T cells that emigrate from the thymus have primarily been studied in vivo using fluorescent dye injection of the thymus. This study examined the properties of thymocytes that emigrate from cultured thymic lobes in organ culture. Under these conditions, thymic emigrants displayed the expected phenotype, that of mature thymocytes expressing high levels of T-cell receptor (TCR-αβ) and either CD4 or CD8, and were observed to emigrate within 24 hours of positive selection. Emigration was inhibited by cytochalasin D, pertussis toxin, orClostridium difficile toxin B, implicating an active motility process. Most of the surface markers on αβ-thymic emigrants (Thy1, CD44, CD69, CD25, leukocyte functional antigen-1, intercellular adhesion molecule-1, α4-integrin, α5-integrin, CD45, and CD28) were expressed at a surface density similar to that on mature intrathymic cells and peripheral splenic T cells. Heterogeneous expression of L-selectin and heat-stable antigen (HSA) suggested that subsets emerge from the thymus with a commitment to different migration patterns. The only marker on emigrants not found on either intrathymic cells or mature spleen T cells was CTLA-4, which could dampen the response of emigrants to peripheral antigens. Antigen responsivenes measured in vitro against allogeneic dendritic cells showed a proliferative response comparable to that of splenic T cells. In vivo, however, thymic emigrants failed to induce an acute graft-versus-host reaction in allogeneic severe combined immunodeficiency recipients. This suggests that a mechanism operating in vivo, perhaps tolerance or migration pattern, attenuates the response of emigrants against antigens that did not induce their deletion in the thymus.
Introduction
The thymus is the site of T-cell development. Lymphoid precursors leave the bone marrow, enter the thymus, undergo a complex series of gene rearrangements, positive and negative selection for T-cell receptor (TCR) specificity, proliferation, and differentiation steps. When this program is complete, the T cell emigrates from the thymus, beginning a pattern of recirculation from blood to lymph to blood in search of its specific antigen in peripheral tissues.
Little is known about the mechanism of emigration of cells from the thymus and many questions remain regarding the markers and functional properties of these emigrants. What is the trigger to emigrate? Where do they go first? How long do they survive and what extrinsic trophic signals maintain them? Are they homogeneous or are there subsets of emigrants? Are they uniquely tolerizable to peripheral antigens? To answer some of these questions, the most direct studies of the properties of thymic emigrants have relied on the injection of fluorescein isothiocyanate (FITC) into the thymus.1 FITC is taken up by thymocytes, and cells leaving the thymus retain the dye for the next few days and can be identified in peripheral sites based on their fluorescence. Using flow microfluorimetry, other markers of these cells can be assessed, and using cell sorting, their functional properties can be examined.
A number of observations have been made regarding thymic emigrants using the FITC method. The labeled cells have a relatively mature single positive phenotype and no unique marker has been found that distinguishes them from either mature thymocytes or peripheral T cells.2 Functionally, the labeled cells are fully responsive to mitogens and alloantigens.3 However, other methods suggest that recent emigrants secrete very little interleukin (IL)-2 compared to mature splenic T cells4 and that emigrants are tolerized by alloantigen contact.5 If thymic emigrants were anergized by contact with antigen, it could explain how self-tolerance is established to those antigens that are not expressed in the thymus.
One limitation of the method of thymus FITC injection is that recirculating mature lymphocytes, and particularly those that have been previously activated, continuously enter the thymus as shown in many studies6-15 and would also be labeled. Many of the FITC-labeled cells that leave the thymus and are detected peripherally could therefore be mature recirculating T cells, rather than cells that recently matured in the thymus. By one estimate, 30% of the cells that are labeled this way and subsequently detected in spleen are mature recirculating cells rather than cells that recently matured in the thymus (P. Matzinger, oral communication, 1999). Thus, the apparent functional maturity of thymic emigrants could be due to contamination with mature, previously activated T cells, making it desirable to develop another method of characterizing thymic emigrants. In the present study, we developed a method for collecting thymocytes that emigrate from the fetal thymus during organ culture in vitro, a technique that avoids the problem of contamination with recirculating mature T cells. We then examined the surface markers and functional properties of these cells.
Materials and methods
Mice
The C57BL/6, Balb/c, C.B-17 SCID/SCID, and B6 SCID/SCID mice were bred at Animal Production Area, Charles River Laboratories, NCI-FCRDC, Frederick, MD. Tap−/−mice were purchased from the Jackson Laboratory (Bar Harbor, ME). To produce timed pregnancy, mice were mated overnight and plugs checked the following day, which was designated as day 1 of gestation. At day 16 of gestation, mothers were killed by CO2 asphyxiation and embryos by chilling on ice. Fetal thymus lobes were removed under a dissecting microscope.
Fetal thymus organ culture
Two fetal thymus lobes were placed on a nylon screen (100 mesh) that was supported by a plastic cylinder in a 24-well tissue culture plate. The organ culture medium was RPMI-1640 supplemented with 2 mM glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% heat-inactivated fetal bovine serum (Hyclone, Logan, UT), and was added at approximately 1.5 mL/well, carefully adjusting the medium level so that the thymic lobes were at the interface of medium and air. The cultures were incubated at 37°C in 5% CO2 and fed every 2 to 3 days by transferring the thymus, mesh, and cylinder to a well with fresh medium. To collect emigrant cells, the cultured fetal thymus lobes were transferred to a new well, then 1 day later, emigrants were harvested from the bottom of the well. For all of the experiments in this study, except the time course shown in Figure1, a standard culture of 12 days was used (11-day culture plus 1 day of collection of emigrants). Emigrant cells were pooled from at least 24 wells (48 thymic lobes). We initiated thymic organ cultures on at least 15 separate dates to characterize the emigrants in this study and never failed to generate such cells. Each type of experiment was conducted at least 3 times.
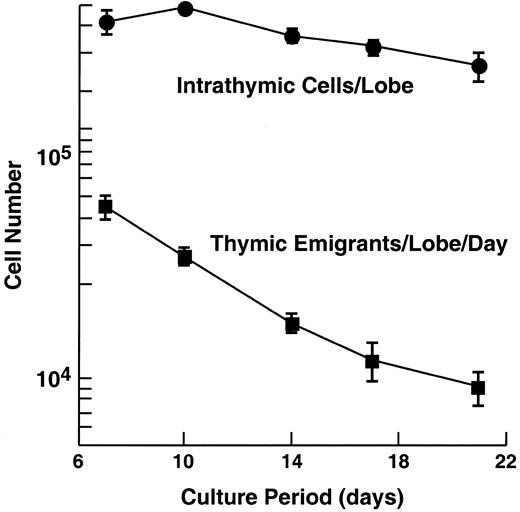
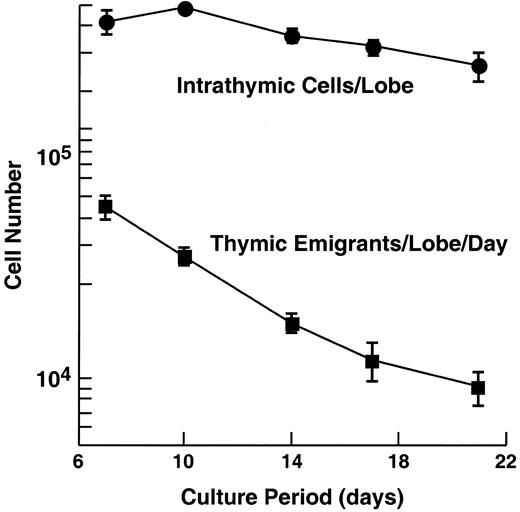
Emigration rate from cultured murine thymic lobes.
