In this study, 2 distinct populations of mature dendritic cells (DCs) were identified in the human thymus. The major population is CD11b−, CD11c+, and CD45ROlowand does not express myeloid-related markers. It displays all the characteristics of mature DCs with a typical dendritic morphology, high surface levels of HLA-DR, CD40, CD83, and CD86, and expression of DC–lysosome-associated membrane glycoprotein messenger RNA (mRNA). In addition, CD11b− thymic DCs do not express macrophage inflammatory protein-1α (MIP-1α) mRNA, but express thymus-expressed chemokine (TECK) mRNA and are able to secrete bioactive interleukin 12 (IL-12) upon stimulation. In contrast, the minor and variable thymic DC population is CD11b+, CD11chigh, and CD45ROhigh and comprises CD83+CD14− mature and CD83−CD14+ immature DCs. It expresses macrophage-colony stimulating factor receptor, MIP-1α mRNA and high amounts of decysin mRNA after CD40 activation, but does not express TECK and is a weak bioactive IL-12 producer. Also identified were the IL-3Rαhigh plasmacytoid cells, which are present in the thymic cortex and medulla. Upon culture with IL-3, granulocyte/macrophage–colony stimulating factor, and CD40 ligand, the plasmacytoid cells can adopt a phenotype resembling that of freshly isolated CD11b− thymic DCs. However, these plasmacytoid-derived DCs fail to secrete bioactive IL-12; therefore, conclusions cannot be made about a direct relation between thymic plasmacytoid cells and CD11b− DCs. Whereas CD11b+ thymic DCs appear to be related to tonsillar germinal-center DCs, the major CD11b− IL-12–secreting human thymus DC population has similarities to mouse CD11b− CD8+ DCs.
Introduction
Dendritic cells (DCs) are professional antigen presenting cells1 that form a dynamic network throughout most tissues and organs and that are crucial for the immune surveillance of the body.2-4 The current model is that Langerhans cells, immature DCs found in epithelia, are the precursors of mature “interdigitating” DCs, found in the T-cell zones of lymphoid organs.5 Immature interstitial DCs in various tissues, such as the dermis, move into germinal centers as germinal center DCs (GCDCs). In the lymph nodes, tonsils, and spleen, the mature DCs present the antigen captured in the periphery to naive T cells and induce immunity. The DCs of the thymus have a somewhat different role, namely, to present self-antigens and induce negative selection of potential auto-reactive T-cell clones.6 Until recently, the interdigitating DCs were considered a single population of mature DCs. However, this laboratory and others showed that distinct DC subsets of different lineage derivation and different functions7-13 could be distinguished in mouse lymphoid organs. On the one hand, CD8+ DCs, lacking myeloid markers such as CD11b and apparently arising from a lymphoid-committed progenitor,14 are typically potent interleukin 12 (IL-12)–secreting mature DCs.10,13,15 CD8+DCs are present at various levels in all mouse lymphoid organs and constitute the major population in the thymus.15 On the other hand, CD8− DCs, which comprise CD4+ and CD4− subsets,16 express CD11b, appear to be myeloid related, and are weak IL-12 producers.10 13
Whereas in mice our concepts of DC life history rely mainly on in vivo studies, investigations of mature human DCs are based mainly on in vitro models of DC generation. Several human DC precursors have been identified, including peripheral blood monocytes17-20 and earlier CD34+ hematopoietic progenitors.21,22All were shown to differentiate into DCs exhibiting high levels of major histocompatibility complex class II and costimulatory molecules upon culture with granulocyte/macrophage–colony stimulating factor (GM-CSF) and IL-4 or tumor necrosis factor α (TNF-α). Recently, the CD4+CD3−CD11c− plasmacytoid cells, whose origin and function had been an enigma, were shown to differentiate into DCs upon culture, albeit with an atypical surface phenotype and without a capacity to secrete IL-12.23-25Such plasmacytoid cells are found in tonsils,23blood,24,26 cord blood,27 and thymus.28 They were subsequently considered to embody the “lymphoid-related” DC lineage in humans as, first, they express the pre–T-cell receptor alpha chain28,29 and, second, in contrast to monocyte- or CD34+ progenitor–derived DCs, plasmacytoid-derived DCs do not express the “myeloid-related” markers CD11b, CD11c, and CD33 and strikingly retain CD45RA expression.23,25,28 Moreover, as in the lymphoid-restricted DC precursor in the mouse,30 growth of plasmacytoid cells requires IL-3 but not GM-CSF.23However, there remains one problem in considering the plasmacytoid cells to be a normal step in DC development. Although they differentiate into DCs in vitro, their mature dendritic form is still to be identified in vivo.
