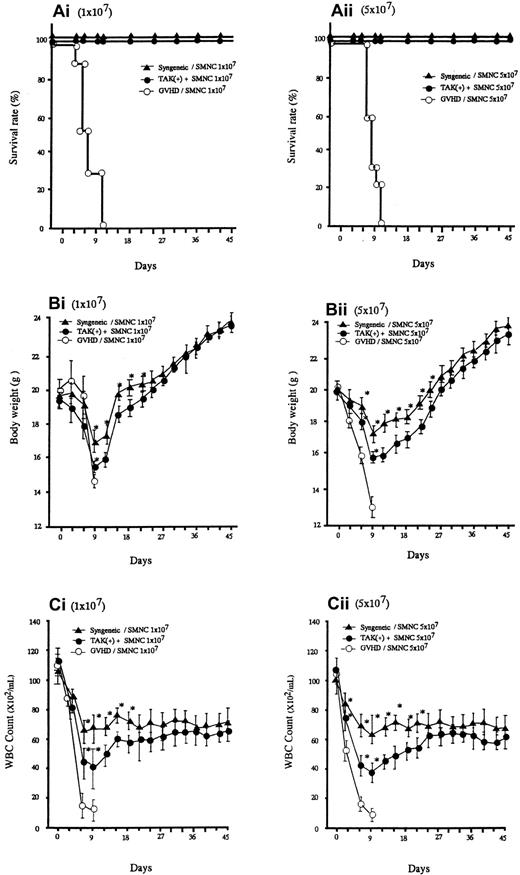
Lethal acute GVHD was induced by intravenous injection of bone marrow and spleen cells from C57BL/6 into BALB/C mice (2 groups, n = 30). As represented in Figure 1A, all the recipients given allogeneic spleen cells with no additional treatment died within 10 days after transplantation. In these mice, clinical symptoms of acute GVHD, such as hair ruffling, lowered mobility, and weight loss became apparent within 6 days. The principal feature of the autopsy was that they all seemed to have died of gross acute GVHD. Administration of TAK-603 markedly reduced the mortality; all mice receiving this treatment, except the 6 mice that were killed on day 6, survived more than 45 days (Figure 1Ai,ii). The protective effect of TAK-603 treatment on weight loss was not apparent until day 9, after which weight began to rise, reaching normal levels by day 45 (Figure 1Bi,ii). All syngeneic transplant mice survived more than 45 days and underwent weight loss similarly to TAK-603–treated GVHD mice, although to a lesser degree (Figure 1A,B).
Survival and serial change of body weight (BW) and total WBC of BALB/c mice after BMT.
Survival (Ai,ii), serial change of BW (Bi,ii) and total WBC count (Ci,ii) of BALB/c mice given 10 Gy TBI followed by 2 × 107 BM cells and 107 or 5 × 107 spleen cells from C57BL/6 mice (○), 2 × 107 BM cells and 107 or 5 × 107 spleen cells from C57BL/6 mice and daily oral administration of TAK-603 (50 mg/kg/100 μL 0.5% methylcellulose solution) from the day of transplantation to 45 days after transplantation (●), and 2 × 107 BM cells and 107 or 5 × 107 spleen cells from BALB/c (syngeneic) mice (▴). The BW (B) and total WBC counts (C) of the survivors were measured once every 3 days from the day of transplantation to 45 days after transplantation. The data represent mean ± SD. *P < .05
Survival and serial change of body weight (BW) and total WBC of BALB/c mice after BMT.