C57Bl/6 embryonic thymus lobes (from day 16 of gestation) were cultured at a medium-air interface by placing the lobe on a nylon mesh supported by a cylinder, adjusting the level of medium to the height of the mesh. Cells that emigrate from the thymus pass through the mesh and fall to the bottom of the tissue culture well. The day before harvest, the lobe and support apparatus were transferred to a new well. The following day, the lobe and cells that had emigrated from it over the preceding day were harvested and counted. In the experiment shown, 60 lobes were initially placed in culture, then, at each time point, 6 were removed to examine intrathymic cells, leaving at the last time point, 36 lobes. At each time point, emigrants were pooled from 60 lobes (beginning) to 36 lobes (end). Error bars (± SD) are shown for 3 experiments.
Emigration rate from cultured murine thymic lobes.
C57Bl/6 embryonic thymus lobes (from day 16 of gestation) were cultured at a medium-air interface by placing the lobe on a nylon mesh supported by a cylinder, adjusting the level of medium to the height of the mesh. Cells that emigrate from the thymus pass through the mesh and fall to the bottom of the tissue culture well. The day before harvest, the lobe and support apparatus were transferred to a new well. The following day, the lobe and cells that had emigrated from it over the preceding day were harvested and counted. In the experiment shown, 60 lobes were initially placed in culture, then, at each time point, 6 were removed to examine intrathymic cells, leaving at the last time point, 36 lobes. At each time point, emigrants were pooled from 60 lobes (beginning) to 36 lobes (end). Error bars (± SD) are shown for 3 experiments.
Fetal thymus lobes obtained from Tap−/− mice were also cultured by the same methods described above. To examine the effect of β2-microglobulin (β2m), the cultured fetal thymus lobes were transferred to a new well supplemented with 5 μg/mL human β2m (Calbiochem, La Jolla, CA) on day 12 of cultivation. Emigrant cells were pooled from at least 6 wells (12 thymic lobes).
Inhibitors of cell motility were tested on emigration by transferring lobes (at day 12 of cultivation) to a well containing the inhibitor, then 6 hours later emigrants were collected and characterized. Cytochalasin D (2 μM; Sigma, St Louis, MO) inhibits microtubule assembly. Clostridium difficile toxin B (50 ng/mL; Oravax, Cambridge, MA) glucosylates and thereby inhibits Rho family small guanosine triphosphatases (GTPases). Pertussis toxin (50 ng/mL; Sigma) catalyzes adenosine diphosphate (ADP)-ribosylation of some G proteins. Control wells contained dimethyl sulfoxide (DMSO; 0.5%) used to dissolve cytochalasin D.
Preparation of cells
Intrathymic cells were isolated from cultured fetal thymus lobes (40-50) by gentle disruption with a pipette after treatment with 0.2% collagenase (Sigma) dissolved in phosphate-buffered saline (PBS) containing 20% heat-inactivated fetal bovine serum (FBS; Hyclone) for 1 hour at 37°C.
Total spleen cells and lymph node cells were prepared from mouse spleen and lymph node, respectively, by gentle disruption between 2 notched slide glasses. The single-cell suspensions were washed with PBS, and then red blood cells were lysed by treatment with ACK lysis buffer (0.15 M NH4Cl, 1.0 mM KHCO3, 0.1 mM EDTA) for 3 minutes. T cells were isolated from total spleen cells by a T-cell enrichment column (Collect-T, Biotex Lab, Alberta, Canada) according to the manufacturer's instructions.
Dendritic cells were generated from mouse bone marrow. Briefly, total bone marrow cells (obtained from 2 femurs by flushing with PBS) were added to a 100-mm Petri dish in 10 mL cell culture medium supplemented with 800 U/mL recombinant murine granulocyte-macrophage colony-stimulating factor (GM-CSF; PeproTech, Rocky Hill, NJ) and 400 U/mL recombinant murine IL-4 (PeproTech). Nonadherent cells were harvested the next day, supplemented with the cytokines, then added to 6-well plates at a density of 5 × 106/well in 5 mL medium and cultured. At day 3 and 4 from the initiation of the culture, nonadherent cells were discarded by a gentle shaking and fresh medium with cytokines added. Aggregates of dendritic cells were usually harvested at day 7, and then disssociated by gentle pipetting.
Flow cytometric analysis
The following mouse monoclonal antibodies were used: anti-CD4 (clone RM4-5), anti-CD8 (clone 53-6.7), anti-TCRα/β (clone H57-597), anti-TCRγ/δ (clone GL3), anti-Thy1.2 (clone 53-2.1), anti-CD44 (clone IM7), anti-CD69 (clone H1.2F3), anti-CD25 (clone 7D4), anti-LFA-1a (clone 2D7), anti-ICAM-1 (clone 3E2), anti-L-selectin (clone MEL-14), anti-α4-integrin (clone R1-2), anti-α5-integrin (clone 5H10-27), anti-CD45 (clone 30F11.1), anti-HSA (clone M1/69), anti-CD28 (clone 37.51), anti-CTLA-4 (UC10-4F10-11), anti-H-Kb (clone AF6-88.5), and anti-CD16 /CD32 (clone 2.4G2). All of these antibodies were purchased from Pharmingen (San Diego, CA).
For staining, cells were washed in a staining solution of PBS containing 5% FBS and 0.1% NaN3, and then resuspended in 50 μL staining solution containing 0.5 μg rat monoclonal antibody 2.4G2 and 10% normal mouse serum to reduce nonspecific binding of antibodies to Fc receptor. Cells were incubated for 10 minutes on ice, mixed with 50 μL prediluted antibody solution that contained 2 kinds of antibodies, and then incubated for 20 minutes on ice. Unbound antibodies were removed by washing the cells twice with staining buffer. If biotinylated antibody was used, the cell pellet obtained from the second wash was resuspended in 50 μL phycoerythrin (PE)- or FITC-conjugated avidin, incubated for 10 minutes on ice, and then washed twice with staining buffer. After staining, cells were fixed in 1% p-formaldehyde in PBS and flow cytometric analysis was performed on a FACStar Plus (Becton Dickinson Immunocytometry System, Mountain View, CA). Dead cells were gated out by their low forward angle light scatter intensity. In most analysis, 10 000 cells were scored.
Mitogenic stimulation
For phorbol myristate acetate (PMA) and ionophore A23187 stimulation, thymic emigrants (2 × 105/well) or purified adult splenic T cells (2 × 105/well) were cultured for 3 days, respectively, in 96-well U-bottom tissue culture plate in the presence of PMA (100 ng/mL), A23187 (1 μg/mL), or both. Cells were pulsed with tritiated thymidine (0.5 μCi/well) for the last 6 hours. For anti-CD3 monoclonal antibody stimulation, thymic emigrants (2 × 105/well) or purified adult splenic T cells (2 × 105/well) were mixed with irradiated (1000 R) syngeneic bone marrow-derived dendritic cells (2 × 103-5 × 104/well), cultured for 3 days, respectively, in 96-well U-bottom tissue culture plate in the presence of 1.0 μg/mL anti-CD3ε monoclonal antibody (clone 145-2C11, PharMingen). Cells were pulsed with tritiated thymidine (0.5 μCi/well) for the last 6 hours.
Primary and secondary mixed lymphocyte response (MLR)
For primary MLR, thymic emigrants (2 × 105/well) or purified adult splenic T cells (2 × 105/well) were mixed with irradiated (1000 R) syngeneic or allogeneic bone marrow-derived dendritic cells (2 × 103-5 × 104/well), respectively, and cultured for 3 days in 96-well U-bottom tissue culture plate. Cells were pulsed with tritiated thymidine (0.5 μCi/well) for the last 6 hours.