Studies of mature DCs in humans ex vivo31-36 have been hampered by the difficulty of isolating these cells. Often, short-term culture steps were required to overcome this problem. In the present study, we use freshly isolated DCs to give an instant picture of a human DC network, adapting a purification procedure used in the mouse15 to human tissue. We have studied human thymus, which represents a good source of mature DCs in terms of the yield and the consistency between donors. We have attempted to compare the mature DC forms found in vivo with those generated in vitro and to compare human with mouse thymus DCs. We provide an extensive phenotype and characterization of these DCs and show that 2 distinct populations of mature DCs, segregated on the basis of CD11b expression, can be isolated from the human thymus. The major population does not express CD11b and shares similarities with thymic plasmacytoid-derived DCs grown in the presence of IL-3, CD40 ligand (CD40L) plus GM-CSF, whereas the minor DC population is CD11b+, arises from immature myeloid DCs, and is related to tonsillar GCDCs.
Materials and methods
Monoclonal antibodies
The following mouse anti–human monoclonal antibodies (mAbs) were purified and conjugated in this laboratory from supernatants of hybridomas obtained from American Type Culture Collection (Manassas, VA): anti-CD3 (OKT3), anti-CD8 (OKT8), anti-CD7 (3A1), anti-CD15 (WEMG1), anti-CD19 (FMC-63), anti-CD20 (B1), anti-glycophorinA (10F7MN), anti-CD40 (G28.5), anti-CD45RO (UCHL-1), fluorescein isothiocyanate–conjugated (FITC-conjugated) anti-CD20 (B1), anti–HLA-DR (2.06), phycoerythrin-conjugated (PE-conjugated) anti-CD20 (B1), Cy5-conjugated anti–HLA-DR (2.06), and biotin-conjugated anti-CD11b (OKM1). The following mAbs were purchased commercially: purified anti-CD86 (BU63) from Ancell (Bayport, MN); FITC-conjugated anti-CD1a (HI149), anti-CD14 (M5E2), anti-CD16 (3G8), anti-CD33 (HIM3-4), anti-CD40 (5C3), anti-CD45RO (UCHL-1), anti-CD64 (10.1), PE-conjugated anti-CD86 (IT2.2), anti–IL-3Rα (9F5, nonblocking), biotin-conjugated anti-CD86 (IT2.2), and anti–IL-3Rα (7G3) from Pharmingen (San Diego, CA); PE-conjugated anti-CD3 (UCHT-1), anti-CD4 (Leu-3a), anti-CD8 (Leu-2a), and anti-CD11c (SHCL-3) from Becton Dickinson (San Jose, CA); FITC-conjugated anti-CD11b (BEAR1), PE-conjugated anti-CD83 (HB15A), and anti–GM-CSFRα (SCO6) from Immunotech (Marseilles, France); purified anti-CD56 (NKH1) from Beckman-Coulter (Fullerton, CA); and PE-conjugated anti-CD45RA (4KB5) and anti-CD56 (MOC1) from Dako (Glostrup, Denmark).
Immunohistological localization of thymic DCs and plasmacytoid cells
Stainings on human tissue sections were performed as previously described.37 Cryostat sections of frozen human thymus were fixed in acetone at 4°C and stored at −20°C. After thawing and remoisturizing in phosphate-buffered saline, sections were double-stained simultaneously with either mouse immunoglobulin (Ig)–G1 anti-CD40 or mouse IgG1 anti-CD86 (BU63), and mouse IgG2a anti–IL-3Rα. The binding of mouse IgG1 antibodies was revealed by sheep anti–mouse IgG1 (The Binding Site, Birmingham, United Kingdom), followed by mouse antialkaline phosphatase-alkaline phosphatase complexes (Dako), the APAAP technique. The binding of mouse IgG2a antibodies was revealed by sheep anti–mouse IgG2a-biotin (The Binding Site) and was followed by incubation with ExtrAvidin-Peroxidase (Sigma Chemical, St Louis, MO). Alkaline phosphatase activity was revealed by the Fast Blue substrate (Sigma), and peroxidase activity by 3-amino-ethylcarbazole (Sigma).
