Abstract
Recipients of allogeneic transplants are at risk of cytomegalovirus (CMV) infection and disease during the period of immune compromise after transplantation. The limitations of current antiviral pharmacotherapy have led to attempts to develop alternative strategies for preventing or treating CMV infection, such as adoptive transfer of donor-derived virus-specific T cells. Methods for generating CMV-specific T cells either use live CMV to infect autologous antigen-presenting cells (APCs) or require some knowledge of the immunodominant peptides involved in the immune response. A novel culture system was developed that does not use live virions and in which the APCs are monocyte-derived dendritic cells (DCs). APCs were pulsed with CMV antigen and cocultured with autologous peripheral blood lymphocytes from donors seropositive for CMV. The culture-output cells consisted of both CD4- and CD8-expressing T cells. Proliferation, as determined by a tritium-thymidine–incorporation assay, showed significant CMV-antigen specificity in cultures from 15 of 15 donors seropositive for CMV. In cytotoxicity assays, cytotoxic T lymphocytes from 10 of 12 cocultures specifically lysed autologous CMV-infected fibroblasts or DCs but not HLA-mismatched or uninfected target cells, and this activity was shown to be blocked by HLA class 1 blocking antibodies. T-cell–receptor spectratyping of cells from the cultures typically showed complex size-distribution patterns, with all size classes of a normal preculture distribution present. However, a few size-class peaks were expanded compared with the preculture patterns and these may have represented expansions of CMV-specific T-cell clones. Advantages of this culture system are that it requires no live virions and no detailed knowledge of the antigenic peptides involved and it is applicable to CMV-seropositive donors of any HLA type.
Introduction
Cytomegalovirus (CMV) reactivation with progression to disease is a major cause of morbidity and mortality in immunocompromised recipients of bone marrow transplants.1,2 Substantial progress has been made in the early diagnosis and treatment of CMV infection and the prevention of CMV disease in such patients.3 Although pre-emptive therapy with ganciclovir reduces the incidence of CMV disease early after transplantation, it may be complicated by severe neutropenia and the onset of late (more than 100 days after transplantation) CMV disease. In spite of these advances, pretransplantation CMV seropositivity and posttransplantation CMV infection are associated with poorer survival after allogeneic transplantation, particularly in recipients of grafts from unrelated donors. Therefore, the development of alternative strategies for prophylaxis and treatment of CMV disease is warranted.
CMV infections occur with great frequency in recipients of marrow transplants because of the severe and prolonged cellular immunodeficiency that occurs in these patients.4 Recovery of CD8+ CMV-specific cytotoxic T cells (CTLs) in the early posttransplantation period abrogates the development of CMV-related disease. In 65% of patients, however, CMV-specific CTLs do not develop during that period, making them at high risk of CMV-related disease.4 CMV-specific CD8+ T cells can be cloned and expanded from the blood of patients seropositive for CMV and adoptively transferred safely to patients without development of graft-versus-host disease (GVHD) and with persistence of adoptive immunity for up to 4 weeks.5
Demonstration that virus-specific T cells might have clinical applications raises several critical issues for further investigation, including questions about practicality and safety. For adoptive immunotherapy for CMV to be widely applicable, rapid and efficient production methods and ready access to the appropriate virus-specific effector cells are required. Several problems must be solved before these criteria can be met. First, many culture systems that have been employed to generate CMV-specific T cells use live CMV. Therefore, there is a risk of viral transmission when the cultured T cells are given to transplant recipients. Second, patients who have received CMV-specific T cells have been given large numbers (up to 1 × 109/m2 of body surface area) of CD8+ cells at regular intervals to maintain in vivo anti-CMV responses.6 This requires several weeks of cell culture and large-scale cloning, which are both time consuming and costly.
The aim of this study was to assess the feasibility of using dendritic cells (DCs) to generate T-cell responses to CMV. DCs are the most potent of the antigen-presenting cells (APCs),7,8 and their role in resistance against experimental malignancies and infections is well documented.9-12 DCs can be generated from bone marrow, cord blood, and peripheral blood. The use of DCs to stimulate CMV-specific T-cell responses in the absence of live CMV may have advantages, with respect to safety, over currently used methods to expand CMV-specific T cells in vitro. We found that antigen-pulsed DCs can be strong stimulators of CMV-specific T-cell responses in vitro and that these T cells have both proliferative and cytotoxic activity.
