Aggregation of the high-affinity IgE receptor induces the tyrosine phosphorylation of subunits of the receptor and the subsequent association with the receptor of the cytosolic protein tyrosine kinase Syk. The current experiments examined the functional importance of membrane association of Syk and the role of the SH2 domain in receptor-mediated signal transduction. Wild-type Syk and chimeric Syk molecules with the c-Src myristylation sequence at the amino-terminus were expressed in a Syk-negative mast cell line. Chimeric Syk with the myristylation sequence was membrane associated, and a small fraction was constitutively colocalized with FcεRI, Lyn, and LAT (linker for T-cell activation) in the glycolipid-enriched microdomains or rafts. However, even under these conditions, the tyrosine phosphorylation of Syk and the downstream propagation of signals required FcεRI aggregation. This chimeric Syk was less active than wild-type Syk in FcεRI-mediated signal transduction. In contrast, a truncated membrane-associated form of Syk that lacked the SH2 domains was not tyrosine phosphorylated by receptor aggregation and failed to transduce intracellular signals. These findings suggest that SH2 domain–mediated membrane translocation of Syk is essential for the FcεRI-mediated activation of Syk for downstream signaling events leading to histamine release. Furthermore, the localization of Syk in glycolipid-enriched microdomains by itself is not enough to generate or enhance signaling events.
Introduction
Aggregation of high-affinity IgE receptors (FcεRI) on mast cells results in biochemical events that eventually lead to the release of histamine. The earliest detectable intracellular change is the phosphorylation of proteins on tyrosine residues.1 Because FcεRI lacks enzymatic activity, cytosolic protein tyrosine kinases (PTKs) are essential for these receptor-induced phosphorylations. The cytoplasmic PTK Syk is essential for FcεRI-mediated signaling in mast cells.2,3 Thus, the expression of Syk reconstitutes FcεRI-mediated tyrosine phosphorylation of phospholipase C-γ (PLC-γ), calcium mobilization, and histamine release in a Syk-negative variant of the RBL-2H3 cells.2 Similarly, mast cells derived from Syk−/− embryos fail to degranulate or to synthesize or release leukotrienes and cytokines after FcεRI aggregation.3 Further analysis of cells from these Syk−/− mice demonstrates that Syk is also essential for signaling from other immune receptors, such as Fcγ receptor, T-cell receptor, and B-cell receptor.4-6
Intracellular signaling depends on protein–protein interactions, one example of which is the binding of molecules by their SH2 (Src homology 2 region) domains to specific phosphotyrosine sequences. For example, the aggregation of FcεRI results in SH2 domain–mediated binding of the cytoplasmic protein tyrosine kinase Syk to the phosphorylated tyrosines in the immunoreceptor tyrosine-based activating motif (ITAM) of FcεRIγ.7-9 In B cells, mutational studies indicate that both SH2 domains of Syk are required for B-cell receptor–mediated tyrosine phosphorylation of Syk and PLC-γ, the generation of inositol 1,4,5 triphosphate, and calcium mobilization.10 Structural studies reveal that the N-terminal SH2 domain of Syk or ZAP-70, the other member of this family of protein tyrosine kinases, binds to the C-terminal pYxxL of the ITAM, whereas the C-terminal SH2 domain binds to the N-terminal pYxxL.11,12 Antibody-mediated aggregation of a chimeric transmembrane protein that has the intracellular domain of FcεRIγ results in tyrosine phosphorylation of Syk and its activation.13 Similarly, antibody-mediated clustering of a chimeric transmembrane protein fused with Syk causes tyrosine phosphorylation of PLC-γ, leukotriene synthesis, degranulation, and expression of cytokine genes.14 In vitro, the binding of Syk to diphosphorylated peptides based on the ITAM of γ or β subunits of FcεRI results in a conformational change and increase in its kinase activity, suggesting the functional importance of the SH2 domain–mediated association of Syk with FcεRI.15,16 By fluorescent microscopy, ZAP-70 is diffusely located throughout the cell but accumulates at the plasma membrane after cellular activation.17 In RBL-2H3 mast cells, FcεRI aggregation results in the translocation of green fluorescent protein-tagged tandem SH2 domains of Syk from the cytosol to the plasma membrane and to the detergent-insoluble microdomains.18 Therefore, these findings suggest that the FcεRIγ–ITAM-mediated recruitment of Syk to the membrane and its activation are essential for downstream signal transduction.
Recent studies suggest that the plasma membrane has microdomains or rafts that are enriched in sphingolipid, cholesterol, and glycosylphosphatidylinositol-anchored proteins.19-21 These glycolipid-enriched microdomains (GEMs) are isolated in the low-density fraction of the Triton-X insoluble cell extract. Various signaling molecules, including the protein tyrosine kinase Lyn, are present in GEMs, suggesting that these rafts may play an important role in signal transduction.22-24 In RBL-2H3 cells, the interaction of cross-linked FcεRI with other molecules in the detergent-resistant rafts is thought to play a role in receptor-mediated signaling.25,26 Similarly, in T cells, the palmitoylation of LAT is required for its localization in GEMs and for the tyrosine phosphorylation of downstream molecules.27 These studies suggest that GEMs may play a critical role in signal transduction from immune receptors.
The current experiments examined the functional importance of membrane association of Syk and the role of the SH2 domain in receptor-mediated signal transduction. Syk, when expressed as a chimeric myristylated molecule, was membrane associated and present in GEMs after transfection of these plasmids into a Syk-negative cell line. However, even under these conditions, the tyrosine phosphorylation of Syk and the downstream propagation of signals required FcεRI aggregation. Membrane-associated Syk that lacked the SH2 domains was not tyrosine phosphorylated by receptor aggregation and failed to transduce intracellular signals.
Materials and methods
Materials and antibodies
Triton X-100 and protein A–agarose beads were obtained from Sigma (St Louis, MO). Polyvinylidene difluoride transfer membrane was purchased from Millipore (Bedford, MA), and the enhanced chemiluminescence reagent, Renaissance, was from NEN Life Science (Boston, MA). Horseradish peroxidase–conjugated mouse antiphosphotyrosine 4G10 monoclonal antibody (mAb) and polyclonal anti-LAT antibody were obtained from Upstate Biotechnology (Lake Placid, NY). The mAb to residues 2-17 of v-Src (LA074), which recognized the myristylation sequence of c-Src, was purchased from Quality Biotech (Camden, NJ). All other antibodies used were previously described.2 28 Plasmid for the expression of the human cytoplasmic domain of erythrocyte band 3 protein (cdb3) was kindly provided by Dr P. S. Low (Purdue University, West Lafayette, IN).