Four-color immunofluorescence staining and flow cytometry
After incubation with directly labeled antibodies, cells were washed twice and stained with streptavidin–Texas Red (Amersham Life Science, Buckinghamshire, United Kingdom) when a biotin-conjugated antibody was used. Analysis was carried out by means of a FacStar Plus (Becton Dickinson), and for each marker, a single-stained control was done to adjust color compensations. At least 8 × 104events were analyzed per sample.
DC purification
Human thymus samples were discarded tissue from young children undergoing corrective cardiac surgery and were obtained according to institutional guidelines. The tissue was cut into small fragments, suspended in 10 mL RPMI 1640 containing 2% fetal calf serum (FCS); collagenase (1 mg/mL, type II) (Worthington Biochemical, Freehold, NJ); and DNAse (0.02 mg/mL; grade II bovine pancreatic DNAseI) (Boehringer Mannheim, Mannheim, Germany) and then digested with intermittent agitation for 15 minutes at 37°C followed by 5 minutes at room temperature with constant agitation. To disrupt DC–T-cell complexes, EDTA was added (to 0.01 M final) to the digest, and incubation with agitation was continued for 5 minutes. The suspension was then passed through a stainless steel sieve to remove aggregates and stromal material. All remaining procedures were performed at 0°C to 4°C and used a buffered NaCl/KCl salt solution lacking divalent metals and containing EDTA (EDTA-SS). The cells were recovered from the digest by centrifugation. Then the pellet was immediately resuspended in Nycodenz medium (Nycomed Pharma, Oslo, Norway) (1.068g/cm3 and iso-osmotic with human serum), and a low-density fraction was collected after centrifugation at 1700g for 10 minutes. The low-density fraction was diluted in EDTA-SS, and the cells were recovered by centrifugation. For DC purification, the cells were then incubated for 25 minutes with a mixture of mAbs, including anti-CD3, anti-CD8, anti-CD7, anti-CD15, anti-CD19, anti-CD20, and anti-glycophorinA in EDTA-SS containing 2% human serum. After incubation, the cells coated with mAbs were removed by 2 cycles of sheep anti–mouse immunoglobulin–coupled magnetic beads (Dynabeads, Dynal, Oslo, Norway). The first cycle was at a 3:1 and the second at a 6:1 bead-to-cell ratio. The cells were then kept overnight at 4°C in EDTA-SS containing 10% FCS. The next morning, the cells were incubated for 25 minutes at 4°C with Cy5-conjugated anti–HLA-DR and biotinylated anti-CD11b in EDTA-SS containing 2% human serum. After 2 washes, the cells were incubated with streptavidin–Texas Red. DC populations were then sorted by means of a FacStar Plus. Purity was always superior to 97% after reanalysis. It was determined that this level of staining with anti–HLA-DR antibody did not interfere with T-cell–stimulation assays.
Plasmacytoid cell purification
For plasmacytoid cell isolation, the undepleted thymic cell preparation was stained with PE-conjugated anti–IL-3Rα (nonblocking) and Cy5-conjugated anti–HLA-DR. Plasmacytoid cells were sorted on the basis of high IL-3Rα and low HLA-DR fluorescence by means of a MoFlow (Cytomation, Fort Collins, CO). Purity was always superior to 98% after reanalysis.
Short-term culture of isolated DCs
Both thymic DC populations (105) were cultured in 96-well plates (Becton Dickinson Labware, Franklin Lakes, NJ), in 150 μL of complete RPMI (RPMI-1640 supplemented with 10% heat-inactivated FCS; Hepes buffer, pH 7.2; 2 mM glutamine; and 10−4 M 2-mercaptoethanol and antibiotics) containing 7.5 μg/mL of agonistic anti-CD40 (G28.5). After 1 to 3 days of activation, DCs were either recovered, washed, and incubated with SS-EDTA for further cell-surface staining, or recovered, washed, and lysed. Measure of CD11b and CD14 expression after sorting and after culture was performed by means of a FACScan (Becton Dickinson). A different clone of anti-CD11b antibody recognizing a different epitope was used to assess CD11b expression after sorting.