For secondary MLR, thymic emigrants (3 × 106/well) or purified adult splenic T cells (3 × 106/well) were first mixed with irradiated (1000 R) syngeneic or allogeneic bone marrow-derived dendritic cells (2 × 106/well), respectively, in wells of 24-well tissue culture plate, and cultured for 2 days. Viable cells were separated by Ficoll-Hypaque centrifugation, counted, and plated to wells of 96-well U-bottom plates at a density of 1 × 105/well, and then rested for 1 day. Secondary stimulation was induced by adding irradiated (1000 R) syngeneic or allogeneic bone marrow-derived dendritic cells (2 × 103-5 × 104/well), respectively, to each primarily stimulated group of cells. The cultures were incubated for 3 days, and were pulsed with tritiated thymidine (0.5 μCi/well) for the last 6 hours.
Graft-versus-host (GVH) reaction
Thymic emigrants (2-4 × 106/recipient) generated from C57BL/6 (H-2b) were injected intravenously into syngeneic severe combined immunodeficiency (SCID) mice or allogeneic CB-17 (H-2d) SCID mice that were treated with anti-asialoGM1 monoclonal antibody and then irradiated with 2.5 Gy. For comparison, unfractionated C57BL/6 lymph node cells (0.5-2 × 106/recipient) and purified splenic T cells (0.5-2 × 106/recipient) were also injected intravenously into the same-treated SCID recipients, respectively. Mortality was monitored every day and body weight was monitored twice a week initially then once a week thereafter. At day 49 after transfer, blood was drawn and hematocrits were performed, after which recipients were killed and skin and liver sections examined for signs of GVH disease (GVHD) by a veterinary pathologist. Thymic emigrants repopulating the lymphoid organs of syngeneic or allogeneic SCID recipients were analyzed by examining the expression of T-cell markers such as CD3, CD4, and CD8, and donor marker H-2Kb. Two complete experiments were performed (on different dates) using in vivo transfers of thymic emigrants.
Results
Thymic organ culture for generating emigrants
To examine cells that emigrate from the thymus during organ culture, a method was devised for collecting thymic emigrants. Previous methods of culturing the embryonic thymus have shown that placing the lobe at the interface of air and medium is much superior for promoting T-cell development compared to submerging the organ.16 The air interface optimizes the availability of O217 because the explanted organ is disconnected from normal vasculature. Previous methods of organ culture generally supported the thymus on a nucleopore membrane that in turn was supported by a gelfoam sponge floating on medium. Cells that emigrate from the thymus in this culture system cannot be easily separated from those that remain in the thymus. We modified this system by placing the thymic lobe on a fine nylon mesh that in turn rested on a cylinder—careful adjustment of the medium height maintained the thymic lobe at the interface of medium and air. Cells that emigrate from the lobe pass freely through the nylon mesh and fall to the bottom of the tissue culture well.
The rate of emigration from cultured thymic lobes is shown in Figure 1. We have analyzed thymic emigrants at various times of organ culture, but the subsequent studies were performed following 12 days of culture for the sake of consistency, and because we occasionally found some immature cells “leaked” at earlier time points. Over the following 18 hours, the number of recovered emigrants numbered approximately 0.5% of the total number of cells in the thymus.
Phenotype of thymic emigrants
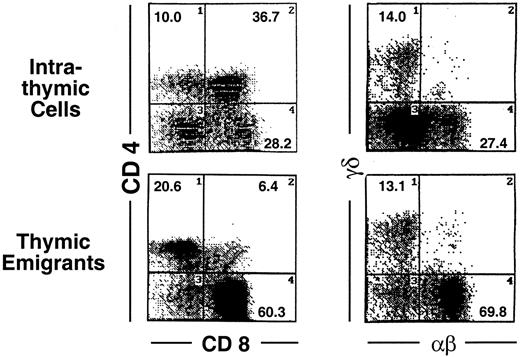
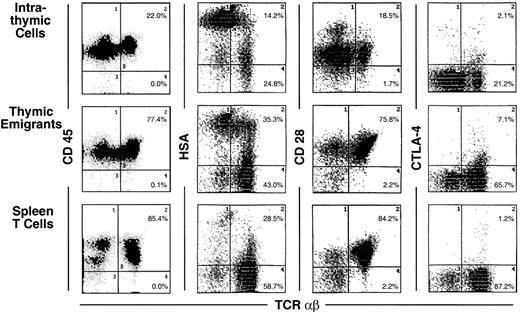
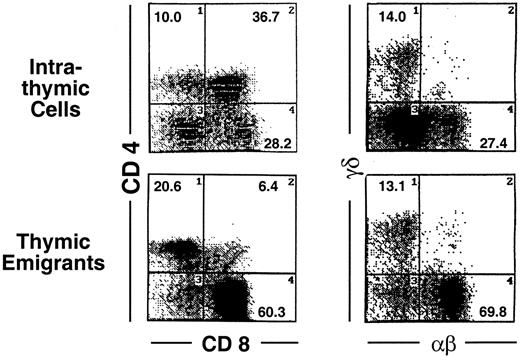
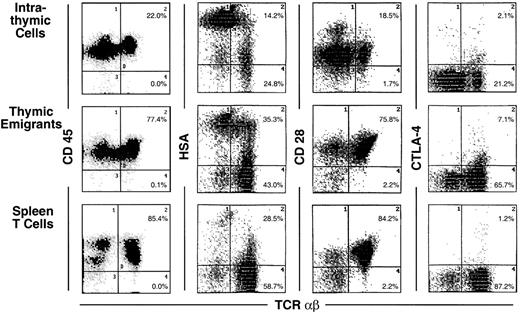
Analysis of these thymic emigrants by flow microfluorimetry revealed characteristics of the most mature thymocytes as indicated in Figure 2 by the criteria of staining with CD4, CD8, TCRαβ and TCRγδ. In the αβ lineage, the most mature thymocytes express high levels of TCRαβ and are single positive for CD4 or CD8, whereas their predecessors express low to intermediate levels of TCRαβ and express both CD4 and CD8. Thus, among emigrants, the proportion of single-positive CD4 cells was 29.1%, compared to 10% within the thymus, and the proportion of single-positive CD8 cells was 51.6% in emigrants, compared to 28.2% within the thymus, whereas double-positive cells accounted for only 6% in emigrants, compared to 36.7% within the thymus. Likewise the proportion of high TCRαβ cells was 63.7% (up to 91% in other experiments) in emigrants compared to 11.2% within the thymus—note the percentages written in Figure 2 refer to both intermediate and high TCRαβ. The TCRγδ cells also emigrated from the thymus, accounting for 13.1% of emigrants. Overall, the emigrant population was primarily comprised of mature thymocytes that had completed development and selection. This method of organ culture, therefore, appeared to generate the types of cells that normally exit the thymus in an intact mouse.
Phenotype of thymic emigrants—expression of CD4 versus CD8 and TCRαβ vs γδ.
Thymic emigrants were generated as in Figure 1. Lobes were cultured for 12 days and transferred to a new well. Thymocytes that emigrated during the following 18 hours were harvested and analyzed by flow microfluorimetry for the indicated markers.
Phenotype of thymic emigrants—expression of CD4 versus CD8 and TCRαβ vs γδ.
Thymic emigrants were generated as in Figure 1. Lobes were cultured for 12 days and transferred to a new well. Thymocytes that emigrated during the following 18 hours were harvested and analyzed by flow microfluorimetry for the indicated markers.
In Figures 3 through 6 (summarized in Table1), the surface markers on thymic emigrants were examined more extensively. These analyses focus on the αβ lineage by counterstaining each marker with TCRαβ; this permits each marker to be visualized on mature thymocytes because they express high levels of TCRαβ. Thus, mature cells within the thymus can be compared to thymic emigrants and to mature peripheral T cells isolated from the spleen. The following comments refer to the mean fluorescence intensity of various markers on mature (high αβ) cells.