Construction of cDNA
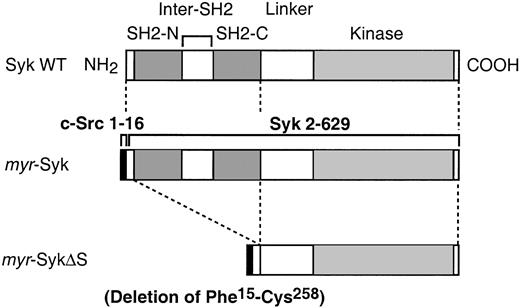
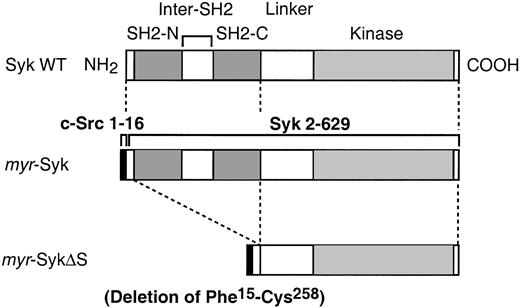
A polymerase chain reaction–based method29 30 was used to prepare a chimeric molecule with the myristylation signal of c-Src joined to Syk. The 16 amino acids of c-Src N-terminus region (c-Src tag: MGSNKSKPKDASQRRR) was fused to the second amino acid in the N-terminal of rat Syk (myr-Syk). Nine additional bases (GCG-AGA-AAT) were added before the initiation codon, which were from the sequence of rat Lyn. This myr-Syk was then used to prepare the dead kinase form by making a point mutation of K396 to R (myr-SykDK) and a truncated form with the deletion of both SH2 domains (Phe15-Cys258, which includes the 2 SH2 domains and the inter-SH2 domain, called myr-SykΔS). All mutations were confirmed by DNA sequencing. Mutant cDNA was then subcloned into the pSVL expression vector (Pharmacia LKB, Piscataway, NJ). The schematic diagram of the constructs used in these experiments is in Figure 1.
Schematic diagram of the mutants of Syk used in the current experiments.
Comparison of wild-type (WT) Syk and the membrane-tagged mutants. Bothmyr-Syk and myr-SykΔS are chimeric molecules of the myristylation signal from c-Src (AA 1-16) joined to the indicated portions of Syk. The myr-SykΔS lacks the 2 SH2 domains of Syk. These cDNAs were subcloned to the pSVL expression vector.
Schematic diagram of the mutants of Syk used in the current experiments.
Comparison of wild-type (WT) Syk and the membrane-tagged mutants. Bothmyr-Syk and myr-SykΔS are chimeric molecules of the myristylation signal from c-Src (AA 1-16) joined to the indicated portions of Syk. The myr-SykΔS lacks the 2 SH2 domains of Syk. These cDNAs were subcloned to the pSVL expression vector.
Cell culture and transfections
Rat basophilic leukemia RBL-2H3 cells and the B2 Syk-negative variant of RBL-2H3 cells have been characterized previously.2,28,31 Stable transfection of the B2 Syk-negative cells was performed as described previously.2Briefly, 20 μg linearized expression constructs and 2 μg pSV2-neo vector were cotransfected into the Syk-negative cells by electroporation, and clones were selected by 0.4 mg/mL active G418 (Life Technologies, Gaithersburg, MD). Cell lines were screened for the level of Syk expression by immunoblotting total lysates with anti-Syk antibody (which recognizes the linker region of Syk), using blotting with anti-FcεRIβ mAb as an internal control.7 Two clones transfected with each kind of cDNA that expressed Syk at a level similar to that in RBL-2H3 cells were selected for further analysis.
RBL-2H3, Syk-negative, and transfected cell lines were maintained as monolayer cultures in minimum essential medium (MEM) with 2 mM L-glutamine, 1% antibiotic-antimycotic (Life Technologies), and 15% heat-inactivated fetal bovine serum (Biofluids, Rockville, MD). Stable transfected cloned cell lines were maintained with 0.4 mg/mL active G418. For activation, the cells were cultured overnight with or without antigen-specific IgE. Cells were washed with release medium (MEM containing 0.1% bovine serum albumin and 10 mM Tris, pH 7.4) and then stimulated with the antigen dinitrophenyl-coupled human serum albumin in the same medium. After 45 minutes of incubation, the medium was removed for histamine analysis as described previously.32 33
Preparation of cell lysates
After stimulation, the monolayers were washed twice with ice-cold phosphate-buffered saline (PBS) containing 1 mM Na3VO4 and 0.1 mM phenylmethylsulfonyl fluoride (PMSF) and then solubilized on ice in 1% Triton buffer (1% Triton X-100, 50 mM Tris, pH 7.4, 150 mM NaCl, 10 mM EDTA, 100 mM NaF, 1 mM Na3VO4, 1 mM PMSF, 90 mU/mL aprotinin, 10 μg/mL leupeptin, and 5 μg/mL pepstatin A). Cell lysates were precleared by mixing with protein A–agarose beads then were incubated with the indicated primary antibody prebound to protein A–agarose. After rotation for 1 hour at 4°C, the beads were washed 4 times with lysis buffer, and the immunoprecipitated proteins were eluted by boiling for 5 minutes with 2 × sample buffer. For the preparation of total cell lysates, monolayers were rinsed as described above with PBS, and cells were lysed by the addition of 2 × sample buffer.
Subcellular fractionation
For the preparation of cytosolic and membrane fractions, cells were washed with ice-cold PBS and resuspended on ice in hypotonic buffer (42 mM KCl, 10 mM HEPES, pH 7.4, 5 mM MgCl2, and protease inhibitors). Cell homogenates were centrifuged (10 minutes at 200g), and the supernatants were centrifuged for 30 minutes at 100 000g.34 Supernatants of the second centrifugation were collected as the cytosolic fraction. The pellet was washed once with hypotonic buffer, then directly solubilized in sample buffer as the membrane fraction.
Detergent-insoluble GEMs were prepared by sucrose density gradient centrifugation essentially as described.27 Cells (107) were lysed on ice in 2.5 mL 0.05% Triton in MNEV buffer (150 mM NaCl, 25 mM MES, pH 6.5, 5 mM EDTA, 1 mM Na3VO4, and protease inhibitors) and dounced 30 times.25 Homogenates were cleared of intact cells and debris by centrifugation for 10 minutes at 200g. The resultant supernatants (2.4 mL) were mixed with equal volumes of 80% sucrose in MNEV buffer (final, 40% sucrose and 0.025% Triton), overlayered by 4.8 mL 30% and 2.4 mL 5% sucrose in MNEV buffer, and then centrifuged for 20 hours at 200 000g (SW41Ti rotor; Beckman). Ten fractions were collected from the top of the gradient. Fraction 3 at the 5%/30% sucrose interface contains detergent-insoluble GEMs, and fractions 7 to 10 contain detergent-soluble fractions.
Immunoprecipitation and immunoblotting
Total cell lysates, immunoprecipitated proteins, and subcellular fractions were separated by SDS-PAGE and electrotransferred to polyvinylidene difluoride membranes. After blocking of the membranes with 4% bovine serum albumin in TBST (10 mM Tris, pH 7.4, 150 mM NaCl, and 0.1% Tween 20), the blots were probed with individual primary antibodies, then incubated with horseradish peroxidase–conjugated secondary antibody or horseradish peroxidase–protein A in TBST. Proteins were visualized by the enhanced chemiluminescence reagent (Renaissance; NEN Life Science).
