Arsenic trioxide is used in clinical trials in the treatment of relapsed and resistant cases of acute promyelocytic leukemia. Adverse effects from arsenic in these studies have been multisystemic. Arsenic is known to cause corrected QT-interval prolongation and T-wave changes, but the potential for serious ventricular arrhythmias is less well understood. Torsades de pointes, a form of ventricular tachycardia, has been reported with arsenic poisoning but not at therapeutic doses used in protocols for hematologic malignancies. We describe 3 patients in whom this arrhythmia developed while they were treated with arsenic trioxide. Early recognition of the arrhythmia or correction of contributory factors is important because arsenic induced ventricular arrhythmias are known to be resistant to most chemical methods and electrical cardioversion.
Introduction
Arsenic trioxide (ATO) induces complete remission in patients with relapsed or refractory acute promyelocytic leukemia.1-7 Arsenic causes partial cytodifferentiation and activation of cysteine proteases (caspases) instrumental in apoptosis.1 Fatal events at therapeutic doses from hepatic toxicity7 or sudden death have been reported (P. Westervelt, personal communication, August 2000, and reference 8). Prolonged corrected QT interval (QTc) is also described.8-10
We are conducting a clinical trial (IND 54209) of ATO in patients with hematologic malignancies. Treatment consists of 10 to 20 mg ATO in 500 mL 5% dextrose/normal saline over 3 hours daily for up to 60 days. We have observed 3 cases of torsades de pointes (TDP) among 19 patients treated to date.
Study design
A 29-year-old man with acute myeloid leukemia (AML) in third relapse was treated. Prior total anthracycline treatment consisted of 72 mg/m2 idarubicin and 36 mg/m2mitoxantrone. He had pancytopenia and was receiving multiple antibiotics and transfusions. Pretreatment QTc and echocardiography findings were normal, and gated imaging showed an ejection fraction of 57%. During treatment with 20 mg/d ATO, drug-related sensory polyneuropathy developed. Blastic leucocytosis (WBC 32 000/μL, 56% blasts) led to increased pulmonary infiltrates that responded to 150-cGy radiation plus hydroxyurea. Diuretics were given for treatment-related fluid overload and capillary leak syndrome.
On day 42 of treatment, the patient was intubated for respiratory distress from progressive leukemia. He subsequently had ventricular premature contractions and ventricular tachycardia (6-21 beats in duration). Serum potassium (K) level was 3.3 mEq/L, and magnesium (Mg) level was 1.7 mg/dL. Electrocardiography (EKG) showed QTc prolonged from baseline (Table 1). He was treated with lidocaine, bretylium, magnesium, and multiple cardioversions to maintain hemodynamic function. Repeat EKG findings were normal. In the next several hours, ventricular tachycardia with the morphology of TDP recurred. Several attempts at electrical cardioversion were made but were unsuccessful.
A 79-year-old woman with pancytopenia was treated with ATO for relapsed myelodysplastic syndrome evolving into AML (no prior anthracycline therapy). Baseline EKG showed sinus tachycardia with normal QTc. Echocardiography revealed normal ventricular function and an ejection fraction of 53%. Twenty-four–hour Holter recording showed sinus rhythm with a single 7-beat run of supraventricular tachycardia. Persantine stress test showed moderate-sized inferolateral and infero-apical fixed perfusion defects with no ischemia or reversibility.
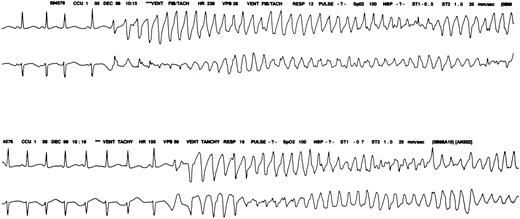
During daily 20 mg ATO treatment, urosepsis and treatment-related fluid overload developed.8 On day 16 of ATO, she required intubation for dyspnea. Chest radiography showed diffuse interstitial infiltrates suspicious for pneumonia or adult respiratory distress syndrome (WBC, 800/μL). Cardiac enzyme levels were normal, but EKG showed a prolongation of QTc from baseline (Table 1). Episodes of TDP developed, and she was treated with intravenous magnesium (Figure1). Her potassium level of 2.4 mg/dL was corrected to 3.1 mEq/L, her magnesium level was 1.7 mg/dL, and the TDP resolved. She later died of hemoptysis, with a cardiac rhythm of sinus bradycardia leading to asystole.
Monitor strip.
Cardiac monitor strip shows torsades de pointes in patient 2.