Materials and methods
Generation of monocyte-derived DCs
Peripheral blood mononuclear cells from donors seropositive for CMV were isolated from heparin-treated blood by means of gradient centrifugation through Ficoll-Paque (Pharmacia, St Albans, United Kingdom [UK]). Monocytes were allowed to adhere in tissue-culture flasks for 2 to 3 hours. The nonadherent peripheral blood lymphocytes (PBLs) were removed and the monocytes were differentiated into DCs in X Vivo 20 medium (Biowhittaker, Wokingham, UK) supplemented with 10% autologous human serum (HS), 100 ng/mL interleukin (IL) 4 (R&D, Abingdon, UK), and 100 ng/mL granulocyte-macrophage colony-stimulating factor for 7 days in a 37°C 5% carbon dioxide humidified incubator.
Testing for CMV infectivity
The CMV antigen (Dade Behring, Marburg, Germany) was produced from human lung fibroblast (FB) cell cultures infected with human CMV (Towne strain) and lyophilized after inactivation with β-propiolactone and the addition of stabilizer. The antigen was screened for CMV infectivity with both conventional virus culture and rapid centrifugation culture with detection of CMV-specific immediate-early antigen fluorescent foci (DEAFF testing). For virus culture, antigen was inoculated onto both human embryonic lung and donor bone marrow–derived FB monolayers that were cultured under routine conditions for 21 days and visually inspected daily for characteristic cytopathic effects. DEAFF testing was performed after rapid centrifugation culture on a human embryonic lung FB monolayer by means of consecutive incubation with a murine monoclonal antibody against CMV early antigen (NEN Life Science Products, Hounslow, UK), a biotinylated antimouse IgG antibody (sheep; Amersham International, Amersham, UK), and streptavidin fluorescein (Amersham International). Each incubation was done at 37°C for 20 minutes, with careful rinsing using phosphate-buffered saline (PBS) between incubations and a final washing step in distilled water. The FB monolayers were examined with an ultraviolet microscope.
Proliferation assays
Cocultures of 10 × 103 DCs and 100 to 200 × 103 PBLs/well were set up in triplicate in 96-well plates with either 1 mg/mL CMV antigen (Dade Behring), 1 mg/mL control antigen (Dade Behring), or no antigen in 200-μL volumes of X Vivo 20 medium with 10% autologous HS. On day 5 of culture, 0.037 MBq (1 μCi) tritium-thymidine (Amersham) was added to each well. After approximately 16 hours, the plates were harvested on a filtermat in an automated harvester. A scintillant sheet (Meltilex; Wallac, Turku, Finland) was melted on the filtermat and the amount of tritium-thymidine incorporation was detected in a scintillation counter (Wallac).
Cocultures of PBLs
Autologous PBLs and monocyte-derived DCs from CMV-seropositive donors were cocultured for 14 to 21 days in X Vivo 20 medium with 10% autologous HS and 1 mg/mL CMV antigen in tissue-culture flasks. Half of the medium was replaced with fresh medium when required. On day 7, the cocultures were restimulated with more autologous DCs and 0.5 mg/mL CMV antigen. Beginning on day 10, 20 U/mL IL-2 (Sigma, Poole, UK) was added to the cultures every 2 days to further stimulate T-cell proliferation. In cultures maintained for longer than 14 days, there was another restimulation with autologous DCs pulsed with CMV antigen on day 14. Cocultures of cells from 6 CMV-seronegative donors were prepared in the same way as those from CMV-seropositive donors. To provide an additional maturation stimulus, coculturing of cells from the CMV-seronegative donors was also done with the addition of 200 IU/mL tumor necrosis factor α (TNF-α; Insight Biotechnology, Middlesex, UK) 24 or 48 hours after CMV-antigen pulsing of the DCs.
Flow cytometric analysis of T-cell subsets
Lymphocytes from 14-day cocultures were dual stained with fluorescein isothiocyanate–conjugated (FITC) anti-CD4 monoclonal antibodies (mAb; Dako, Ely, UK) plus phosphatidylethanolamine (PE)-conjugated anti-CD8 mAb (Dako) and with FITC-conjugated anti-CD45 (Dako) mAb plus PE Cy5-conjugated anti-CD3 mAb (Dako) for 30 minutes on ice. After washing, the cells were resuspended in PBS with 2% formaldehyde. The samples were analyzed in a flow cytometer (Beckman Coulter, High Wycombe, UK).