In vitro kinase assay
Washed anti-Syk immunoprecipitates from the different cell lines were incubated in kinase buffer (30 mM HEPES, pH 7.5, 10 mM MgCl2, 2 mM MnCl2, 4 μM adenosine triphosphate (ATP), 4 μCi [γ-32P] ATP, and 2.5 μg substrate cdb3) for 30 minutes at room temperature.28Radiolabeled proteins were separated by SDS-PAGE and visualized by autoradiography.
Results
Generation of stable cell lines expressing Syk with a membrane targeting sequence
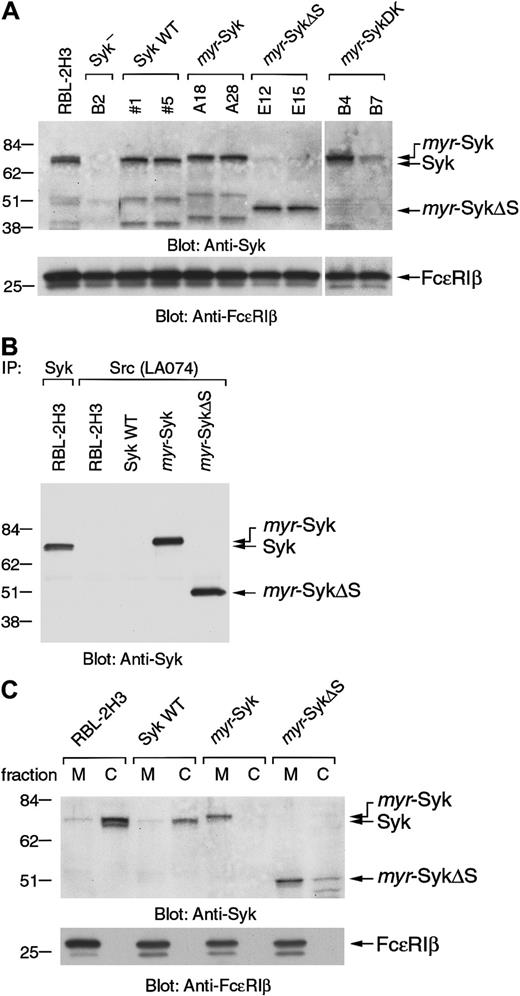
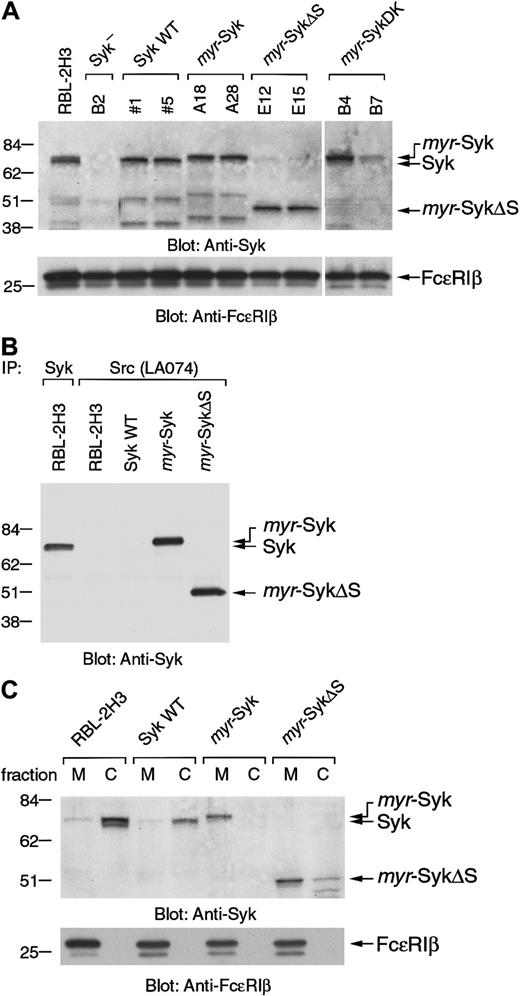
To investigate the role of receptor-induced membrane translocation of Syk in signal transduction, we generated stable cell lines expressing Syk mutants that constitutively localized in the plasma membrane. The myristylation of cytoplasmic proteins by the addition of 16 amino acids from the N-terminus of c-Src (c-Src tag) can direct molecules to the plasma membrane.29,30 The c-Src tag was added to the N-terminus of wild-type Syk (myr-Syk), its dead kinase (DK) mutant (myr-SykDK), and a truncated form of Syk (myr-SykΔS) that had the deletion of the 2 SH2 domains (Figure 1). Wild-type and mutant forms of Syk were stably transfected into the Syk-negative variant of the RBL-2H3 cells.2 31Cloned lines were then screened by immunoblotting with anti-Syk and anti-FcεRIβ antibodies. For further analysis, 2 cloned lines transfected with each cDNA were selected in which the level of expression of Syk was similar to that in the RBL-2H3 cells (Figure2A). The mAb to the c-Src tag (LA074) immunoprecipitated myr-Syk and myr-SykΔS that had been tagged with the 16 N-terminal amino acids from c-Src but did not precipitate wild-type Syk from RBL-2H3 or transfected cells (Figure2B). The c-Src–tagged Syk was localized in the membrane fraction, as shown by analysis of the subcellular fractions (Figure 2C). The small amount of myr-SykΔS in the cytosolic fraction could be attributed to degradation (Figure 2C, lane 8). In the following experiments, each of these lines was examined, though some figures present the results from only one representative cell line.
Generation of the stable cell lines expressing membrane-associated forms of Syk.
(A) The Syk-negative variant of the RBL-2H3 cells (B2 cells) was stably transfected with the various Syk cDNAs shown in Figure 1 and selected with G418. Two positive cloned lines expressing each of the different forms of Syk were selected for further analysis. Cell lysates from RBL-2H3, parental B2, and selected cloned lines were immunoblotted with anti-Syk and anti-FcεRIβ antibodies. Molecular size markers are indicated at the left in kilodaltons. (B) Cell lysates from RBL-2H3 cells and the transfected cell lines expressing wild-type Syk (clone 5), myr-Syk (A28), and myr-SykΔS (E15) were immunoprecipitated (IP) with either anti-Syk or anti-Src (LA074) antibody. Immunoprecipitated proteins were analyzed by immunoblotting with anti-Syk antibody. (C) Membrane (M) and cytosolic (C) fractions of RBL-2H3 cells and the transfected cell lines were analyzed by immunoblotting with anti-Syk and anti-FcεRIβ antibodies.
Generation of the stable cell lines expressing membrane-associated forms of Syk.