Survival (Ai,ii), serial change of BW (Bi,ii) and total WBC count (Ci,ii) of BALB/c mice given 10 Gy TBI followed by 2 × 107 BM cells and 107 or 5 × 107 spleen cells from C57BL/6 mice (○), 2 × 107 BM cells and 107 or 5 × 107 spleen cells from C57BL/6 mice and daily oral administration of TAK-603 (50 mg/kg/100 μL 0.5% methylcellulose solution) from the day of transplantation to 45 days after transplantation (●), and 2 × 107 BM cells and 107 or 5 × 107 spleen cells from BALB/c (syngeneic) mice (▴). The BW (B) and total WBC counts (C) of the survivors were measured once every 3 days from the day of transplantation to 45 days after transplantation. The data represent mean ± SD. *P < .05
Effect of TAK-603 on GVHD-associated histopathology
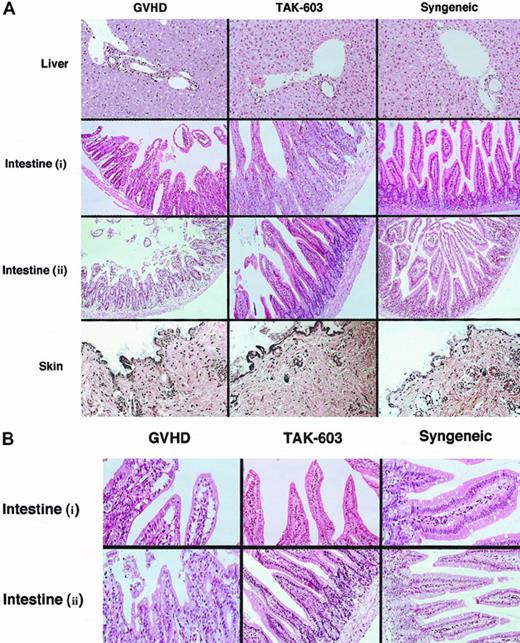
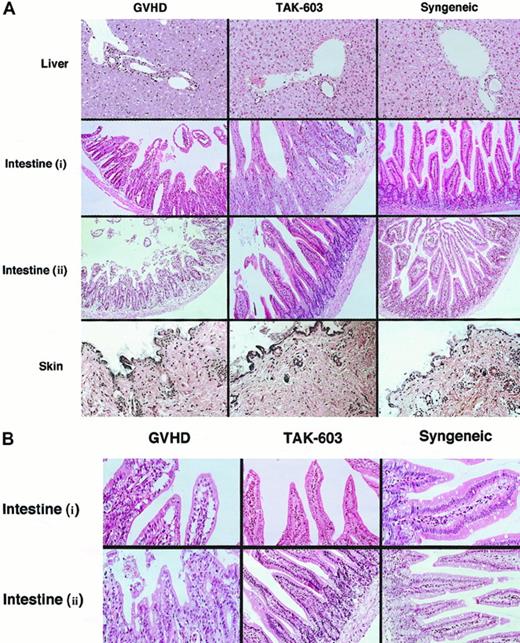
To evaluate the effect of TAK-603 on histopathology of GVHD, tissue sections of target organs (liver, gut, skin) were prepared on day 6. Day 6 was selected because it is known to be the peak time of donor T-cell expansion.25 In the liver from the GVHD mice, a slight infiltration of mononuclear cells was observed mainly in the portal areas (Figure2). The lymphocyte infiltration was decreased in the livers from TAK-603–treated GVHD mice and control mice (Figure 2). The guts from GVHD mice exhibited dilatation, flattening of the villi, and elevation and atrophy of the crypts, which are characteristics of intestinal GVHD (Figure 2). In TAK-603–treated mice, structural integrity of the villi and crypts was partially improved; however, mononuclear cell infiltration was observed to the same degree as that in GVHD mice and control mice. The skin from the mice undergoing GVHD exhibited loss of hair follicles. Such changes were not observed in TAK-603–treated mice.
Histopathologic examination.
Induction of lethal acute GVHD and administration of TAK-603 were performed as described in “Materials and methods.” On day 6, 3 mice from each of the 6 groups were killed. Paraffin sections of the skin, liver, and small intestine were stained by hematoxylin and eosin (original magnification: A, × 200; B, × 400. Intestine (i): intestine from BALB/c mice receiving 2 × 107 nucleated BM cells and 1 × 107 nucleated spleen cells. Intestine (ii): intestine from BALB/c mice receiving 2 × 107nucleated BM cells and 5 × 107 nucleated spleen cells.