For IL-12 production, isolated DCs (5 × 104 or 105) were cultured in 96 round-bottom plates (Becton Dickinson Labware) in a final volume of 200 μL complete RPMI containing recombinant human (rh)GM-CSF (50 ng/mL) (kind gift from Dr D. Lynch, Immunex, Seattle, WA); rhIL-4 (20 ng/mL) (Peprotech, Rocky Hill, NJ); rh interferon (IFN)–γ (20 ng/mL) (Peprotech); and soluble trimeric CD40L (1 μg/mL) (kind gift from Dr D. Lynch, Immunex).
Culture of isolated plasmacytoid cells
Plasmacytoid cells (5 × 104) were cultured in flat-bottom 96-well plates (Becton Dickinson Labware) in 200 μL complete RPMI containing 25 ng/mL rhIL-3 (Peprotech) and 1 μg/mL of soluble trimeric CD40L, with or without 100 ng/mL rhGM-CSF. On day 2 of culture, 100 μL supernatant was recovered for IL-12 enzyme-linked immunosorbent assay (ELISA), and fresh medium was added. On day 5, plasmacytoid-derived DCs were restimulated with either fresh soluble CD40L (1 μg/mL) alone or CD40L (1 μg/mL), rhGM-CSF (100 ng/mL), rhIL-4 (20 ng/mL), and rhIFN-γ (20 ng/mL). Supernatants were then recovered on day 7 for IL-12 ELISA.
Reverse transcriptase–polymerase chain reaction
Total cytoplasmic RNA was prepared from freshly sorted or activated DCs by means of RNeasy Mini kit (Qiagen, Hilden, Germany). First-strand complementary DNA (cDNA) was synthesized by means of random hexamers, deoxynucleotide triphosphate (dNTP) mixture, rRNAsin ribonuclease inhibitor, and Moloney murine leukemia virus reverse transcriptase (all reagents from Promega, Madison, WI). The polymerase chain reaction (PCR) reaction was carried out in a final volume of 50 μL containing 1 μL cDNA, 1.5 mM MgCl2, thermo-reaction buffer (Promega), dNTP mixture (0.2 mM each dNTP) (Promega), 0.5 mM each oligonucleotide primer (Geneworks Pty, Adelaide, Australia), and 2 U Taq DNA polymerase (Boehringer Mannheim). Each set of 25 to 35 cycles was performed by means of a PerkinElmer thermal cycler (PerkinElmer/Cetus, Norwalk, CT). After 3 minutes of inactivation at 94°C, each cycle consisted of 30 seconds of denaturation at 94°C, 30 seconds of annealing at 60°C, and 30 seconds of extension at 72°C. A final extension at 72°C was carried out for 5 minutes. For analysis, 10 μL of each reaction was electrophoresed through a 2% agarose gel. Monocyte-derived DCs (MoDCs) used as controls were produced from peripheral blood monocytes selected by adherence followed by depletion of contaminants by means of anti-CD3, anti-CD8, anti-CD20, anti-CD15, anti-CD56, and anti-glycophorinA mAbs. Monocytes were then cultured for 6 days with rhGM-CSF (100 ng/mL) and rhIL-4 (20 ng/mL). MoDCs were activated by means of an agonistic anti-CD40 (7.5 μg/mL) for 24 hours. T cells and B cells used as controls were sorted from tonsils on the basis of CD3 and CD20 expression, respectively.
The following primers were used for the PCR: β-actin forward primer 294-325: ATC TGG CAC CAC ACC TTC TAC AAT GAG CTG CG; β-actin reverse primer 1100-1131: CGT CAT ACT CCT GCT TGC TGA TCC ACA TCT GC; DC–lysosome-associated membrane glycoprotein (DC-LAMP) forward primer 530-549: 5′-GTA ACC AGA CCA CCC TTC CA-3′; DC-LAMP reverse primer: 1129-1148 5′-AGG CTT GAA GTT GGA CAT CG; decysin forward primer 693-712: AGA CTT TCT TCG GGC ACA GA; decysin reverse primer 1175-1194: CCA GAG GGA CAC TTG GTG TT; M-CSFR forward primer 2719-2751: TCC AAC TAC ATT GTC AAG GGC AAT GCC CGC CT; M-CSFR reverse primer 3047-3078: CAG ATT GGT ATA GTC CCG CTC TCT CCT GTC CT; thymus-expressed chemokine (TECK) forward primer 404-423: 5′-GCA GCA AGA GGA ATG TCT CC-3′; TECK reverse primer 798-817: 5′-TCT GGT CAT TCA AGG ATC CC-3′; MIP-1α38 forward primer 5′-CGA GCC CAC ATT CCG TCA CC-3′; MIP-1α reverse primer 5′-CGC ATG TTC CCA AGG CTC AGG-3′.