(A) The Syk-negative variant of the RBL-2H3 cells (B2 cells) was stably transfected with the various Syk cDNAs shown in Figure 1 and selected with G418. Two positive cloned lines expressing each of the different forms of Syk were selected for further analysis. Cell lysates from RBL-2H3, parental B2, and selected cloned lines were immunoblotted with anti-Syk and anti-FcεRIβ antibodies. Molecular size markers are indicated at the left in kilodaltons. (B) Cell lysates from RBL-2H3 cells and the transfected cell lines expressing wild-type Syk (clone 5), myr-Syk (A28), and myr-SykΔS (E15) were immunoprecipitated (IP) with either anti-Syk or anti-Src (LA074) antibody. Immunoprecipitated proteins were analyzed by immunoblotting with anti-Syk antibody. (C) Membrane (M) and cytosolic (C) fractions of RBL-2H3 cells and the transfected cell lines were analyzed by immunoblotting with anti-Syk and anti-FcεRIβ antibodies.
Targeting by the SH2 domains, but not localization of Syk in the plasma membrane, is critical for FcεRI-induced histamine release
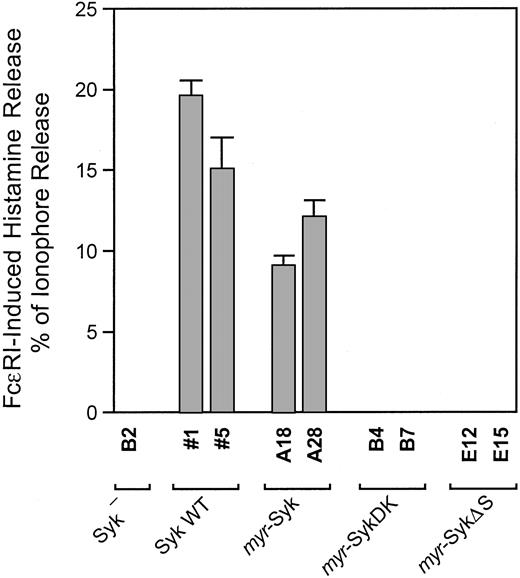
The FcεRI-mediated histamine release is reconstituted by the expression of wild-type Syk in the Syk-negative variant of the RBL-2H3 cells.2 To examine the role of Syk translocation to the plasma membrane in signal transduction, we compared the FcεRI-induced histamine release using the stable cloned lines shown in Figure 2A. The Syk-negative cells and the different transfected cell lines were stimulated either by FcεRI-aggregation or the calcium ionophore A23187 (Figure 3). Histamine release induced by the calcium ionophore was similar among all these clones and was, therefore, used as an internal control. As we previously reported, the lack of FcεRI-induced histamine release in the Syk-negative cells (B2) was reconstituted by the expression of wild-type Syk (clones 1, 5).35 Expression of myr-Syk in Syk-negative cells (clones A18 and A28) also reconstituted the FcεRI-mediated histamine release, though this release was significantly lower than that in cells transfected with wild-type Syk. Kinase activity was still required by the membrane-targeted Syk because the cells that expressedmyr-SykDK failed to release histamine (clones B4, B7). In contrast, there was no FcεRI-induced histamine release in the cells that expressed myr-SykΔS, the membrane-localized myristylated Syk that lacked the 2 SH2 domains (clones E12, E15). This defect in release did not result from the addition of the c-Src tag because the cells that expressed myr-Syk were capable of reconstituting antigen-induced histamine release. Thus, the membrane localization of Syk is not enough for signal transduction but still requires the SH2 domains. Furthermore, the function of the SH2 domains is not simply to recruit Syk to the plasma membrane by binding to the phosphorylated ITAM of FcεRI.
SH2 domains, but not membrane localization of Syk, are critical for FcεRI-induced histamine release.
Syk-negative cells and cells expressing the different forms of Syk were cultured overnight with antigen-specific IgE and then stimulated with antigen or with calcium ionophore A23187. Maximum antigen-induced histamine release is presented as a percentage of that with 1 μM A23187. Results are the mean values ± SE from 3 independent experiments. There was no antigen-induced release above background with the parental B2, myr-SykDK, and the myr-SykΔS cloned lines. The difference in antigen-induced release between the Syk WT and myr-Syk cell lines was statistically significant (.05 per t test). The A23187-induced average release as a percentage of the total cellular histamine content in the different cell lines was 67% (Syk-negative B2), 67% (Syk WT-1), 67% (Syk WT 5), 76% (myr-Syk A18), 78% (myr-Syk A28), 76% (myr-SykDK B4), 63% (myr-SykDK B7), 84% (myr-SykΔS E12), and 83% (myr-SykΔS E15). In nonstimulated cells incubated for 45 minutes, the histamine release as a percentage of the total cellular content was as follows: 4% (Syk-negative B2), 4% (Syk WT 1), 4% (Syk WT 5), 4% (myr-Syk A18), 3% (myr-Syk A28), 3% (myr-SykDK B4), 5% (myr-SykDK B7), 3% (myr-SykΔS E12), and 4% (myr-SykΔS E15).
SH2 domains, but not membrane localization of Syk, are critical for FcεRI-induced histamine release.
Syk-negative cells and cells expressing the different forms of Syk were cultured overnight with antigen-specific IgE and then stimulated with antigen or with calcium ionophore A23187. Maximum antigen-induced histamine release is presented as a percentage of that with 1 μM A23187. Results are the mean values ± SE from 3 independent experiments. There was no antigen-induced release above background with the parental B2, myr-SykDK, and the myr-SykΔS cloned lines. The difference in antigen-induced release between the Syk WT and myr-Syk cell lines was statistically significant (.05 per t test). The A23187-induced average release as a percentage of the total cellular histamine content in the different cell lines was 67% (Syk-negative B2), 67% (Syk WT-1), 67% (Syk WT 5), 76% (myr-Syk A18), 78% (myr-Syk A28), 76% (myr-SykDK B4), 63% (myr-SykDK B7), 84% (myr-SykΔS E12), and 83% (myr-SykΔS E15). In nonstimulated cells incubated for 45 minutes, the histamine release as a percentage of the total cellular content was as follows: 4% (Syk-negative B2), 4% (Syk WT 1), 4% (Syk WT 5), 4% (myr-Syk A18), 3% (myr-Syk A28), 3% (myr-SykDK B4), 5% (myr-SykDK B7), 3% (myr-SykΔS E12), and 4% (myr-SykΔS E15).