Histopathologic examination.
Induction of lethal acute GVHD and administration of TAK-603 were performed as described in “Materials and methods.” On day 6, 3 mice from each of the 6 groups were killed. Paraffin sections of the skin, liver, and small intestine were stained by hematoxylin and eosin (original magnification: A, × 200; B, × 400. Intestine (i): intestine from BALB/c mice receiving 2 × 107 nucleated BM cells and 1 × 107 nucleated spleen cells. Intestine (ii): intestine from BALB/c mice receiving 2 × 107nucleated BM cells and 5 × 107 nucleated spleen cells.
TAK-603 lowered the enhanced level of Th1 cytokines in acute GVHD mice
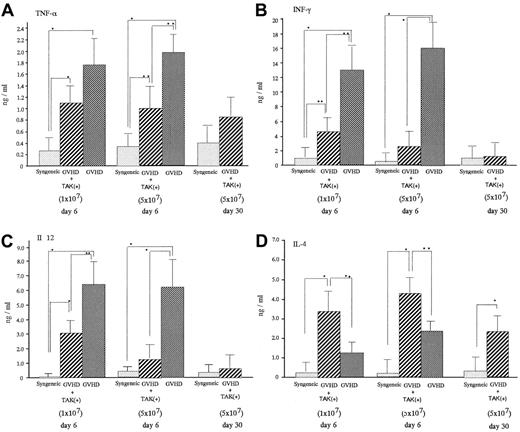
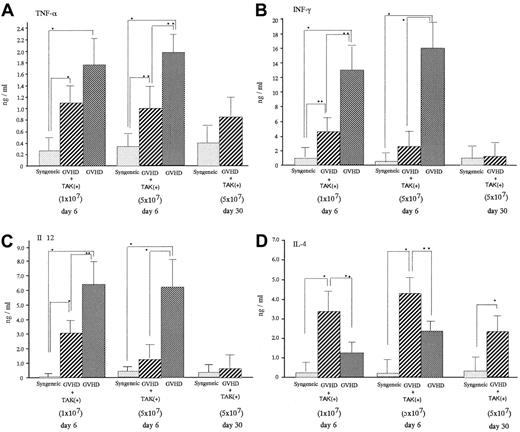
On day 6 after transplantation, acute GVHD mice were characterized by significantly increased serum levels of TNF-α, INF-γ, and IL-12 compared with levels in mice receiving syngeneic bone marrow cell and spleen cell infusion (syngeneic transplant mice) (Figure 3). Administration of TAK-603 significantly lowered the enhanced serum level of INF-γ and IL-12. TNF-α was also lowered, though to a much lesser extent. Serum IL-4 level was suppressed somewhat more in GVHD mice than in syngeneic transplant mice and significantly increased in TAK-603–treated GVHD mice compared to that in GVHD mice or syngeneic transplant mice.
Effect of TAK-603 on cytokine production during the course of acute GVHD.
TNF-α (A), IFN-γ (B), IL-12 (C), and IL-4 (D) concentration in sera from BALB/c mice given 10 Gy TBI followed by 2 × 107 BM cells and 1 × 107 or 5 × 107 spleen cells from C57BL/6 (GVHD; ▩), 2 × 107 BM cells and 1 × 107 or 5 × 107 spleen cells from C57BL/6 and daily oral administration of TAK-603 (GVHD+TAK; ▨) and 2 × 107 BM cells and 1 × 107 or 5 × 107 spleen cells from BALB/c mice (syngeneic; ░) on day 6 after transplantation. On day 30 after transplantation, cytokine concentrations in sera from BALB/c mice (GVHD+TAK) were also evaluated. The data represent mean ± SD. *P < .01; **P < .05.
Effect of TAK-603 on cytokine production during the course of acute GVHD.