T-cell stimulation assays
Naive CD4+ T cells were purified from peripheral blood obtained from healthy volunteers. Mononuclear cells were recovered after centrifugation over Ficoll-hypaque (Ficoll-Paque Plus) (Pharmacia Biotech, Uppsala, Sweden). Monocytes/macrophages were subsequently removed by a 1-hour adherence step in RPMI-1640 at 37°C in 150-cm2 culture flasks (Nunc, Roskilde, Denmark) at 106 cells per milliliter. The nonadherent cells were then collected by gently washing the flasks. The naive CD4 T cells were depleted of contaminating cells by incubation with a mixture of mAbs including anti-CD19, anti-CD20, anti-CD45RO, anti-CD8, anti-CD11b, anti-CD15, anti-glycophorinA, and anti-CD56, followed by 2 rounds of Dynabeads (Dynal). The purity of CD4+CD3+CD45RA+ T cells was greater than 90%. Sorted DCs were used as stimulator cells for naive allogeneic CD4 T cells. DCs (10 to 3000) were cocultured with 1.5 × 104 T cells in round-bottom 96-well plates (Becton Dickinson Labware) by means of complete RPMI. After 5 days of coculture, cells were pulsed for 9 hours with 1 μCi3H-TdR per well and then harvested, and thymidine incorporation was measured by liquid scintillation counting. Assays were performed in triplicate, and results were expressed as mean counts per minute (SD).
IL-12 p70 quantitation by ELISA
Aliquots of DCs and plasmacytoid cell culture supernatants were assayed by 2 site ELISAs. Briefly, 96-well polyvinyl chloride microtiter plates (Dynatech Laboratories, Chantilly, VA) were coated with purified capture mAb (anti–human IL-12 p70; 20C2) (Pharmingen). Cytokine binding was then detected with a biotinylated detection mAb (anti–human IL-12 p40; C8.6) (Pharmingen). The readout was then obtained by using streptavidin-horseradish peroxidase conjugate (Amersham Life Science) and a substrate solution containing 548 mg/mL ABTS (2,2′-Azino-bis 3-ethylbenz-thiazoline-6-sulfonic acid) (Sigma) and 0.001% hydrogen peroxide (Ajax Chemicals, Auburn, Australia) in 0.1 M citric acid, pH 4.2, followed by scanning the optical density at 405 to 490 nm.
Results
The surface phenotype of mature human thymic DCs
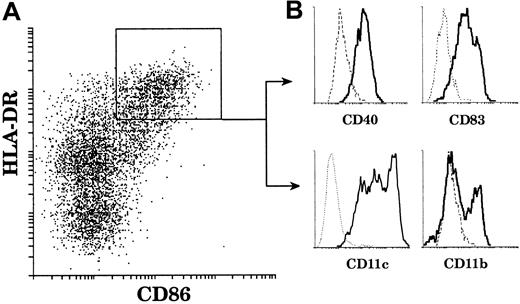
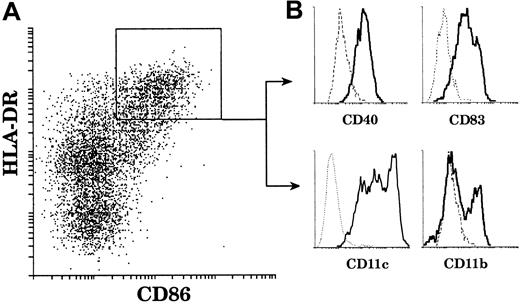
In order to study mature thymic DCs, which represent only a small fraction of thymic cells (0.02%), we first enriched the light-density cells (3%) after collagenase digestion and EDTA treatment of the thymic tissue. All detectable DCs were in this fraction. We then carefully depleted non-DC contaminants and in particular CD4−CD19+CD20+ B-cell populations, which expressed surprisingly high levels of costimulatory molecules (data not shown). Finally, we stained the preparation for immunofluorescence using 4 colors, analyzed the preparation by flow cytometry, and focused attention on cells expressing high levels of HLA-DR as our primary criteria for mature DCs. Other markers were then used to confirm a mature DC phenotype. As shown in Figure1, thymic DCs gated on HLA-DRhighCD86high were also CD40high and CD83high. Thus, these thymic DCs displayed a surface phenotype typical of mature DCs. Furthermore, HLA-DRhighCD86high DCs were all CD11c+. The broad CD11c profile suggested the existence of different DC populations but did not allow clear segregation for further sorting. In contrast, CD11b expression revealed 2 populations of mature DCs: the major population was CD11b−, and the minor one CD11b+. The proportion of the respective populations varied among donors, but the proportion of the CD11b− DC population was always above 65% of the HLA-DRhighCD86high mature DCs.