Cytotoxicity assays
PBLs from the 14- to 21-day cocultures were used as effector cells in lactate dehydrogenase (LDH)–release cytotoxicity assays (Cytotox96 kit; Promega, Southampton, UK). One of 3 different types of target cells was used: autologous CMV-infected FBs, autologous CMV-infected DCs, and autologous CMV-antigen–pulsed DCs. Target FBs were infected with CMV strain AD169 in a small volume of RPMI 1640 medium (Sigma) for 3 hours on the day before the assay. Control FBs were mock infected by using the same volume of medium. DCs were infected in the same way by using a clinically isolated endotheliotropic strain of CMV. For the antigen-pulsed DCs, 1 mg/mL CMV antigen was added to DCs on the day before the assay. Autologous DCs, either unpulsed or pulsed with control antigen (1 mg/mL), were used as negative controls for the CMV-antigen–pulsed DCs. Additional controls used were allogeneic HLA-mismatched; infected or antigen-pulsed target cells and uninfected or unpulsed target cells. Interferon γ (50 U/mL; Sigma) was added to all target cells and control target cells to promote HLA class 1 expression.
Cytotoxicity assays (done in triplicate) were set up according to the manufacturer's instructions in round-bottomed 96-well plates in volumes of 200 μL RPMI 1640 medium without phenol red (Sigma), supplemented with 5% CMV-seronegative HS. There were 3000 target cells per well, effector cells at various effector-to-target (E:T) ratios, and the appropriate controls, ie, target cells alone with (target total release) and without (target spontaneous release) lysis solution and effector cells alone (effector spontaneous release). The plates were incubated for 4 hours at 37°C. Then, 50 μL of supernatant was transferred to flat-bottomed 96-well plates, 50 μL of a chromogenic substrate for LDH was added, and the plates were incubated for 20 minutes in the dark for color development. Subsequently, 50 μL of stop solution was added and the plates were read in an enzyme-linked immunosorbent assay plate reader. The percentage of target-cell lysis was calculated by using the following formula: [(experimental LDH release) − (target spontaneous LDH release) − (effector spontaneous LDH release) × 100] / [(target total LDH release) − (target spontaneous LDH release)].
HLA class 1 blocking experiments
Target cells for cytotoxicity assays were incubated with 20 μL of a murine antihuman HLA class 1 antibody (W6/32, which recognizes a monomorphic epitope on the 45-kd polypeptide products of the HLA-A, HLA-B, and HLA-C loci; Dako) for 30 minutes at room temperature. Cytotoxicity assays were then done in triplicate as described earlier.
T-cell receptor CDR3 spectratyping
RNA was extracted from preculture and postculture PBLs by using Ultraspec RNA (BiotecX Laboratories, Houston, TX) according to the manufacturer's instructions. Complementary DNA (cDNA) was generated from 1 μg RNA in a 20-μL reaction using random hexanucleotide primers for reverse transcription with reverse transcriptase (Superscript; Gibco BRL, Paisley, UK). Each of 22 functionally rearranged BV gene subfamilies was amplified across the constant-variable junctions by using the 24 BV-subfamily–specific primers described previously by Maslanka et al,13 as well as a fluorescent dye–conjugated (FAM; Perkin Elmer, Cambridge, UK) BC-region–specific primer. Some of the BV primers amplify short polymerase chain reaction (PCR) products and others amplify longer products. Short and long BV primers were combined in duplex PCRs as follows: BV 5.1 plus 1, BV2 plus 12, BV8 plus 3, BV4 plus 5.4, BV13 plus 7, BV9 plus 14, BV11 plus 20, BV17 plus 15, BV16 plus 21, BV18 plus 23, and BV24 plus 22. BV6.1 and BV6.2 were used unpaired.