SH2 domains, but not membrane localization of Syk, are essential for FcεRI-induced tyrosine phosphorylation of Syk and its enzymatic activation
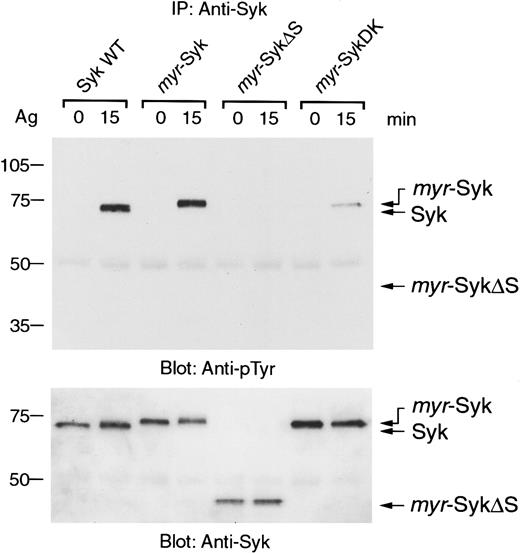
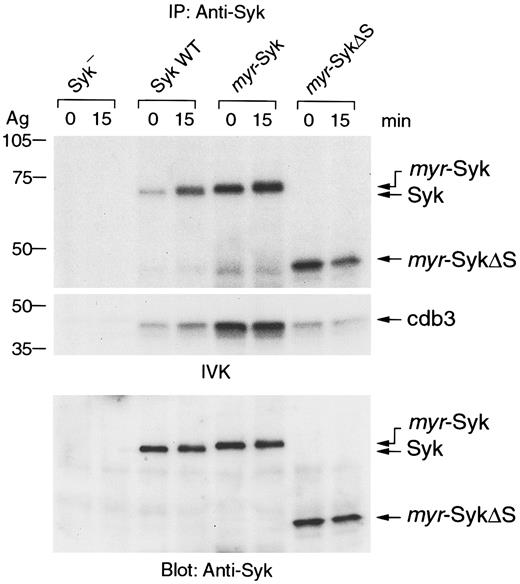
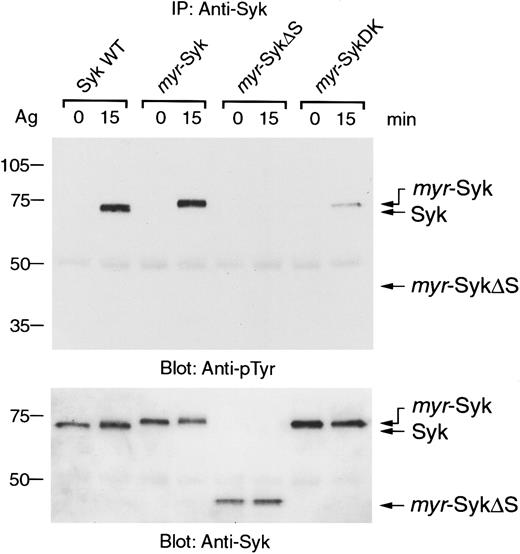
Aggregation of FcεRI induces tyrosine phosphorylation and activation of Syk.7 36 Because the aggregation-induced tyrosine phosphorylation of the β and γ subunits of FcεRI were similar in these cell lines expressing the different forms of Syk (data not shown), we examined the receptor-mediated tyrosine phosphorylation of Syk. In nonstimulated cells, the different forms of Syk were not tyrosine phosphorylated (Figure 4, lanes 1, 3, 5, 7). Antigen-induced tyrosine phosphorylation was detected in wild-type and myr-Syk, but not in myr-SykΔS (Figure 4, lanes 1-6). The myr-SykDK was also slightly tyrosine phosphorylated by FcεRI stimulation (Figure 4, lanes 7, 8). Therefore, the SH2 domains, but not membrane localization of Syk, are critical for the FcεRI-mediated tyrosine phosphorylation of Syk.
Deletion of the SH2 domains abrogates the FcεRI-mediated tyrosine phosphorylation of plasma membrane–associated Syk.
Cell lines expressing different forms of Syk were stimulated with antigen (Ag), and cell lysates were then immunoprecipitated with anti-Syk antibody. Immunoprecipitates were analyzed by immunoblotting with antiphosphotyrosine (pTyr) (top) and anti-Syk antibodies (bottom). Similar results were obtained when the other cloned lines were tested in 5 independent experiments.
Deletion of the SH2 domains abrogates the FcεRI-mediated tyrosine phosphorylation of plasma membrane–associated Syk.
Cell lines expressing different forms of Syk were stimulated with antigen (Ag), and cell lysates were then immunoprecipitated with anti-Syk antibody. Immunoprecipitates were analyzed by immunoblotting with antiphosphotyrosine (pTyr) (top) and anti-Syk antibodies (bottom). Similar results were obtained when the other cloned lines were tested in 5 independent experiments.
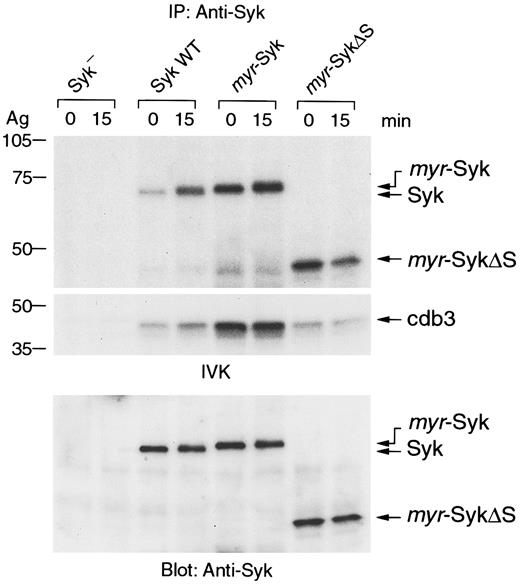
The intrinsic kinase activity of the Syk mutants was examined in an in vitro kinase assay. Syk was immunoprecipitated from nonstimulated and stimulated cells, and the washed immunoprecipitates were incubated with [γ-32P]ATP and the exogenous substrate cdb3 (Figure5). As previously reported,2 28 receptor aggregation enhanced the kinase activity of wild-type Syk as indicated by the increase in Syk autophosphorylation and the phosphorylation of the cdb3 substrate (Figure 5, lanes 1-4). Interestingly, myr-Syk andmyr-SykΔS from both nonstimulated and stimulated cells had more autophosphorylation activity than wild-type Syk (Figure 5, lanes 5-8). Similar differences in activity were observed when these forms of Syk were immunoprecipitated after transient expression in COS-1 cells (data not shown). There was no detectable increase in the kinase activity of myr-Syk after receptor aggregation. Moreover,myr-Syk was more active than myr-SykΔS in phosphorylating the exogenous substrate cdb3. Therefore, the membrane targeting of Syk results in constitutive activation. These results indicate that the SH2 domains of Syk are critical for the FcεRI-mediated enzymatic activation of Syk.
Effect of Syk mutation on its intrinsic kinase activity.
Syk was immunoprecipitated from antigen-stimulated (Ag) or nonstimulated cells and tested by the in vitro immune-complex kinase (IVK) assay. Radiolabeled proteins were detected by autoradiography (top, autophosphorylation of Syk; middle, phosphorylation of the substrate cdb3). Amounts of precipitated proteins were confirmed by anti-Syk immunoblotting (bottom). Similar results were obtained when the other cloned lines were tested in 4 independent experiments.
Effect of Syk mutation on its intrinsic kinase activity.
Syk was immunoprecipitated from antigen-stimulated (Ag) or nonstimulated cells and tested by the in vitro immune-complex kinase (IVK) assay. Radiolabeled proteins were detected by autoradiography (top, autophosphorylation of Syk; middle, phosphorylation of the substrate cdb3). Amounts of precipitated proteins were confirmed by anti-Syk immunoblotting (bottom). Similar results were obtained when the other cloned lines were tested in 4 independent experiments.