TNF-α (A), IFN-γ (B), IL-12 (C), and IL-4 (D) concentration in sera from BALB/c mice given 10 Gy TBI followed by 2 × 107 BM cells and 1 × 107 or 5 × 107 spleen cells from C57BL/6 (GVHD; ▩), 2 × 107 BM cells and 1 × 107 or 5 × 107 spleen cells from C57BL/6 and daily oral administration of TAK-603 (GVHD+TAK; ▨) and 2 × 107 BM cells and 1 × 107 or 5 × 107 spleen cells from BALB/c mice (syngeneic; ░) on day 6 after transplantation. On day 30 after transplantation, cytokine concentrations in sera from BALB/c mice (GVHD+TAK) were also evaluated. The data represent mean ± SD. *P < .01; **P < .05.
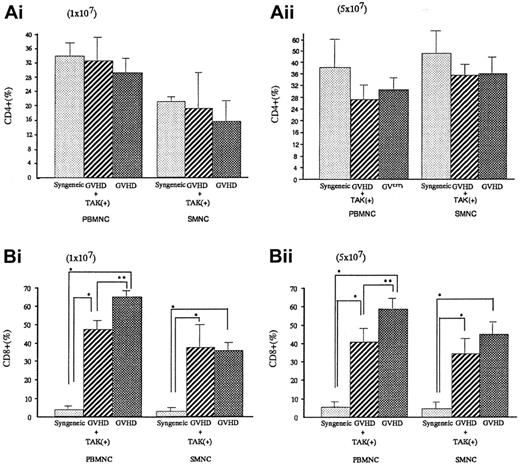
Alteration of lymphocyte subpopulation in vivo
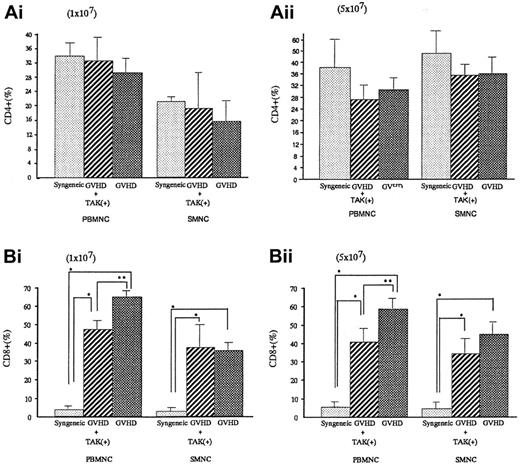
To investigate the lymphocyte subpopulations, we conducted FACS analyses. As shown in Figure 4, CD4+ populations either in spleen cells or in PBMNC were almost equal in syngeneic transplant mice and GVHD mice with or without TAK-603 treatment. In contrast, CD8+ populations were significantly higher in GVHD than those of syngeneic transplant mice (P < .05). TAK-603 treatment did reduce the increase of CD8+ populations in PBMNC in acute GVHD mice.
CD4+ or CD8+ T lymphocyte populations in peripheral blood and spleen from BALB/c mice after BMT.
Percentages of CD4+ or CD8+ T lymphocytes in peripheral blood or spleen from BALB/c mice given 10 Gy TBI followed by 2 × 107 BM cells and 1 × 107 (Ai,Bi) or 5 × 107 (Aii, Bii) spleen cells from C57BL/6 (GVHD; ▩), 2 × 107 BM cells and 1 × 107(Ai,Bi) or 5 × 107 (Aii,Bii) spleen cells from C57BL/6 and daily oral administration of TAK-603 (GVHD+TAK; ▨) and 2 × 107 BM cells and 1 × 107 (Ai,Bi) or 5 × 107 (Aii,Bii) spleen cells from BALB/c mice (syngeneic; ░) on day 6 after transplantation. The data represent mean ± SD. *P < .01; **P < .05.
CD4+ or CD8+ T lymphocyte populations in peripheral blood and spleen from BALB/c mice after BMT.