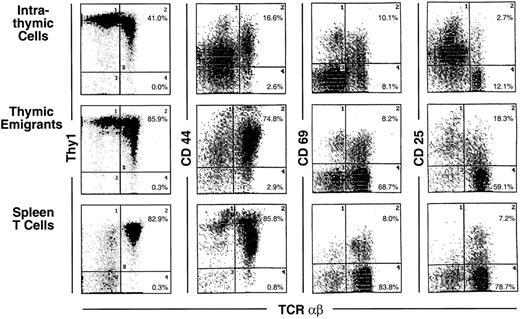
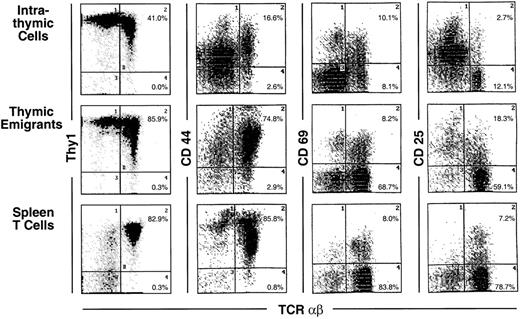
Phenotype of thymic emigrants—expression of Thy1, CD44, CD69, and CD25 versus TCRαβ.
Thymic emigrants were generated as in Figure 2 and analyzed for the indicated markers. For comparison, intrathymic cells and mature peripheral T cells isolated from spleen are shown.
Phenotype of thymic emigrants—expression of Thy1, CD44, CD69, and CD25 versus TCRαβ.
Thymic emigrants were generated as in Figure 2 and analyzed for the indicated markers. For comparison, intrathymic cells and mature peripheral T cells isolated from spleen are shown.
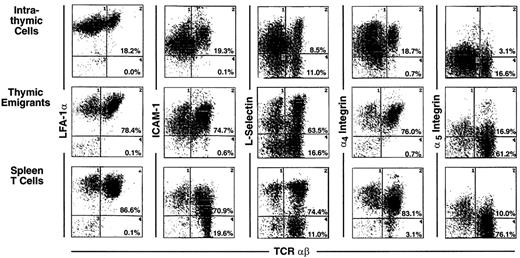
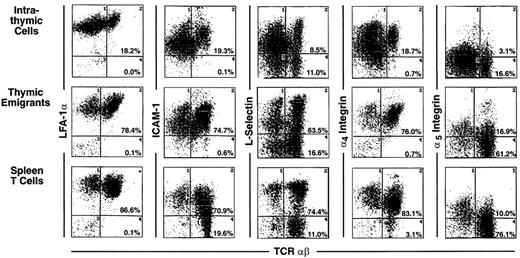
Most markers showed the same level of expression on emigrants as on mature intrathymic or splenic T cells. These markers include CD44 (Figure 3), LFA-1α, L-selectin, α4-integrin, and α5-integrin (Figure4), and CD45 and CD28 (Figure5). CD25 was expressed on a higher proportion of emigrants than intrathymic high αβ cells (Figure 3).
Phenotype of thymic emigrants—expression of LFA-1, ICAM-1, L-selectin, α4 integrin, and α5integrin versus TCRαβ.
Thymic emigrants were generated as in Figure 2 and analyzed for the indicated markers in comparison with intrathymic cells and mature peripheral T cells isolated from spleen.
Phenotype of thymic emigrants—expression of LFA-1, ICAM-1, L-selectin, α4 integrin, and α5integrin versus TCRαβ.
Thymic emigrants were generated as in Figure 2 and analyzed for the indicated markers in comparison with intrathymic cells and mature peripheral T cells isolated from spleen.
Phenotype of thymic emigrants—expression of CD45, HSA, CD28, and CTLA-4 versus TCRαβ.
Thymic emigrants were generated as in Figure 2 and analyzed for the indicated markers in comparison with intrathymic cells and mature peripheral T cells isolated from spleen.
Phenotype of thymic emigrants—expression of CD45, HSA, CD28, and CTLA-4 versus TCRαβ.
Thymic emigrants were generated as in Figure 2 and analyzed for the indicated markers in comparison with intrathymic cells and mature peripheral T cells isolated from spleen.
Several markers that were examined are more highly expressed on intrathymic cells than on splenic T cells. Thus, it would be possible for emigrants to either express the high thymic level or the low peripheral level. Whereas thymic emigrants more resembled intrathymic cells for their high expression of ICAM-1 (Figure 4), they more resembled splenic T cells for their low expression of CD69 (Figure 3). Emigrants showed intermediate expression (between intrathymic cells and splenic T cells) for Thy1 (Figure 3) and HSA (Figure 5).
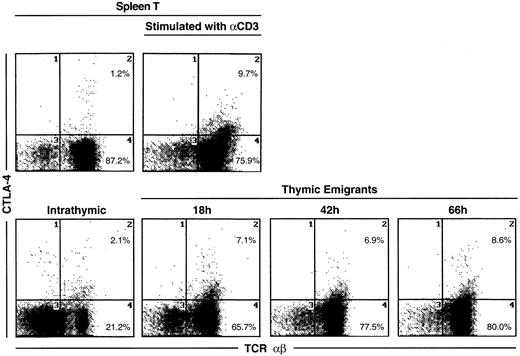
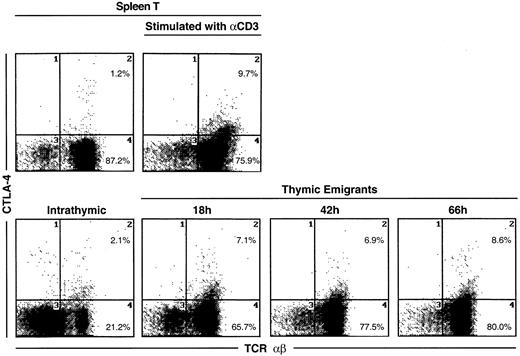
The most unique marker detected on thymic emigrants was CTLA-4 (Figure5), which was virtually undetectable on peripheral T cells and only slightly visible on intrathymic cells. Although emigrants expressed a low level of CTLA-4 (relative to other markers), the level was similar to that on activated peripheral T cells (Figure6). Thus, the level of CTLA-4 on emigrants could be sufficiently high to deliver similar signals to that in mature T cells, in which it performs a largely inhibitory role.18
CTLA-4 expression on thymic emigrants.
Thymic lobes were cultured as in Figure 2 and beginning on day 12, emigrants were collected for 18, 42, or 66 hours, then analyzed for expression of CTLA-4. For comparison, splenic T cells were activated for 72 hours with different concentrations of anti-CD3, then analyzed for CTLA-4 expression.
CTLA-4 expression on thymic emigrants.
Thymic lobes were cultured as in Figure 2 and beginning on day 12, emigrants were collected for 18, 42, or 66 hours, then analyzed for expression of CTLA-4. For comparison, splenic T cells were activated for 72 hours with different concentrations of anti-CD3, then analyzed for CTLA-4 expression.
It is important to point out that expression of many of these markers is surprisingly heterogeneous on thymic emigrants. For example, L-selectin (Figure 4) or HSA (Figure 5) expression on emigrants ranged from brightly positive cells to negative cells. This may indicate that different subsets of cells emerge from the thymus with commitments to different functions and migration patterns. Thus, thymic emigrants expressing high L-selectin levels may be already committed to home to lymph nodes, whereas those with low levels may home elsewhere.