The total PCR volume (20 μL) contained Genamp PCR buffer (Perkin Elmer), 2 mM magnesium chloride, 0.2 mM of each deoxyribonucleoside triphosphate, 1 mM of each primer, and 1 μL cDNA (equivalent to approximately 25 000 cells). For the hot start, 0.5 U Amplitaq DNA polymerase (Perkin Elmer) was added after a 5-minute denaturation step at 95°C. Optimal cycling conditions were 95°C for 30 seconds, 58°C for 30 seconds, and 72°C for 45 seconds for 30 cycles, followed by a final extension at 72°C for 5 minutes. PCR product (1 μL) was denatured in 12 μL formamide and electrophoresed through Performance Optimized Polymer 4 (Perkin Elmer) on an ABI 110 automated sequencer (Perkin Elmer) in the presence of a Tamra 500 size standard (Perkin Elmer). Genescan 2.1 software (Perkin Elmer) was used to analyze the data.
Statistical methods
The Wilcoxon signed rank test for paired nonparametric data was used to compare the results of proliferation assays using cultures with CMV antigen, control antigen, or no antigen.
Results
Culture growth and phenotypic analysis
PBLs and autologous DCs from donors seropositive for CMV were cocultured for 2 to 3 weeks in the presence of CMV antigen. On day 4 to day 9 of culture, the lymphocytes proliferated rapidly and fresh medium was usually required every 1 to 2 days. During this time, lymphocytes accumulated in clusters around the DCs and the number of DCs in the culture decreased over time. Therefore, the cultures were restimulated with more antigen-pulsed DCs on day 7. After day 9, lymphocyte proliferation slowed down considerably, with an increase in the number of dead cells demonstrated by fluorescence-activated cell-sorter analysis (data not shown). Beginning on day 10, IL-2 was added in an attempt to selectively stimulate growth of CMV-activated lymphocytes. At the end of the culture period, there was a median 2.1-fold (0.3-fold to 4.0-fold) expansion in the numbers of T cells, with a median total T-cell number of 1 × 107 cells.
Phenotypic analysis of the nonadherent cells at the end of the coculture period using fluorescently labeled monoclonal antibodies revealed that most cells expressed CD45 and CD3. All cultures contained both CD4- and CD8-expressing T cells. The proportion of CD8-expressing cells varied greatly among donors, ranging from 5% to 67% at time points up to 3 weeks of coculture (Table1) and from 5% to 39% (median, 14%) at 2 weeks.
Antigen-specific proliferation
Proliferation assays were done on day 6 of culture to measure T-cell proliferation in response to CMV antigen presented by autologous DCs. The CMV antigen used was a crude preparation from a CMV-infected human lung FB cell line. Attempts to culture virus from this inactivated preparation using conventional culture techniques, rapid centrifugation cultures, and DEAFF testing failed to demonstrate any evidence of CMV infectivity. Dose-response curves for CMV-antigen concentration in relation to tritium-thymidine incorporation showed an optimal antigen concentration of 1 mg/mL (Figure1). DCs stimulated more T-cell proliferation than did their precursor monocytes when used as APCs (Figure 1A). To determine the extent to which lymphocyte proliferation was stimulated by CMV antigen rather than antigens derived from the human cell line, a control antigen preparation derived from the human lung FB cell line and not infected with CMV was used (Figure 1B and Figure 2). In 15 donor cultures studied, T-cell proliferation in response to the control antigen was significantly less than that in response to the CMV antigen (P = .0007, Wilcoxon signed rank test), but it was often slightly greater than proliferation without any antigen (P = .01, Wilcoxon signed rank test).
Dose-response curves for CMV and control antigen in cocultures, measured with proliferation assays.
Tritium-thymidine–incorporation assays done on day 6 of coculture showed an optimal CMV-antigen concentration of 1 mg/mL with DCs as APCs (⧫). (A) Autologous monocytes pulsed with CMV antigen stimulated less proliferation when used in the same manner as APCs (●). (B) Control antigen produced minimal stimulation of proliferation with DCs as APCs (▴).
Dose-response curves for CMV and control antigen in cocultures, measured with proliferation assays.
Tritium-thymidine–incorporation assays done on day 6 of coculture showed an optimal CMV-antigen concentration of 1 mg/mL with DCs as APCs (⧫). (A) Autologous monocytes pulsed with CMV antigen stimulated less proliferation when used in the same manner as APCs (●). (B) Control antigen produced minimal stimulation of proliferation with DCs as APCs (▴).