Constitutive presence of myristylated Syk in GEMs
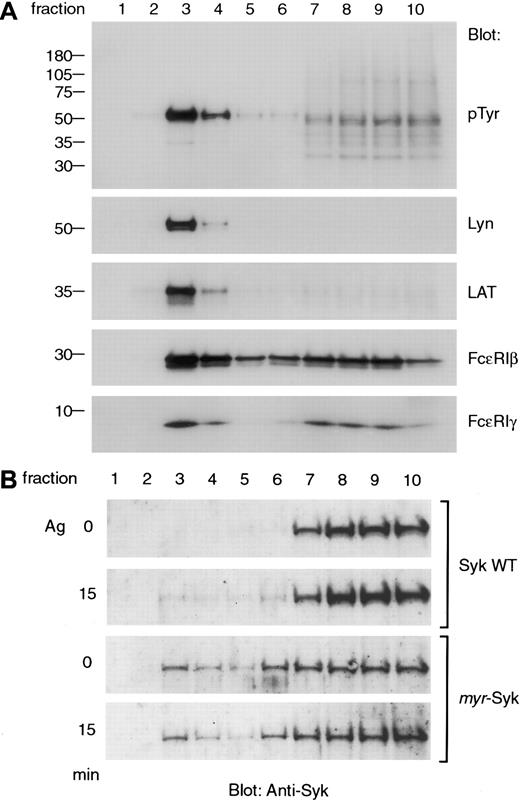
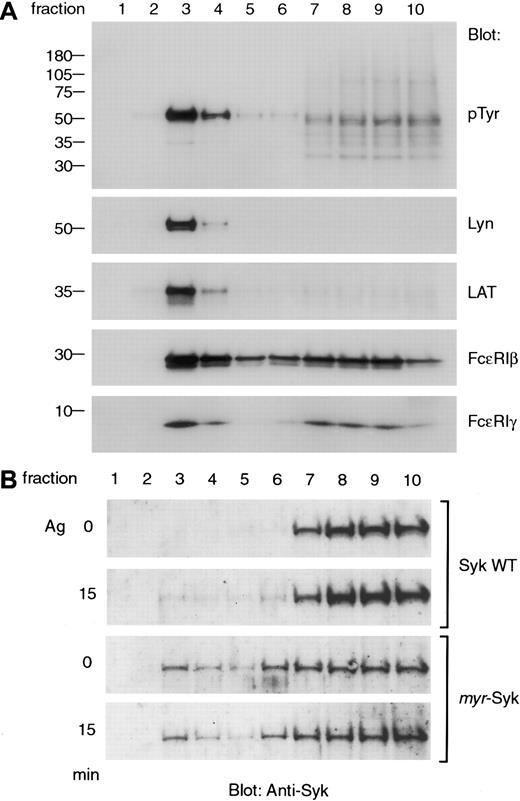
Various signaling molecules are present in GEMs, and, after receptor aggregation, the tyrosine-phosphorylated β and γ subunits of FcεRI are selectively increased in the GEMs.18 25 In the current experiments, the analysis of sucrose density gradient fractions of nonstimulated cells showed that Lyn, LAT, and the β and γ subunits of FcεRI were present in GEMs (Figure6A). Among these signaling molecules, Lyn and LAT were predominantly localized in the GEM fractions (Figure 6A, lane 3). Aggregation of FcεRI did not alter the restricted localization of Lyn and LAT in GEMs (data not shown). Because Syk is known to associate with tyrosine-phosphorylated FcεRI after receptor aggregation, we examined for the presence of wild-type and myristylated Syk in the GEMs (Figure 6B). Wild-type Syk in RBL-2H3 cells was in the detergent-soluble fractions in nonstimulated cells. After antigen stimulation, a small fraction was detectable in the GEM fraction (Figure 6B, lane 3). Unlike wild-type Syk, the myr-Syk was constitutively present in the GEM fraction with no change in localization after receptor aggregation (Figure 6B, lane 3). Therefore, colocalization of Syk with other signaling molecules in GEMs neither activated signal transduction for histamine release in nonstimulated cells nor enhanced these signals after antigen activation.
Some of the myristylated Syk constitutively localizes to the GEM fraction along with Lyn, LAT, and FcεRI.
(A) The association of signaling molecules in the GEM fraction in RBL-2H3 cells. Lysates from nonstimulated RBL-2H3 cells were fractionated by sucrose density gradient centrifugation, and 12 μL each fraction was analyzed by immunoblotting with anti-pTyr, Lyn, LAT, FcεRIβ, and γ antibodies. (B) RBL-2H3 cells and cells expressingmyr-Syk (A28) were either nonstimulated or stimulated with antigen (Ag), and cell homogenates were fractionated by sucrose density gradient centrifugation and analyzed by immunoblotting with anti-Syk antibody. Similar results were obtained in 3 independent experiments.
Some of the myristylated Syk constitutively localizes to the GEM fraction along with Lyn, LAT, and FcεRI.
(A) The association of signaling molecules in the GEM fraction in RBL-2H3 cells. Lysates from nonstimulated RBL-2H3 cells were fractionated by sucrose density gradient centrifugation, and 12 μL each fraction was analyzed by immunoblotting with anti-pTyr, Lyn, LAT, FcεRIβ, and γ antibodies. (B) RBL-2H3 cells and cells expressingmyr-Syk (A28) were either nonstimulated or stimulated with antigen (Ag), and cell homogenates were fractionated by sucrose density gradient centrifugation and analyzed by immunoblotting with anti-Syk antibody. Similar results were obtained in 3 independent experiments.