Emigration from the thymus following positive selection
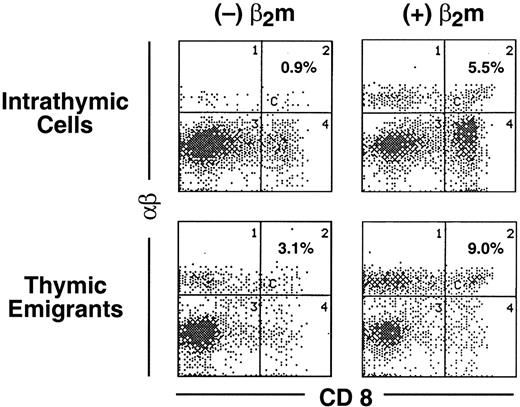
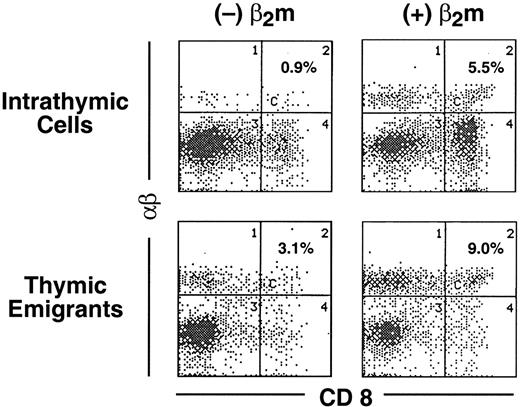
The phenotype of thymic emigrants of the αβ lineage indicated they had completed positive selection (they were single positive for CD4 and CD8 and expressed high levels of TCRαβ). To determine how rapidly emigration occurred after positive selection, we modified the organ culture such that positive selection was induced synchronously, then examined how rapidly mature emigrants emerged. Our approach took advantage of the block in positive selection of CD8 cells that occurs in the thymus from Tap−/− mice, which is due to the instability of the class I major histocompatibility complex (MHC)/peptide complex that is required for positive selection of CD8 cells. Synchronous positive selection of CD8 cells can be induced in cultures of Tap−/− thymus organs by adding β2m,19 which stabilizes class I molecules (presumably together with extracellular peptides present in the culture). As shown in Figure 7, within 1 day following addition of β2m to these cultures, mature (high TCRαβ) CD8 cells appeared both within the thymus (rising from 0.9% to 5.5%) and among emigrants (rising from 3.1% to 9.0%). Thus, emigration can occur within a day of positive selection.
Emigration of positively selected CD8+ cells from Tap−/− thymic lobes following treatment with β2m.
Thymic lobes from Tap−/− mice were cultured as in Figure1. On day 12, lobes and support apparatus were transferred to a new well, human β2m was added (or omitted from the control) and emigrants collected for the subsequent 24 hours. Cells were analyzed for expression of CD8 versus TCRαβ.
Emigration of positively selected CD8+ cells from Tap−/− thymic lobes following treatment with β2m.
Thymic lobes from Tap−/− mice were cultured as in Figure1. On day 12, lobes and support apparatus were transferred to a new well, human β2m was added (or omitted from the control) and emigrants collected for the subsequent 24 hours. Cells were analyzed for expression of CD8 versus TCRαβ.
Inhibitors of cell motility block emigration
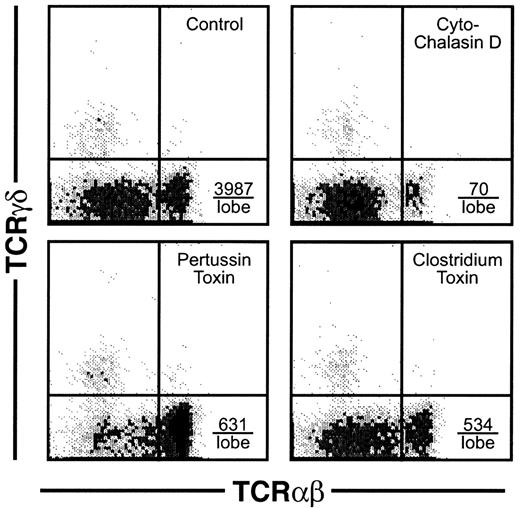
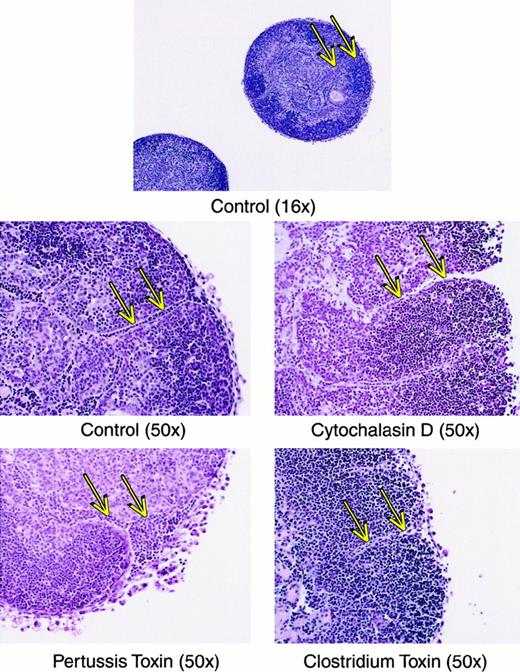
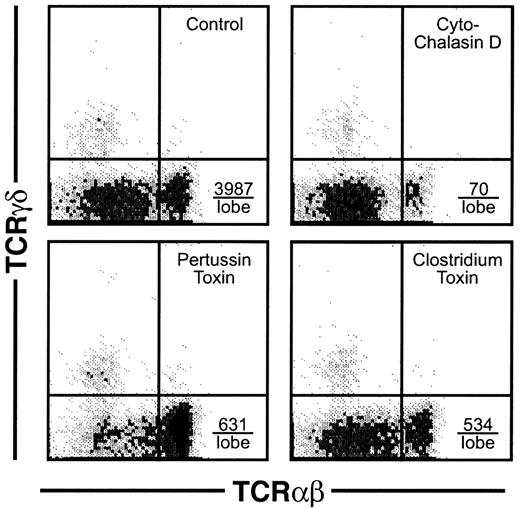
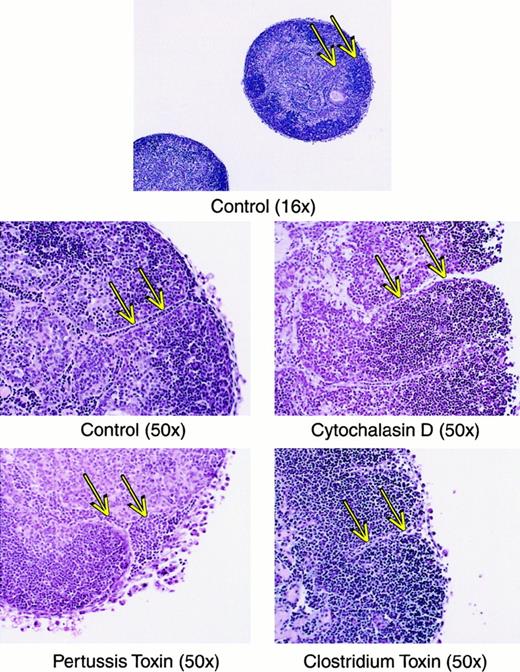
For mature thymocytes to emigrate from the thymus, an active motility process would seemingly be involved. This assumption was verified using several types of inhibitors of lymphocyte motility, which dramatically blocked the emigration process during a 6-hour exposure to the drugs. As shown in Figure8, cytochalasin D blocked emigration of mature thymocytes by over 98%. Inhibition of G proteins via pertussis toxin blocked emigration by over 84%. Inhibition of the Rho family of small GTPases by C difficile toxin B blocked emigration by over 86%. These inhibitors blocked the emigration of high TCRαβ cells, rather than reducing the surface expression of TCRαβ, because the expression of TCRαβ on intrathymic cells was not reduced (not shown). We then compared histologic sections of these treated lobes, which suggested a possible route of emigration. As shown in Figure 9, comparison of normal versus drug-inhibited lobes showed differences in the lymphocyte content of radially oriented cords that could represent tracks of emigrating mature thymocytes. In normal lobes, these tracks could represent a string of outwardly migrating thymocytes, whereas in cytochalasin D- orClostridium toxin-treated lobes, fewer cells were observed within these cords. Pertussis toxin treatment on the other hand induced apparent “congestion” within the cords. However, these are static pictures of a dynamic process and other interpretations are possible. The control sections also indicate the integrity of the capsule in lobes maturing in these culture conditions, arguing against a passive “leak” of cells and supporting our view that cells that emerge from these organs do so by an active emigration process.