Proliferation as measured by tritium-thymidine–incorporation assays.
Proliferation assay results for samples from 15 donors, with assays done on cultures stimulated with DCs pulsed with the CMV antigen (1 mg/mL), control antigen (1 mg/mL), or no antigen. The results for each donor under the 3 different culture conditions are shown linked. In each case, there was significantly more proliferation with the CMV antigen than with the control antigen derived from the human cell line used to produce the CMV antigen (P = .0007, Wilcoxon signed rank test).
Proliferation as measured by tritium-thymidine–incorporation assays.
Proliferation assay results for samples from 15 donors, with assays done on cultures stimulated with DCs pulsed with the CMV antigen (1 mg/mL), control antigen (1 mg/mL), or no antigen. The results for each donor under the 3 different culture conditions are shown linked. In each case, there was significantly more proliferation with the CMV antigen than with the control antigen derived from the human cell line used to produce the CMV antigen (P = .0007, Wilcoxon signed rank test).
Cocultures of cells derived from 6 CMV-seronegative donors did not show significant proliferation, despite prolongation of the culture period for up to 6 weeks and continued weekly restimulation with autologous CMV-antigen–pulsed DCs. Similarly, the use of TNF-α to induce DC maturation and up-regulation of costimulatory molecules did not result in lymphocyte proliferation.
Cell-mediated cytotoxicity
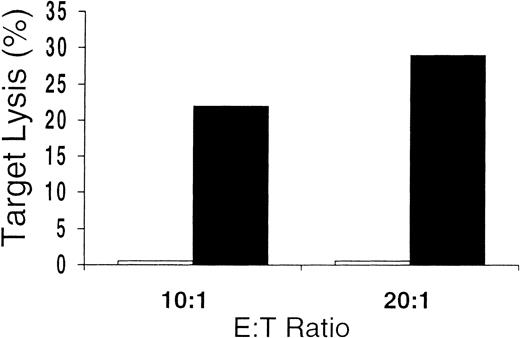
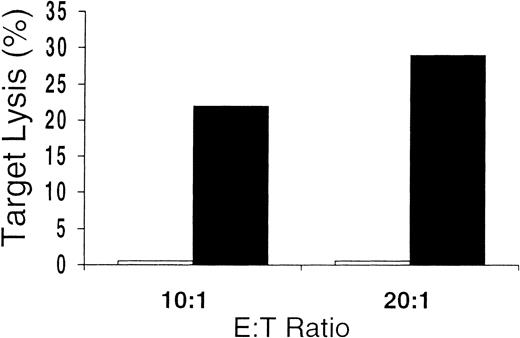
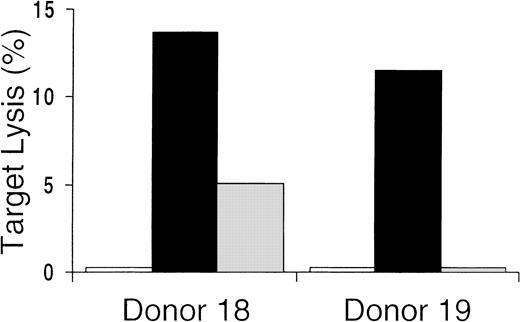
Unseparated mononuclear cells obtained from the culture were used as effectors in cytotoxicity assays. Killing of CMV-infected cells by CD8+ CTLs is thought to play a major role in the immunologic control of CMV infection in vivo. We found that 10 of 12 in vitro cultures from CMV-seropositive donors tested showed CMV-specific cytotoxicity against CMV-infected FBs or DCs or antigen-pulsed DCs (Table 1). At various E:T ratios, there was substantial lysis of autologous CMV-infected or pulsed targets but very little or no killing of uninfected or unpulsed autologous targets or allogeneic HLA-mismatched targets. Control-antigen–pulsed autologous DCs were also not lysed (Figure 3). These data indicate that, in general, the culture conditions promoted the development of CMV-specific cytotoxicity. In addition, killing was HLA restricted, since only autologous—not allogeneic—HLA-mismatched target cells were lysed. The presence of class 1 dependence was also indicated by the abrogation of lysis shown by preincubating targets with HLA class 1 blocking antibodies (Figure4). These data also suggest that any contribution of CD4-expressing cells to target-cell lysis was minimal, since this would probably have been mediated by HLA class 2 receptor interactions. Because only a minority of the culture-output cells expressed CD8, the CD8 ratios of CTL to target were much lower than is indicated in Table 1 and Figures 3 and 4.