A 43-year-old man with refractory AML, unresponsive to 4 cycles of standard induction treatment, was referred for ATO treatment (total idarubicin, 72 mg/m2; mitoxantrone, 36 mg/m2). He had pancytopenia and was receiving antibiotics and transfusions. Baseline EKG showed normal QTc, and echocardiography findings were normal. This patient was given 10 mg/d ATO for 7 days when serial prolongation of QTc was noted. Arsenic was discontinued after 7 doses, and QTc intervals gradually shortened.
Twelve days after ATO treatment was started, the patient was intubated for respiratory distress. Later ventricular tachycardia with the morphology of TDP developed, and cardioversion was attempted multiple times because of hemodynamic instability. Serum electrolytes were maintained at high-normal levels (K = 4.0 mEq/L; Mg = 2.5 mg/dL), but the patient had recurrent arrhythmias and died.
Results and discussion
TDP, observed in arsenic poisoning,11 has not been reported to date in patients on therapeutic trials of ATO.8,9 Cardiac toxicity reported in arsenic poisoning includes QTc prolongation and T-wave inversion,6,12second-degree heart block,1 and complete heart block requiring pacemaker insertion.13 T-wave alternans—a condition in which the T wave is prolonged, morphologically bizarre, and varied from beat to beat—is reported.13 14
TDP is a ventricular tachycardia with QRS complexes of changing amplitude that twist around the isoelectric baseline (Figure 1). Arrhythmia may be associated with prolongation of QTc interval or prominent U waves. This signifies an abnormality in repolarization of the ventricles, which enhances the probability of ventricular re-entry.
The causative association of ATO administration with our cardiac findings is suggested by the following: (1) before ATO therapy, all patients had normal QTc intervals; (2) prolongation of QTc interval was noted before the administration of any antiarrhythmic agent; (3) continuous prolongation of QTc interval was observed throughout treatment with ATO; (4) other possible causes, such as electrolytes, infection, and hypoxia, were being treated or corrected, though they might have been contributory.
In contrast to TDP induced by antiarrhythmic agents, which commonly occurs within 48 to 72 hours of drug initiation,15 we observed its occurrence at later time points (42, 16, and 12 days of treatment). This delayed onset may result from an accumulation of tissue levels of ATO.16 High concentrations of the compound in heart and lungs at blood levels, safe for short treatment protocols, have been reported 6.
The mechanism of QTc prolongation by arsenic is unclear. It is unknown whether neuropathy of the cardiac sympathetic system is involved because arsenic is known to affect the peripheral nervous system and to cause sympathetic input imbalance.17,18Direct myocardial effects have not been studied. Interactions of arsenic with magnesium may be important in the genesis or resistance of the arrhythmia to conventional treatment. Magnesium suppresses triggered arrhythmias by decreasing the amplitude of early after-depolarizations (calcium-inflow blockade) to subthreshold values.17,19,20 In membrane studies, arsenic affects the total conductance of ions, which is not corrected with supplemental magnesium.20
Treatment of ventricular arrhythmias with prolonged QTc caused by arsenic is problematic. Tachycardia is made worse by quinidine and procainamide, which prolong QTc intervals. Phenytoin, lidocaine, and β-blockers do not cause prolongation of QTc and can be used, though their efficacy in arsenic toxicity has been controversial. In the short term, isoproterenol is the drug of choice because it shortens the QTc interval by its effect on membranes and by increasing the heart rate. If medical therapy fails, overdrive pacing may be useful.20
A very long QTc (longer than 600 msec) is a marker of increased risk. However, there is significant overlap between innocuous and arrhythmogenic prolongation, and the QTc interval is of limited value in predicting TDP. The value of monitoring is to identify warning signs, such as the appearance of prominent U waves, extra-systole, U-wave augmentation after extra-systole, ventricular bigeminy, prolongation of QTc intervals from baseline, and abnormal T waves (biphasic T waves, T-wave alternans).14 18 Factors that contribute to QTc prolongation should be aggressively corrected (electrolytes) or removed (drugs).
Patients administered ATO should be monitored by electrocardiography and by the assessment of QTc intervals, preferably daily while they are in treatment. Patients with increased QTc intervals or prolongation of QTc intervals from baseline may benefit from continuous electrocardiographic monitoring. Additional data regarding the predictability and degree of QTc prolongation and TDP are needed, as is further definition of a therapeutic approach. If ATO accumulation is contributory, as some studies suggest, intermittent schedules of ATO should be investigated. Lipid encapsulation of the agent should be considered a potential method to reduce cardiotoxicity by providing a slower release formulation.
Supported by NIH grant R03-CA79399, FDA Orphan Drug Grant FD-R-001699, and Leukemia Society of America Translational Research Award 6121-99.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Janice P. Dutcher, Our Lady of Mercy Cancer Center, 600 East 233rd St, Bronx, NY 10466; e-mail: jpd4401@aol.com.