Effect of inhibitors of motility on thymic emigration.
Thymic lobes were cultured as in Figure 2 and beginning on day 12, various inhibitors were added (42 lobes per treatment group) and emigrants collected for the subsequent 6 hours. Treatments were control (DMSO only), cytochalasin D (2 μM), pertussis toxin (50 ng/mL), orClostridium toxin (50 ng/mL). The total number of emigrants per lobe/6 hours was control (6667), cytochalasin D (952), pertussis toxin (2619), and Clostridium toxin (2857), and, calculated from the percentage, the number of high CD3 is indicated in the lower right quadrant for each treatment group.
Effect of inhibitors of motility on thymic emigration.
Thymic lobes were cultured as in Figure 2 and beginning on day 12, various inhibitors were added (42 lobes per treatment group) and emigrants collected for the subsequent 6 hours. Treatments were control (DMSO only), cytochalasin D (2 μM), pertussis toxin (50 ng/mL), orClostridium toxin (50 ng/mL). The total number of emigrants per lobe/6 hours was control (6667), cytochalasin D (952), pertussis toxin (2619), and Clostridium toxin (2857), and, calculated from the percentage, the number of high CD3 is indicated in the lower right quadrant for each treatment group.
Histologic sections of thymic lobes treated with inhibitors of motility.
Lobes were treated as in Figure 8, imbedded and stained with hematoxylin and eosin. Magnification is indicated in parentheses. Arrows indicate tracks of lymphoid cells that may represent a route of emigration, and show that in cytochalasin D or Clostridiumtoxin groups, these tracks are relatively empty, whereas in the pertussis toxin-treated group the tracks are relatively congested.
Histologic sections of thymic lobes treated with inhibitors of motility.
Lobes were treated as in Figure 8, imbedded and stained with hematoxylin and eosin. Magnification is indicated in parentheses. Arrows indicate tracks of lymphoid cells that may represent a route of emigration, and show that in cytochalasin D or Clostridiumtoxin groups, these tracks are relatively empty, whereas in the pertussis toxin-treated group the tracks are relatively congested.
Function of emigrants in vitro
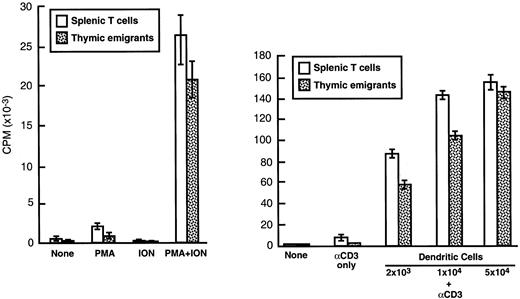
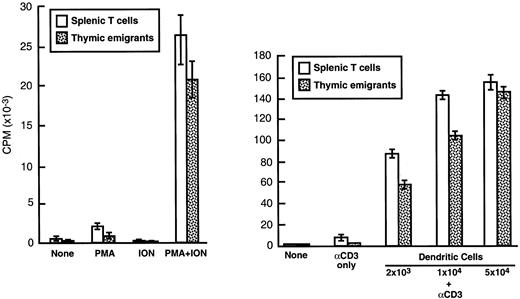
The ability of thymic emigrants to proliferate in response to polyclonal activators was compared to that of mature peripheral T cells. As shown in Figure 10, the combination of PMA and ionophore elicited a response in emigrants that was similar in magnitude to that of splenic T cells. The combination of anti-CD3 and syngeneic dendritic cells also triggered a strong proliferative response in emigrants, although it was slightly lower than that of splenic T cells. The ability to recognize alloantigens was measured using the MLR. In a primary MLR (Table2), emigrants mounted a vigorous proliferative response to allogeneic dendritic cells, although the magnitude was slightly lower than that of splenic T cells. In a secondary MLR (Table 3), proliferation of emigrants was virtually the same as splenic T cells. Thus, the functional capacities of emigrants were similar to those of mature peripheral T cells, at least regarding their proliferative response in vitro to dendritic cell stimulation. Hence, the CTLA-4 expression on emigrants (noted above) did not greatly impede proliferation of emigrants in vitro.
Proliferation of thymic emigrants in response to polyclonal stimuli.
Thymic emigrants were generated as in Figure 2. Cells were cultured (2 × 105/well) for 72 hours with (left panel) PMA, A23187, or (right panel) anti-CD3 and the indicated numbers of syngeneic dendritic cells (which were generated from bone marrow by culture in GM-CSF and IL-4). Cultures were pulsed for 6 hours with3H-TdR and incorporation was measured. For comparison, mature T cells isolated from spleen are shown.
Proliferation of thymic emigrants in response to polyclonal stimuli.
Thymic emigrants were generated as in Figure 2. Cells were cultured (2 × 105/well) for 72 hours with (left panel) PMA, A23187, or (right panel) anti-CD3 and the indicated numbers of syngeneic dendritic cells (which were generated from bone marrow by culture in GM-CSF and IL-4). Cultures were pulsed for 6 hours with3H-TdR and incorporation was measured. For comparison, mature T cells isolated from spleen are shown.
Function of emigrants in vivo
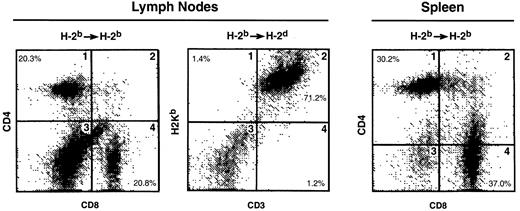
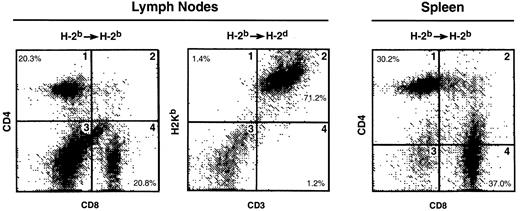
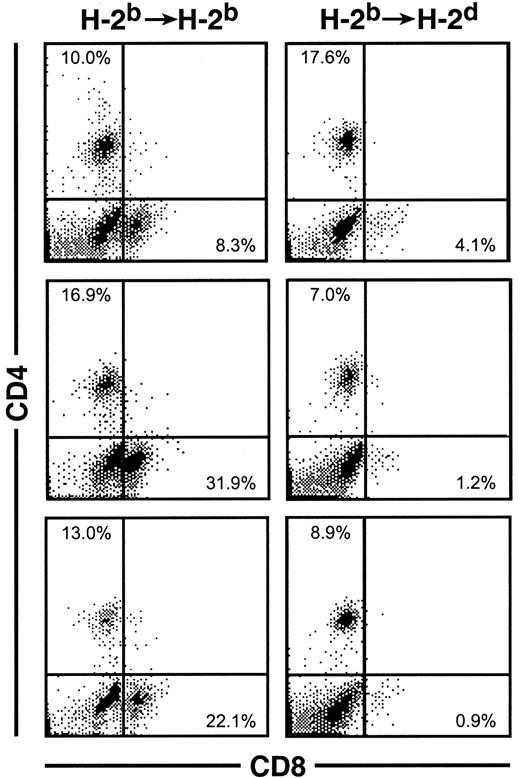
To determine whether the thymic emigrants generated in this organ culture system could function in vivo, they were transferred into syngeneic or allogeneic SCID mice. As shown in Figure11, repopulation of both lymph nodes and spleen was observed in either syngeneic or allogeneic hosts. Both CD4 and CD8 cells were found in syngeneic lymph node (panel 1) and spleen (panel 3). In allogeneic recipients, donor cells were observed in lymph node (panel 2) and spleen (Figure12). Although CD4 cells repopulated both syngeneic and allogeneic recipients similarly, CD8 repopulation was much lower in allogeneic recipients (Figure 12).