Cytolytic assay with CMV-antigen–pulsed and control-antigen–pulsed autologous DC targets.
Antigen specificity of the culture-output cells demonstrated by failure to lyse autologous DCs pulsed with control antigen (■), even in cases showing the greatest amounts of lysis of autologous DCs pulsed with CMV antigen (▪).
Cytolytic assay with CMV-antigen–pulsed and control-antigen–pulsed autologous DC targets.
Antigen specificity of the culture-output cells demonstrated by failure to lyse autologous DCs pulsed with control antigen (■), even in cases showing the greatest amounts of lysis of autologous DCs pulsed with CMV antigen (▪).
Cytolytic assays with CMV-antigen–pulsed autologous DC targets preincubated with HLA class 1 blocking antibody.
HLA restriction of the cytotoxic activity of the culture-output cells demonstrated by prevention of lysis of autologous CMV-antigen–pulsed targets preincubated with anti-HLA class 1 antibody (■) compared with preincubation with a control anti-CD19 antibody (▪). ░ indicates autologous unpulsed controls.
Cytolytic assays with CMV-antigen–pulsed autologous DC targets preincubated with HLA class 1 blocking antibody.
HLA restriction of the cytotoxic activity of the culture-output cells demonstrated by prevention of lysis of autologous CMV-antigen–pulsed targets preincubated with anti-HLA class 1 antibody (■) compared with preincubation with a control anti-CD19 antibody (▪). ░ indicates autologous unpulsed controls.
T-cell receptor CDR3 spectratyping
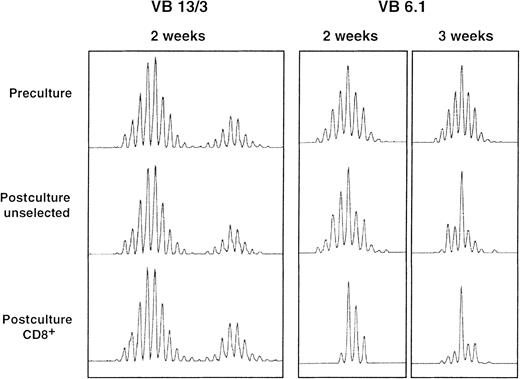
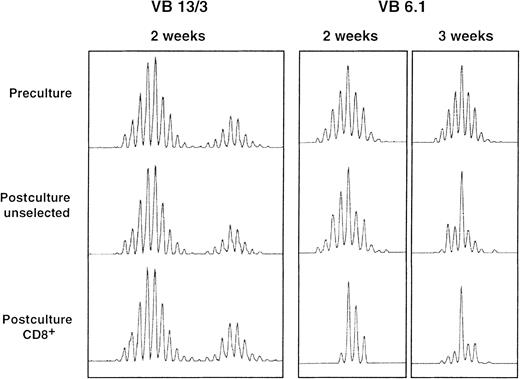
A comparison of preculture and postculture T-cell receptor (TCR) CDR3 spectratypes showed that postculture T-cell repertoires were still polyclonal, ie, most BV spectratypes had a complete set of size peaks. Although most postculture spectratypes were similar to the preculture spectratypes, some BV spectratypes contained one or more predominant size peaks after culture. These differences were more pronounced when analysis was restricted to the CD8+ subset of cells and when the culture period was prolonged to 21 days (Figure5). Also, they were more common in the BV13, BV8, and BV6.1/6.2 gene subfamilies.
TCR BV spectratyping results for 3 BV gene family members from a single patient.
Full BV spectratypes were generated for preculture, postculture unselected, and postculture CD8+ selected samples. In most cases, postculture spectratype appearances were similar to preculture appearances, as is shown for BV13 and BV3 (in duplex PCR reactions). Some spectratypes had one or more dominant size-class peaks after culture and this was often more pronounced after selection for cells bearing CD8 and with increasing culture duration, as is shown for BV6.1 after 2 and 3 weeks of culture.