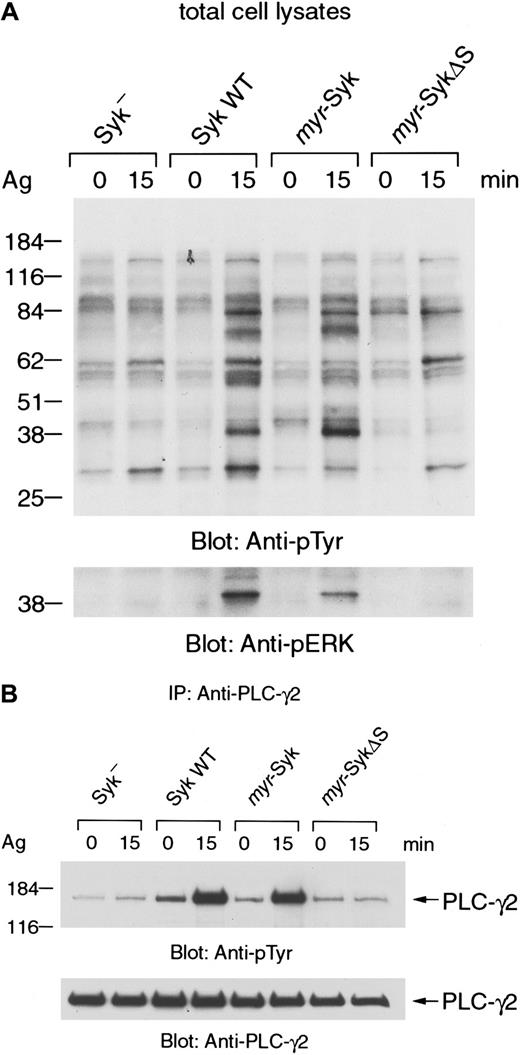
Membrane localization of Syk is not sufficient for FcεRI-mediated tyrosine phosphorylation of cellular proteins
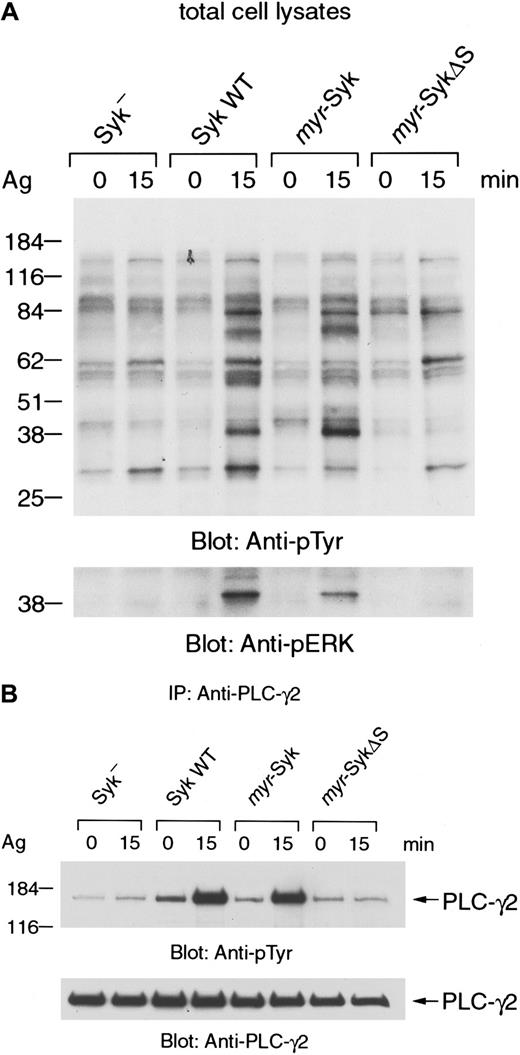
Next we examined the FcεRI-mediated tyrosine phosphorylation of cellular proteins that are known to be downstream of Syk (Figure7). The FcεRI-induced tyrosine phosphorylation of total cellular proteins was similar in themyr-Syk and wild-type transfected cells (Figure 7A, upper panel, lanes 1-6). In contrast, there was minimal receptor-induced tyrosine phosphorylation in cells that expressed myr-SykΔS (Figure 7A, lanes 1, 2 vs lanes 7, 8). There was no increase in the basal histamine release or tyrosine phosphorylation of cellular proteins in the nonstimulated cells, indicating that myr-Syk and myr-SykΔS were not constitutively signaling in these cells. Furthermore, the histamine content of the different transfected cells was not less than the parental cell line, again suggesting that there was no constitutive degranulation in nonstimulated cells. Therefore, membrane localization of Syk by itself does not lead to constitutive signaling.
FcεRI-induced tyrosine phosphorylation of cellular proteins.
(A) Cells were stimulated with antigen (Ag), and total cell lysates were analyzed by anti-phosphotyrosine (top) and antiphospho-ERK immunoblotting (bottom). (B) Antigen-stimulated or nonstimulated cell lysates were immunoprecipitated (IP) with anti-PLC-γ2 antibody. Immunoprecipitates were analyzed by immunoblotting with anti-phosphotyrosine (top) and anti-PLC-γ2 antibodies (bottom). Similar results were obtained when the other cloned lines were examined in 3 independent experiments.
FcεRI-induced tyrosine phosphorylation of cellular proteins.
(A) Cells were stimulated with antigen (Ag), and total cell lysates were analyzed by anti-phosphotyrosine (top) and antiphospho-ERK immunoblotting (bottom). (B) Antigen-stimulated or nonstimulated cell lysates were immunoprecipitated (IP) with anti-PLC-γ2 antibody. Immunoprecipitates were analyzed by immunoblotting with anti-phosphotyrosine (top) and anti-PLC-γ2 antibodies (bottom). Similar results were obtained when the other cloned lines were examined in 3 independent experiments.
Syk is essential for the FcεRI-mediated tyrosine phosphorylation of phospholipase C-γ and the rise in intracellular calcium levels.2 Furthermore, the tyrosine phosphorylation of extracellular signal-regulated kinase (ERK) is also downstream of Syk.3 37 Therefore, we examined FcεRI-mediated tyrosine phosphorylation of ERK and PLC-γ in Syk-transfected cells (Figure 7A lower panel and 7B). The receptor-mediated tyrosine phosphorylation of ERK and PLC-γ2 was similar in the cells that expressedmyr-Syk and wild-type Syk (Figure 7A lower panel and 7B, lanes 3-6). In contrast, myr-SykΔS failed to reconstitute the receptor-induced phosphorylations of ERK and PLC-γ2 in the Syk-negative cells (Figure 7A lower panel and 7B, lanes 1, 2, 7, 8). Similar results were obtained when the receptor-induced tyrosine phosphorylation of Vav was examined in the different cell lines (data not shown). These results indicate that the SH2 domains, but not membrane localization of Syk, are critical for FcεRI-induced tyrosine phosphorylation of cellular proteins, including ERK, PLC-γ, and Vav.
Discussion
These results demonstrate that the c-Src–tagged full-length Syk (myr-Syk), similar to wild-type Syk, was tyrosine phosphorylated after FcεRI aggregation and did activate downstream signals, such as the tyrosine phosphorylation of PLC-γ, ERK, and Vav. Receptor activation caused minimal tyrosine phosphorylation of the membrane-associated but kinase-inactive Syk (myr-SykDK), presumably by Lyn. Therefore, most of the receptor-induced tyrosine phosphorylation of myr-Syk was due to auto-phosphorylation and was regulated similarly to that of wild-type Syk. In contrast to these results, there is constitutive tyrosine phosphorylation of Syk family PTKs when they are expressed as a chimeric protein with intracellular Syk or ZAP-70 fused to the transmembrane and extracellular domain of CD16 or CD2, respectively.14 38The reason for the variation in phosphorylation of these 2 forms of membrane-associated Syk could be the differences in the level of protein expression, localization, or protein–protein interaction.