Percentages of CD4+ or CD8+ T lymphocytes in peripheral blood or spleen from BALB/c mice given 10 Gy TBI followed by 2 × 107 BM cells and 1 × 107 (Ai,Bi) or 5 × 107 (Aii, Bii) spleen cells from C57BL/6 (GVHD; ▩), 2 × 107 BM cells and 1 × 107(Ai,Bi) or 5 × 107 (Aii,Bii) spleen cells from C57BL/6 and daily oral administration of TAK-603 (GVHD+TAK; ▨) and 2 × 107 BM cells and 1 × 107 (Ai,Bi) or 5 × 107 (Aii,Bii) spleen cells from BALB/c mice (syngeneic; ░) on day 6 after transplantation. The data represent mean ± SD. *P < .01; **P < .05.
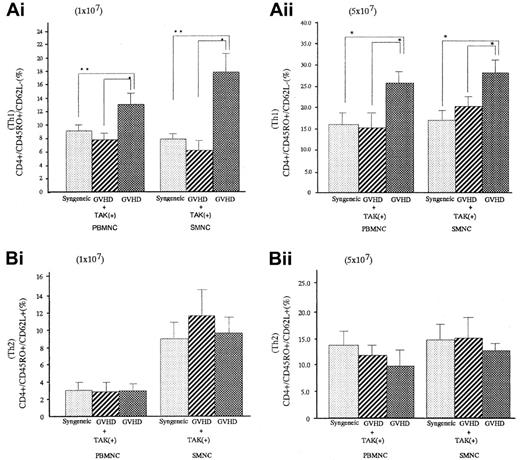
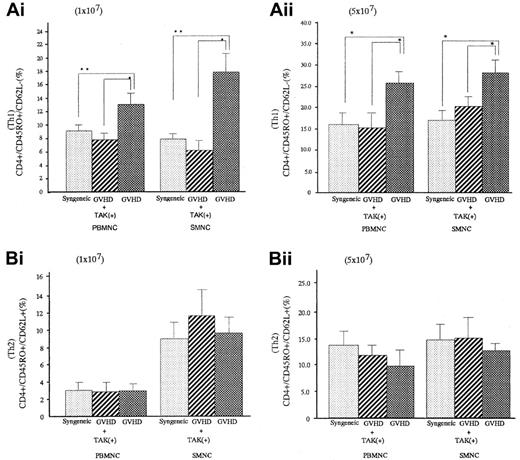
Percentages of Th1-like cell populations in spleen cells and PBMNCs of GVHD mice were significantly greater than those of syngeneic transplant mice (P < .05 for spleen cells and P < .05 for PBMNC), and they were significantly decreased in GVHD mice who received TAK-603 (P < .05 for spleen cells andP < .05 for PBMNC). There were no differences in Th2-like cell populations in spleen cells and PBMNC between GVHD mice with or without TAK-603 treatment (Figure 5).
Effect of TAK-603 on the population of Th1 (CD45RO+CD62L−) or Th2 (CD45RO+CD62L+)-like cells in CD4+cells from PBMNC and SMNC.
The percentage of Th1-like cell (A) or Th2-like cell (B) population in CD4+ cells of PBMNC or SMNC from BALB/c mice given 10 Gy TBI followed by 2 × 107 BM cells and 1 × 107 (Ai,Bi) or 5 × 107 (Aii,Bii) spleen cells from C57BL/6 (GVHD; ▩), 2 × 107 BM cells and 1 × 107 (Ai,Bi) or 5 × 107 (Aii,Bii) spleen cells from C57BL/6 and daily oral administration of TAK-603 (GVHD+TAK; ▨) and 2 × 107 BM cells and 1 × 107 (Ai,Bi) or 5 × 107 (Aii,Bii) spleen cells from BALB/c mice (syngeneic; ░). The data represent mean ± SD of 3 mice in each group. *P < .01; **P < .05.
Effect of TAK-603 on the population of Th1 (CD45RO+CD62L−) or Th2 (CD45RO+CD62L+)-like cells in CD4+cells from PBMNC and SMNC.