TCR BV spectratyping results for 3 BV gene family members from a single patient.
Full BV spectratypes were generated for preculture, postculture unselected, and postculture CD8+ selected samples. In most cases, postculture spectratype appearances were similar to preculture appearances, as is shown for BV13 and BV3 (in duplex PCR reactions). Some spectratypes had one or more dominant size-class peaks after culture and this was often more pronounced after selection for cells bearing CD8 and with increasing culture duration, as is shown for BV6.1 after 2 and 3 weeks of culture.
Discussion
In recent years, there has been a decrease in morbidity and mortality due to CMV after allogeneic transplantation.3,14-16 The initial reason for this was the development of newer and more effective pharmacologic antiviral agents, such as ganciclovir and foscarnet.17-19 However, although early randomized studies in which ganciclovir was used to provide antiviral prophylaxis did show a reduction in CMV-associated mortality, there was no overall survival advantage in the group given the agent.20,21 This was attributed to ganciclovir-related myelosuppression and an increase in associated bacterial and fungal infections. The development of more sensitive, rapid, and reliable surveillance techniques using CMV PCR analysis or assessments of antigenemia in combination with pre-emptive use of antiviral drugs has reduced unnecessary exposure of some patients to these side effects.22,23 However, because of the high rates of CMV reactivation when either the donor or the recipient is seropositive for CMV, ganciclovir-related cytopenia, recurrent infection, and late CMV disease continue to complicate the pre-emptive approaches.22-26 The true positive predictive value of tests for the development of CMV disease is unclear, but it is estimated that perhaps only 50% of episodes of detected CMV would have led to CMV-related disease27-30 and that pre-emptive therapy results in overtreatment of many patients. The limitations of antiviral pharmacotherapy have promoted an interest in adoptive immunotherapy as an alternative means for preventing and treating CMV disease in patients given allogeneic transplants.
The potential efficacy of adoptive immunotherapy has been demonstrated by the complete regression of immunoblastic lymphoma in allograft recipients after the infusion of Epstein-Barr virus (EBV)–specific CTLs.31,32 A similar proof of principle for the prevention of CMV disease was provided by the studies of Riddell and colleagues.5,6,33,34 In these studies, CMV-specific CD8+ CTLs used for infusions into patients were cloned from bulk cultures to minimize the risk of alloreactivity.35CMV-specific T-cell clones were adoptively transferred to recipients of allografts and resulted in restoration of CMV-specific cellular immunity and prevention of CMV infection in patients at risk of CMV disease. Although this process virtually eliminates the risk that the adoptively transferred cells will cause GVHD, it is time consuming and requires substantial logistical support. These practical difficulties may partly account for why these studies have not led to the widespread use of adoptive immunotherapy for preventing and treating CMV infection. In addition, they confirmed the requirement for CD4+ T-cell–helper function to restore longer-term immune memory, as was suggested by earlier work in murine models.6 36
Conventional methods for generating CMV-specific T cells use a culture technique in which the APCs are autologous skin FBs infected with live CMV.35 Thus, there has been concern about the potential for viral reinfection concurrent with adoptive cellular therapy. Regulatory and safety issues also complicate the use of fetal-calf serum (FCS) in cultures destined for transfusion to patients. Culture techniques that avoid these risks are being developed on the basis of an increasing understanding of the principles governing presentation of peptides by HLA class 1 molecules and recognition of HLA-peptide complexes by CTLs by means of the TCR.37-39 The human CTL response to CMV is dominated by structural protein pp65, which is targeted by 70% to 90% of CMV-specific CTLs.40,41 Other immunogenic peptides that account for a smaller part of the overall response include elements of the major immediate-early gene product (IE-1), the matrix protein pp150, and virion envelope glycoprotein B.42,43 The immunodominant nonapeptides from the pp65 matrix glycoprotein that are restricted to specific HLA molecules are being identified,41 and these can be used to load class 1 molecules of transformed lymphoblastoid cell lines for the generation of CMV-specific CD8+ CTL in the absence of live virus. However, this technique does not stimulate CD4+ T cells and is limited to patients with HLA types for which the immunodominant nonamer is delineated.