Repopulation of lymphoid organs by thymic emigrants.
C57Bl/6 (H-2b) thymic emigrants were generated as in Figure2. Emigrant cells (4 × 106/recipient) were injected intravenously into syngeneic SCID mice or allogeneic CB.16 (H-2d) SCID mice that were treated before transfer with sublethal irradiation and anti-asialoGM1. Two months later, lymphoid organs were analyzed for the indicated markers.
Repopulation of lymphoid organs by thymic emigrants.
C57Bl/6 (H-2b) thymic emigrants were generated as in Figure2. Emigrant cells (4 × 106/recipient) were injected intravenously into syngeneic SCID mice or allogeneic CB.16 (H-2d) SCID mice that were treated before transfer with sublethal irradiation and anti-asialoGM1. Two months later, lymphoid organs were analyzed for the indicated markers.
Splenic repopulation by thymic emigrants.
Emigrants were transferred into syngeneic or allogeneic recipients as in Figure 11. Forty-nine days later, spleen cells were analyzed for CD4 and CD8 expression. Three individual recipients of each type are shown whose total nucleated spleen cell numbers were: syngeneic (2, 3, and 5 × 106) versus allogeneic (0.4, 1, and 4 × 106). The percentage of CD8 cells, indicated in the lower right quadrant, was notably lower in allogeneic recipients.
Splenic repopulation by thymic emigrants.
Emigrants were transferred into syngeneic or allogeneic recipients as in Figure 11. Forty-nine days later, spleen cells were analyzed for CD4 and CD8 expression. Three individual recipients of each type are shown whose total nucleated spleen cell numbers were: syngeneic (2, 3, and 5 × 106) versus allogeneic (0.4, 1, and 4 × 106). The percentage of CD8 cells, indicated in the lower right quadrant, was notably lower in allogeneic recipients.
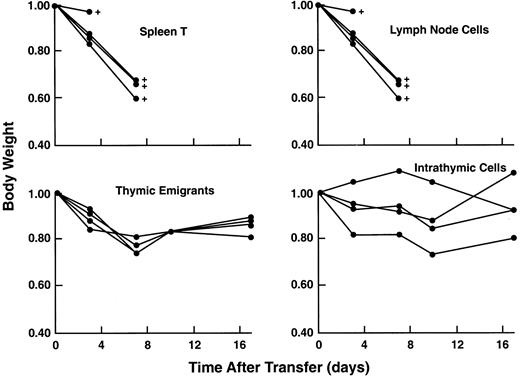
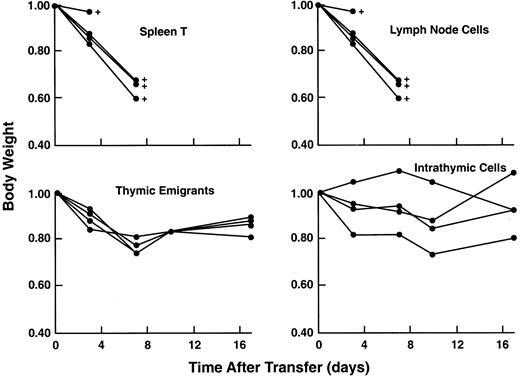
Acute GVHD can be induced by very small numbers of mature peripheral T cells when injected into a SCID mouse (that was previously sublethally irradiated and treated with anti-asialoGm1). Thymic emigrants did not elicit acute GVHD as shown in Figure13. It was surprising, given the vigorous proliferative response of thymic emigrants to alloantigens in vitro (Tables 1 and 2), that they did not induce an acute GVHD even when administered at 8 times the number of splenic T cells that would elicit a lethal acute GVH syndrome. Skin and liver sections from 3 syngeneic and 3 allogeneic recipients were examined (blind) by a veterinary pathologist who reported no indications of GVHD in allogeneic recipients; in fact, syngeneic recipients showed more inflammation and lymphocytic infiltration of both skin and liver. As another possible indicator of T-cell activation in allogeneic hosts, spleen cells were analyzed for expression of CD25 but it was not detected. On the other hand, hematocrits did reflect a potential GVH reaction; 2 of 3 allogeneic recipients (with 31, 34.5, 49.5) fell below normal, whereas all 3 syngeneic recipients (with 43.5, 46, 48.5) were normal and comparable to control mice similarly treated and injected with syngeneic lymph node cells (with 40 and 47.5). This suggests that, although in vivo allogeneic reactivity of emigrants is sufficiently attenuated to protect from acute lethality, significant reactivity in hematopoietic tissues may remain.
Lack of acute GVHD in recipients of thymic emigrants.
C57Bl/6 thymic emigrants (4 × 106) were transferred into allogeneic SCID recipients as in Figure 9 and examined for induction of acute GVHD as indicated by loss of body weight and mortality. For comparison, unfractionated C57Bl/6 lymph node cells (0.5 × 106), purified splenic T cells (0.5 × 106), or unfractionated intrathymic cells (4 × 106) were injected.
Lack of acute GVHD in recipients of thymic emigrants.
C57Bl/6 thymic emigrants (4 × 106) were transferred into allogeneic SCID recipients as in Figure 9 and examined for induction of acute GVHD as indicated by loss of body weight and mortality. For comparison, unfractionated C57Bl/6 lymph node cells (0.5 × 106), purified splenic T cells (0.5 × 106), or unfractionated intrathymic cells (4 × 106) were injected.
Discussion
The properties of thymic emigrants were examined using a new method for collecting cells that emigrate from the fetal thymus during organ culture. Phenotypic analysis indicated that most of the cells that emigrate had a mature phenotype, in that they were single positive for CD4 or CD8 and expressed high levels of TCR. The profile of most surface markers coincided with that of mature splenic T cells, except that CTLA-4 was expressed at a higher level, similar to that on activated T cells. Emigration was induced within 24 hours after positive selection and was blocked by several different inhibitors of cell motility. Functional analysis in vitro indicated full proliferative responsiveness to alloantigens and polyclonal activators. Transferred in vivo, thymic emigrants repopulated lymphoid organs. Unlike mature peripheral T cells, thymic emigrants failed to induce acute GVHD in allogeneic SCID mice. It should be emphasized that this system may only relate to emigration from the fetal or newborn thymus, in that these emigrants correspond to postnatal day 8.
A question of major theoretical interest regarding thymic emigrants is whether they are tolerized by peripheral self-antigens not expressed in the thymus.4,5,20 Thus, although clonal deletion of self-reactive cells would be effective for epitopes expressed in the thymus, a peripheral tolerance mechanism is presumably needed for epitopes not expressed in the thymus. One hypothesis that could account for peripheral tolerance would be that the thymic emigrant is rendered tolerant by antigen contact. It has previously been shown using the intrathymic injection of FITC to tag emigrants, that labeled cells found in the periphery are fully reactive against lectins and alloantigens in vitro.3 However, as noted above, it is unclear how many of the cells detected by this method are mature T cells that were passing through the thymus, rather than newly generated T cells. Hence, the antigen responsiveness previously detected could have been attributable to the presence of recirculating mature T cells, rather than true emigrants.