CD11b− and CD11b+ mature thymic DCs.
Mature thymic DCs comprise 2 populations segregated by CD11b expression. DCs were released from a thymus tissue sample, light-density cells selected, and non-DC lineages including T and B cells depleted. The enriched DCs were then immunofluorescent stained and analyzed. (A) DCs were gated on the basis of high HLA-DR (Cy5) and positive CD86 (Texas Red) fluorescence. (B) HLA-DRhighCD86+ DCs express CD40 (FITC) and CD83 (PE). All express CD11c (PE); however, a major population (65%) lacks CD11b (FITC) expression and a minor population (35%) is CD11b+. This figure is representative of 4 separate thymuses.
CD11b− and CD11b+ mature thymic DCs.
Mature thymic DCs comprise 2 populations segregated by CD11b expression. DCs were released from a thymus tissue sample, light-density cells selected, and non-DC lineages including T and B cells depleted. The enriched DCs were then immunofluorescent stained and analyzed. (A) DCs were gated on the basis of high HLA-DR (Cy5) and positive CD86 (Texas Red) fluorescence. (B) HLA-DRhighCD86+ DCs express CD40 (FITC) and CD83 (PE). All express CD11c (PE); however, a major population (65%) lacks CD11b (FITC) expression and a minor population (35%) is CD11b+. This figure is representative of 4 separate thymuses.
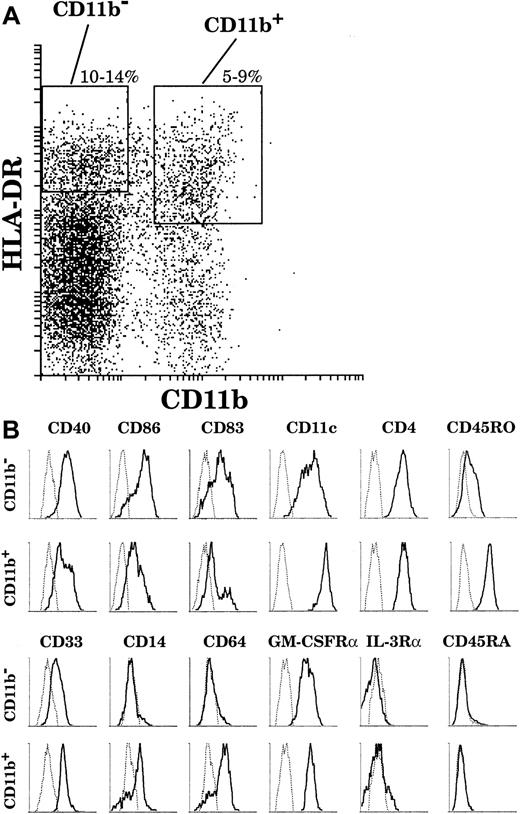
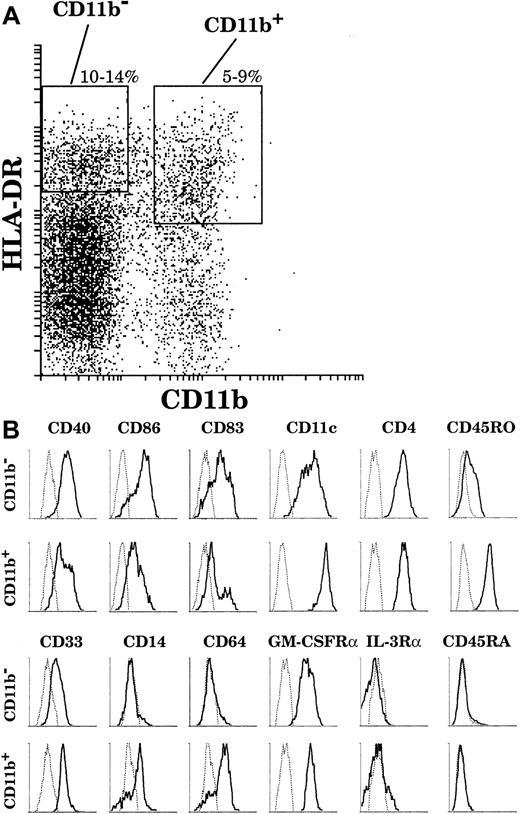
On the basis of this analysis, we adopted the sorting strategy shown in Figure 2A to collect sufficient numbers of cells to study both CD11b− and CD11b+ DC populations. Both populations were simultaneously sorted on the basis of high HLA-DR and negative or positive CD11b fluorescence, together with characteristic high forward and side scatter. In order to recover a sufficient number of CD11b+ cells, we chose to include among the CD11b+ cells some expressing slightly lower levels of HLA-DR, although these would then be expected to include some less mature DCs.