An elegant method of generating CMV and EBV-specific T cells in a single culture was recently described.44 This involves the transduction of lymphoblastoid cell lines with a recombinant retrovirus encoding pp65. Endogenous intracellular synthesis and processing of viral proteins avoids the necessity of knowing the presented peptide sequences. However, the technique is time consuming and its dependence on the use of recombinant retrovirus is likely to limit the number of centers that could employ this innovative approach.
The use of monocyte-derived DCs as APCs and as target cells in our study circumvented the problem of procuring skin biopsy specimens from the donors and the time required to grow the FB monolayers. In addition, the use of donor autologous serum eliminated the need to use FCS in the culture. Furthermore, the absence of live virions in the culture minimizes the risk of CMV infection due to the transfer of the CMV-specific T cells. Indeed, the CMV antigen failed to show evidence of a cytopathic effect in FB cultures and the results of CMV DEAFF testing were negative. DCs have the unique ability to process exogenously supplied antigen efficiently and present peptides on both class 1 and class 2 HLA molecules along with an array of costimulatory molecules.8 45 The presentation of both helper and CTL-defined epitopes means that both CD4+ and CD8+ CMV-specific T cells will be generated. This allows both the generation of cytolytic effector function and the potential for re-establishment of longer-term immune memory, which may be important in preventing subsequent viral reactivation.
The lack of an absolute need to know the presented peptides means that our technique can be used for patients of any HLA type and will trigger T-cell reactivity to undefined immunogenic determinants, thereby allowing a greater potential for augmentation of a broader T-cell response. This will reduce the possibility that selective pressure will be applied to CMV in vivo. It may be particularly important with the emergence of more evidence that other targets, such as IE-1, may play a more important role in CMV-directed immune responses than previously recognized.46 Use of our technique can also be extended to other infective agents for which the immunodominant peptides are not yet known. Indeed, further study of culture-output cells may help to delineate other specific features of the immune responses to various pathogens. Most important, the short time required to generate the CMV-specific T-cell lines, together with a relatively simple method, should allow widespread use of adoptive immunotherapy for CMV.
Some of the polyclonal cell lines expanded in the DC cocultures showed relatively small amounts of lysis during cytotoxicity assays. However, it should be noted that in many of the cases in which the percentage of lysis was under 20% and in which CD8+ cell numbers were enumerated, CD8+ cells accounted for less than 20% of the total lymphocyte count. Therefore, the E:T ratios were clearly not comparable to those observed with clonal CTL cultures in cytotoxicity assays. Even if all the CD8+ cells were CMV-specific CTLs, the effective maximal E:T ratios would be about 2:1 to 4:1, and it is probable that the proportion of CMV-specific CTLs was far smaller than this. Whether the enrichment for CMV-specific T cells, including CD4+ T-helper cells, will allow more profound in vivo expansions to enable clinical efficacy while avoiding GVHD is currently unclear and must be addressed in clinical studies.
Despite the complex postculture spectratypic appearances, the T cells generated from most donors in this study did not lyse uninfected and allogeneic targets, thereby suggesting that the time-consuming process of T-cell cloning may not be necessary. However, the presence of potentially alloreactive size-class peaks and the low positive predictive value of most in vitro tests for subsequent development of GVHD47 48 necessitate initial dose-escalation clinical studies with the aim of dissociating an antiviral effect from a graft-versus-host effect.
So far, all the CMV-specific T-cell lines generated with our culture system have been from seropositive rather than seronegative individuals. This limitation has been mentioned by others who used alternative APCs.44 49 However, the proposed central role of DCs in the generation of the primary immune response in vivo should make these cells the ideal candidate for this in vitro task. Using culture conditions identical to those used for the seropositive donors, we found no significant proliferation of lymphocytes for up to 6 weeks of culture. Additional maturation stimuli may be required to increase the efficacy of antigen presentation and up-regulate costimulatory signals to generate a primary immune response with this culture system. Therefore, we are currently attempting to identify suitable stimuli that will allow more widespread application in high-risk CMV-seropositive patients with seronegative donors.
Supported by the Leukaemia Research Fund, London, United Kingdom.
K.P. and S.V. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Stephen Mackinnon, Department of Haematology, University College Hospital, 98 Chenies Mews, London WC1E 6HX, United Kingdom; e-mail: s.mackinnon@ucl.ac.uk.