Our results addressing functional reactivity of thymic emigrants clearly show that, on one hand, emigrants tested in vitro show vigorous proliferative responses against polyclonal activators and alloantigens in primary and secondary MLRs induced by dendritic cell stimulators. Our results, thus, confirm the conclusions reached using the FITC method3 that showed emigrants were equal to mature peripheral T cells in their ability to proliferate to lectins and lyse allogeneic target cells. This reactivity would argue against a simple peripheral tolerance model in which the emigrant is killed or anergized by antigen. On the other hand, emigrants did not induce acute GVHD in SCID mice in vivo, as do fully mature T cells from spleen or lymph node. There are a number of possible explanations for the difference in emigrant functions measured in vivo versus in vitro. (1) Emigrants may be prone to tolerance induction in vivo, perhaps based on their expression of CTLA-4, a primarily negative regulator of T-cell activation,18 or other mechanisms such as preferentially encountering nonprofessional antigen-presenting cells (APCs).21 (2) They may produce a spectrum of cytokines that is less injurious to the host. Thus, although not actually rendered tolerant, their impact would be less severe than mature T cells. Consistent with this explanation, using the FITC method, labeled cells produced more IL-4 and less IL-2 than mature peripheral cells.22 23 (3) Perhaps their migratory pattern does not take them to sites of major GVH injury, such as the intestine or liver. There was no sign of GVHD in liver or skin of allogeneic recipients, although several individuals showed depressed hematocrits that could represent GVHD in hematopoietic tissue. Thus, although our findings show that the emigrant does not acutely harm the allogeneic host, it remains to be shown whether this is actually based on a tolerance mechanism.
Acute GVHD has been induced by both the HSA-positive and HSA-negative fractions of CD4+ thymocytes from adult thymus,24 which seemingly predicts that emigrants would also have the potential to induce acute GVHD. One explanation of the differences from our findings, showing no acute GVH induction by emigrants, is that the adult thymus contains substantial numbers of recirculating mature T cells, even small numbers of which might be capable of inducing acute GVHD. Another explanation is that the embryonic thymocytes used in our studies have different properties from their adult counterparts. However, the same study also observed that unfractionated newborn thymocytes induced acute GVHD. This would seem to indicate a difference in the GVHD model used in those studies compared to ours, because in unfractionated thymus from adults25 26 or embryos (data not shown), we have not observed induction of acute GVHD. That study induced acute GVHD by injecting C3H thymocytes into sublethally irradiated AKR mice (a barrier of minor histocompatibility and minor lymphocyte stimulating antigens [MLS]). Our studies used C57BL/6 thymic emigrants transferred to CB.17 SCID mice (a barrier of MHC, minor histocompatibility, and MLS antigens) that had been sublethally irradiated and treated with anti-asialoGM1.
Fewer CD8 cells were detected in allogeneic recipients than syngeneic recipients, whereas the numbers of CD4 cells were similar in both syngeneic and allogeneic recipients. Perhaps this reflects a requirement for CD8 emigrants to obtain survival signals from the correct MHC in the periphery. On the other hand, expansion of cell numbers must occur after transfer of emigrants, perhaps through stimulation with environmental antigens, and CD8 emigrants may fail to recognize foreign antigens in the allogeneic host and thus fail to expand.
The surface markers on emigrants generated using our method are consistent with markers detected using the in vivo methods,2,27 both methods concluding that mature cells emigrate from the thymus. In our study, heterogeneity of thymic emigrants was noted particularly for expression of L-selectin and HSA. This suggests that subsets develop in the thymus and will have different properties after they leave. For example, L-selectin promotes exit of lymphocytes from the blood and had previously been found on all spleen and lymph node cells labeled by the FITC method.27Our study partly confirms this, in that more emigrants expressed L-selectin than did their mature intrathymic counterparts; nevertheless, 20% of the mature αβ T-cell emigrants did not express L-selectin, and were therefore not detected in the spleen or lymph nodes because they went elsewhere. High HSA expression has sometimes been used as a marker on peripheral cells that identified recent thymic emigrants, whereas low HSA expression on thymocytes has been used as a predictor of whether a single positive thymocyte would soon emigrate.24 However, although our study confirms that high HSA expression does indeed identify some emigrants, the majority of emigrants expressed low or undetectable levels—similar conclusions were reached using the FITC method.2
There were several differences in emigrants previously described in vivo2 versus our in vitro method. The CD4/CD8 ratio was high in vivo and low in vitro, and we detected quite a few (13.1%) γδ cells. We suspect that this difference is because the fetal thymic organ in culture produces more CD8 and γδ cells than does the adult thymus in vivo. Another difference is that the organ culture produces declining numbers of emigrants with time, which parallels decreased thymic cellularity (Figure 1). Perhaps this represents exhaustion of the thymic stem cell (of fetal liver origin), which would normally be replaced by a bone marrow-derived stem cell at about this time after birth. Another possibility would be that the stroma loses its properties with age in organ culture. We also observed that CD25 was expressed on more emigrants than the mature intrathymic cells, suggesting that activation might accompany emigration.
The mechanism of thymic emigration has not been elucidated and this culture system can be used to study this process. Three inhibitors that blocked the emigration process, cytochalasin D, pertussis toxin, andC difficile toxin B, suggest possible mechanisms of emigration as follows.
Emigrants expressed high levels of the β2-integrin, LFA-1, and the β1-integrin, VLA-4. Lymphocyte motility can involve integrins, which reversibly bind their ligands on other cells and on extracellular matrix28 and the integrins on thymocytes demonstrate this reversible binding29; this type of process could be involved in the migration out of the thymus. Because the same integrins are also expressed on immature thymocytes, they would not account for the change that occurs on positive selection, which must relate to other components of the cellular motility machinery. Our finding that pertussis toxin blocks emigration, implicating Gi proteins in thymic emigration, supports a previous report that expression of a pertussis toxin transgene in thymocytes inhibited both their ability to emigrate (they accumulated in the thymus) and to enter lymphoid organs (following intravenous injection they failed to home to lymphoid organs).30 In our study, pertussis toxin appeared to induce congestion of thymocytes in the radial tracks that may be the path of emigration. Perhaps Gi proteins are involved in an integrin-based emigration mechanism along these tracks, because they have been shown to participate in LFA-1–dependent arrest of lymphocytes in high endothelial venules.31
Emigration was blocked by C difficile toxin B an inhibitor of the Rho family of small GTPases (Rho, Rac, and Cdc42) via monoglucosylation. This is consistent with a recent study showing that a dominant negative form of Cdc42 in T-cell lines blocked their migration induced by the chemokine stromal-derived factor 1 (SDF-1).32 This effect could relate to a role for Rho family members in altering the affinity of integrins for their substrates,33 consistent with a role for integrins in the emigration process as suggested above.
Positive selection apparently initiates differentiation changes in a thymocyte that lead to emigration. Differentiation changes previously shown to be induced following positive selection include down-regulation of CD4 or CD8, up-regulation of the CD3 complex, transient up-regulation of CD69, apoptosis resistance, a rise in bcl-2 expression, and other changes. Our results add to this list, showing that a motility response occurs within 24 hours of positive selection. This does not mean that all thymocytes emigrate promptly on positive selection (some estimates place the average medullary residency, in a large adult thymus, at 6-8 days).34 The physiologic significance of this motility change is to retain immature thymocytes within the organ and to trigger their departure when differentiation is complete. We have previously examined the spontaneous motility of thymocytes and noted that, whereas thymocytes are cessile within the organ, when removed from the thymic microenvironment, all the major subsets of thymocytes showed a high level of motility (Kitazawa and Durum, unpublished observations). The fact that only the most mature thymocytes are actually motile in the intact thymus suggests that the motility of the immature subsets is repressed by the thymic environment, whereas positive selection releases the cell from this repression. These signals are now amenable to study using this organ culture system.
We thank L. Finch for flow analysis, R. Wyles for technical assistance and Dr J. Oppenheim for comments on the manuscript.
Laboratory of Molecular Immunoregulation, Division of Basic Sciences, National Cancer Institute and Intramural Research Support Program, Science Application International Corporation, Frederick, MD.
Submitted May 3, 2000; accepted November 9, 2000.
Supported in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract no. N01-CO-5600.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Scott K. Durum, Section of Cytokines and Immunity, National Cancer Institute, Building 560 Room 31-71, Frederick, MD 21702-1201; e-mail: durums@mail.ncifcrf.gov.