The binding of ligand to its cognate receptor protein tyrosine kinase brings about dimerization, which then results in transphosphorylation of the 2 molecules on tyrosine residues. These phosphorylated tyrosines become the docking sites for SH2 domain-containing proteins, which then propagate the downstream signals. In contrast, multi-subunit antigen receptors such as FcεRI lack kinase activity and have to recruit molecules such as Syk to the membrane.39,40 The Syk associated with the FcεRI could then interact and phosphorylate other adaptor membrane–associated proteins, such as LAT, SLP-76, or BLNK, to form a receptor-signaling complex.41-44 By sucrose density gradient centrifugation, a small fraction of wild-type Syk was detectable in the detergent-soluble fractions after receptor activation (Figure 6B). Similarly, by immunoprecipitation, a small fraction of Syk is found to be associated with FcεRI after receptor aggregation.7 Unlike native wild-type Syk that is cytoplasmic, the addition of a myristylation signal (myr-Syk) resulted in its membrane association with a small fraction of myr-Syk in the GEM fractions (Figure 6B). Although the receptor-induced tyrosine phosphorylation of wild-type Syk and myr-Syk were similar, myr-Syk was less efficient in downstream propagation of intracellular signals. Thus, there was less receptor-induced histamine release, though the tyrosine phosphorylation of downstream molecules such as PLC-γ, ERK, and Vav in the cells expressing myr-Syk was only slightly less than in cells expressing wild-type Syk (Figures 3, 7). Localization of Syk in GEMs without receptor aggregation was insufficient for signaling downstream events such as the tyrosine phosphorylation of molecules or degranulation. Therefore, localization in the membrane does not make Syk more efficient for signal transduction.
Myristylation of proteins targets them to the membrane, and, as observed here, a small fraction of myr-Syk was in the GEM fraction.30 Previously it was reported that native myristylated c-Src was not detected in the GEM fractions prepared from RBL-2H3 suspension cells.45 The difference between our observations with myr-Syk and this previous report on the localization of c-Src could be due to several reasons. First, localization of molecules in the GEM fraction may depend not only on myristylation but on multiple other factors, such as the interaction of Syk or Src with membrane molecules. Second, there could be differences in the binding capacity of the antibodies used to detect Syk and c-Src. Third, the experimental conditions were different; suspension cells were used when c-Src was found to be absent from the GEM fraction, whereas we used adherent cells. Changes occur in cell morphology and capacity for stimulation when RBL-2H3 cells are detached and placed in suspension; therefore, the use of adherent cells reflects more closely the natural environment of mast cells in connective tissue. Fourth, c-Src interacts with caveolin, an integral protein in GEMs, suggesting that c-Src can be present in these microdomains.46 Nevertheless, it is clear from our experiments that the Syk in the GEM fraction was not enough for signal transduction, though it was greater than the amount of wild-type Syk associated with FcεRI after receptor aggregation. Therefore, targeting to the cell membrane with some in the GEM fraction did not make Syk more efficient for signal transduction.
Syk is a cytosolic PTK recruited to the FcεRI in the plasma membrane because of the interaction of the 2 SH2 domains with the phosphorylated ITAM.7,8,47 This critical role for the SH2 domains is demonstrated by experiments in which the 2 SH2 domains inhibit FcεRI-mediated signal transduction in permeabilized cells.48 These experiments suggest that the SH2 domains are required to recruit cytoplasmic Syk to the membrane. The current experiments, however, indicate a more complex role for the SH2 domains of Syk. Although the truncated myristylated form of Syk (myr-SykΔS) that lacked the 2 SH2 domains was membrane associated, it was neither tyrosine phosphorylated nor activated after receptor aggregation and, hence, failed to transmit downstream signals. The lack of tyrosine phosphorylation of myr-SykΔS signifies that it is a poor substrate for both autophosphorylation and phosphorylation by other kinases such as Lyn. Thus, the SH2 domains of Syk are essential for it to become tyrosine phosphorylated after receptor aggregation. This suggests that phosphorylation of Syk, whether it is the result of autophosphorylation (or transphosphorylation) or phosphorylation by Lyn, requires association with FcεRI. It is possible that the aggregation of FcεRI results in a unique spatial scaffold essential for the phosphorylation and activation of Syk and the propagation of downstream signals.
When immunoprecipitated from quiescent cells, the membrane-associated forms of Syk (both myr-Syk and myr-SykΔS) had greater autophosphorylation activity than wild-type Syk (Figure 5).Myr-Syk but not myr-SykΔS was also more active in phosphorylating the exogenous cdb3 substrate (Figure 5, lanes 5-8). The binding of Syk to phosphorylated ITAM peptides or the tyrosine phosphorylation of Syk results in a conformational change recognized by anti-SykC antibody.15 Nonphosphorylatedmyr-SykΔS was immunoprecipitated with this anti-SykC antibody from lysates of nonstimulated cells, again suggesting that it is in an activated state (data not shown). These results indicate that by in vitro assays, membrane-associated forms were constitutively more active than wild-type Syk. However, in nonstimulated cells there was no increase in basal tyrosine phosphorylation of cellular proteins and no evidence of increased spontaneous degranulation in the cell lines expressing myr-Syk or myr-SykΔS. Several reasons may explain why in vivo the more active membrane-associated Syk did not constitutively signal. In nonstimulated cells, the membrane-associated forms of Syk were not tyrosine phosphorylated. Tyrosine phosphorylation of Syk, especially of the activation loop, appears to be important for signal transduction in cells.28 Such phosphorylation may result from transphosphorylation by Syk bound to the gamma chain of different FcεRI that have been aggregated by antigen or can be due to another kinase, such as Lyn, that is also receptor associated. Without binding to the tyrosine-phosphorylated ITAM of FcεRI, the local concentration of Syk may not allow transphosphorylation of the activation loop. The propagation of intracellular signals may also depend on the formation of aggregates of signaling molecules on the cytoplasmic domains of the receptor. Without the prior activation of Lyn, it is possible that such aggregates of molecules cannot develop for signaling. Another possibility is that molecules such as protein-tyrosine phosphatases or the adaptor protein Cbl may be more activate in regulating Syk function in the absence of these aggregates of signaling molecules.49 Nevertheless, these results suggest that the activation of pathways downstream of Syk after receptor aggregation is not caused by its membrane recruitment but by its escape from negative regulation.
The 40-kd proteolytic fragment of Syk purified from spleen cells has increased kinase activity compared to the full-length molecule.50 This suggests that there are intramolecular mechanisms to regulate Syk enzyme activity. Similarly, intramolecular interactions regulate the catalytic activity of the Src kinases.51 The inter-SH2 domain of Syk family PTK has a coiled-coil structure that may be important in protein–protein interactions and the regulation of kinase activity.12 The immunoprecipitation of myr-SykΔS with anti-SykC antibody that recognizes the active conformational form of Syk suggests that the tandem SH2 or inter-SH2 domains may mask or modify the accessibility of the carboxyl-terminus of the kinase domain. Although GST-Syk SH2 domains (Met1-Gly269) did not precipitatemyr-Syk or myr-SykΔS (data not shown), it is still possible that there are weak intramolecular interactions in Syk family PTKs that control kinase functions.
In summary, SH2 domain–mediated targeting, but not membrane localization, is critical for membrane-compartmentalized activation of Syk and downstream events in FcεRI-mediated signaling pathways leading to histamine release.
We thank Drs Zhi-hui Xie, Maher M. Haddad, and Akiko Sada for helpful discussions and criticism of the manuscript. We thank Greta Bader for histamine analysis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kiyonao Sada, Receptors and Signal Transduction Section, Oral Infection and Immunity Branch, National Institutes of Dental and Craniofacial Research, National Institutes of Health, Bldg 10/1N-106, Bethesda, MD 20892-1188; e-mail: rs53x@nih.gov.