The percentage of Th1-like cell (A) or Th2-like cell (B) population in CD4+ cells of PBMNC or SMNC from BALB/c mice given 10 Gy TBI followed by 2 × 107 BM cells and 1 × 107 (Ai,Bi) or 5 × 107 (Aii,Bii) spleen cells from C57BL/6 (GVHD; ▩), 2 × 107 BM cells and 1 × 107 (Ai,Bi) or 5 × 107 (Aii,Bii) spleen cells from C57BL/6 and daily oral administration of TAK-603 (GVHD+TAK; ▨) and 2 × 107 BM cells and 1 × 107 (Ai,Bi) or 5 × 107 (Aii,Bii) spleen cells from BALB/c mice (syngeneic; ░). The data represent mean ± SD of 3 mice in each group. *P < .01; **P < .05.
Discussion
In the present study, we found a profound ameliorating effect of TAK-603, a Th1 inhibitor, in a murine model of lethal acute GVHD. Oral administration of TAK-603 almost completely inhibited GVHD, including associated pathologies in the intestine, liver, and skin, resulting in 100% reduction of mortality associated with GVHD.
Acute GVHD begins when donor T cells recognize alloantigens presented by host APCs. Following this recognition, most T cells are activated through a signal from CD28, which interacts with CD80 or CD86 located on the APCs. The activated T cells then express the CD40 ligand, which in turn interacts with CD40 on the APCs, and stimulates them to produce IL-12. IL-12 subsequently activates Th0 to become Th1 cells, which secrete Th1 cytokines such as IL-2 and IFN-γ. At the same time, Th1 cytokines stimulate monocytes to secrete monokines such as TNF-α and IL-1. These cytokines eventually cause tissue destruction and bring about acute GVHD.
Thus, attempts to block the interaction between T cell and APC or neutralize cytokines with corresponding antibodies have been made by several investigators.32-35 Blazar et al32and Wallace et al33 reported that administration of antibodies against CD80 (B7.1) plus CD86 (B7.2)32 or CTLA4Ig (CD28)33 have inhibited the interaction between CD28 and B7 and have been effective in reducing the mortality rate of acute GVHD. However, in each of these reports, the mortality rate was reduced at best by 80%, and neither body weight loss nor leukocytopenia associated with GVHD was changed. A possible explanation for such an incomplete prevention of GVHD is that not all T cells are dependent on CD28/B7 costimulation.18 In our experiments, on the other hand, TAK-603 inhibited Th1 function regardless of CD28/B7 interaction.
The neutralization of cytokines derived from activated Th1 cells such as IFN-γ, TNF-α, and IL-2 by treating mice with specific mAbs against each of these cytokines has been reported.20 22However, similarly to the above-mentioned reports, results showed mortality rate reductions of only 80%, 50%, and 60%, respectively, as well as poor restoration of GVHD-related weight loss and leukocytopenia, most likely because each mAb neutralizes only a single cytokine.
In this context, TAK-603 is known to inhibit production of all the Th1 cytokines.29 In fact, in the present study, TNF-α and IFN-γ, which normally exist at high levels in the circulation of GVHD mice, were simultaneously suppressed by TAK-603 treatment. Furthermore, in addition to these Th1 specific cytokines, elevated IL-12 was also suppressed (Figure 3), as some portion of the elevated IL-12 in GVHD mice was speculated to be derived from Th1, which is also capable of releasing IL-12.
Completely blocking IL-12 with mAbs or anti-CD40 antibodies in acute GVHD mice may have favorable results for Th1 inhibition, but at the cost of compromising immune defense and hematopoiesis soon after BMT because IL-12 plays a critical role in proliferation and differentiation of lymphohematopoietic progenitors36 and in the activation of NK cells.37 In fact, anti–IL-12 treatment using the anti-CD40 or anti–IL-12 antibodies did not prolong the survival of acute GVHD mice as expected.34 38 With TAK-603, however, the degree of IL-12 inhibition was not complete (Figure 3) suggesting immune defense or hematopoiesis was not compromised. Even though only a partial reduction of IL-12 was obtained, it may lead to the reduction of Th1.
To substantiate this assumption, we investigated the Th1/Th2-like cell populations in CD4+ T cells using 3-color FACS analyses. T cells that express the CD45RO antigen, which discriminates between CD4+ memory T cells and CD4+ naive T cells, may be further subdivided into 2 populations based on the presence of a lymph node homing receptor, L-selectin (CD62L).30 These 2 populations behaved differently in the differentiation of B cells into Ig-producing cells.39 Thus, cells that were L-selectin+ CD45RO+ T cells produced higher levels of cytokines characteristic of Th2 (ie, IL-4 and IL-5) than cells that were L-selectin− CD45RO+ T cells, which synthesized cytokines characteristic of Th1 cells such as IFN-γ.30 We have already reported that 3-color immunofluorescent analysis using CD4, CD45RO, and CD62L was useful in monitoring immunologic backgrounds in patients with ulcerative colitis40 or myelodysplastic syndrome.41 With this analysis, we revealed a significant increase of the Th1-like cell population in acute GVHD mice, and this increment was completely suppressed to a normal level by oral administration of TAK-603. This also may indicate a clinical relevance of 3-color FACS analysis in monitoring lethal acute GVHD. Such potency to suppress multifactorial cytokines may explain the superiority of TAK-603 in terms of reducing the mortality rate over previously used antibody therapies against GVHD.
Although TAK-603 has a considerable effect on inflammation and tissue disruption and dysfunction, it has little effect on lymphoid cell immigration and infiltration. As Abhyankar et al42 and other investigators43-45 have reported, IL-2, the quintessential T-cell cytokine, was produced only during the first week after transplantation, and only in the spleen. In other primary target organs of acute GVHD, such as intestine or skin, IL-2 was never produced above levels found in normal cells.42 This absence of IL-2 is consistent with the paucity of lymphocyte infiltrates observed in acute GVHD. Several inflammatory cytokines, including TNF-α, IFN-γ, and IL-1, are produced during GVHD and are considered to be responsible for the cytopathic and immunosuppressive characteristics of the disease.45 Thus, it may not be surprising that TAK-603 did not affect the lymphocyte infiltration but suppressed intestinal injury because the excess production of these tissue-damaging cytokines do not enhance T-cell activation.
An alternative approach to preventing acute lethal GVHD without using antibodies is the administration of a Th1-specific immunosuppressant. Rapamycin has been effective in reducing the mortality rate of acute GVHD in murine models.46 However, rapamycin induced an autoimmune-like syndrome consisting of ulcerative dermatitis with alopecia, hepatic bile duct proliferation, and nondestructive peribronchiolitis. In contrast, TAK-603 did not induce any complications in the present study. According to a toxicity test report using rats, no toxic side effects were observed until after 13 weeks of daily TAK-603 oral administration, indicating that TAK-603 is a safe immunosuppressant.27 Taking these factors into account, TAK-603 increases the survival rate because it prevents the increase of Th1 cytokines such as IFN-γ and TNF-α, which cause intestinal destruction.
Regarding the effect of TAK-603 on chronic GVHD, we postulate that TAK-603 does not increase the probability that chronic GVHD will occur because IL-4 levels on day 30 were significantly lower than that on day 6 (Figure 3). However, this is only speculation because there is no effective chronic GVHD mouse model.
In conclusion, the results from this study demonstrate that prevention of Th1-type cytokine production is an effective therapy for murine lethal acute GVHD. TAK-603 prevents Th1-dependent lethality by polarizing the alloimmune response toward a Th2 phenotype. Administration of TAK-603, therefore, may be of potential benefit in the clinical setting for prevention of lethal acute GVHD after BMT.
Acknowledgment
The authors wish to thank Kevin Litton for his editorial assistance.
Supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan. Y. L. and S. S. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Yoshiro Niitsu, Fourth Department of Internal Medicine, Sapporo Medical University School of Medicine, South-1, West-16, Chuo-ku, Sapporo, 060-8543, Japan; e-mail:niitsu@sapmed.ac.jp.