The surface phenotype of thymic DCs.
(A) By means of 4-color analysis, DCs were gated as HLA-DRhigh(Cy5)CD11b− (Texas Red) and HLA-DRhigh/intCD11b+. (B) Both fractions were then analyzed by means of FITC and PE channels. Particular cases: CD86 and IL-3Rα expressions were revealed in Texas-Red after the gating was carried out with FITC-conjugated anti-CD11b antibody. This analysis is representative of 6 separate thymus samples.
The surface phenotype of thymic DCs.
(A) By means of 4-color analysis, DCs were gated as HLA-DRhigh(Cy5)CD11b− (Texas Red) and HLA-DRhigh/intCD11b+. (B) Both fractions were then analyzed by means of FITC and PE channels. Particular cases: CD86 and IL-3Rα expressions were revealed in Texas-Red after the gating was carried out with FITC-conjugated anti-CD11b antibody. This analysis is representative of 6 separate thymus samples.
As revealed by means of 4-color staining (Figure 2B), CD11b− DCs were CD40high, CD86high, and CD83high and expressed CD11c and CD4. These cells did not express the myeloid markers CD14 and CD64 and expressed only a low level of CD33. Interestingly, in contrast to the phenotype of plasmacytoid cells, CD11b− DCs expressed the GM-CSFRα but not the IL-3Rα; did not express the marker of immature leucocytes, CD45RA; and expressed only low levels of the marker of mature leucocytes, CD45RO. On the other hand, the CD11b+ DC fraction comprised mature CD40highCD86highCD83highCD14−CD64−cells as well as less mature CD40lowCD86lowCD83lowCD14+CD64+DCs. All CD11b+ DCs expressed CD4 and the GM-CSFRα, but did not express either the IL-3Rα or CD45RA. Importantly, in contrast to CD11b− DCs, all CD11b+ DCs expressed high levels of CD11c, CD33, and CD45RO, regardless of their state of maturation.
Both CD11b− and CD11b+ populations display functional and morphological characteristics of DCs
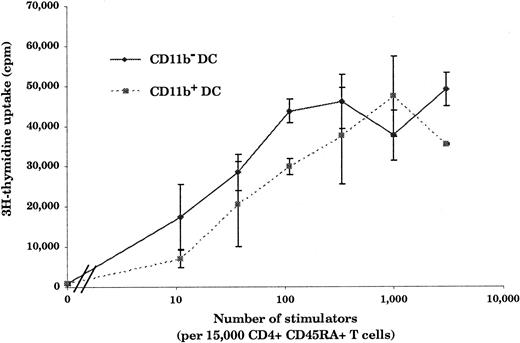
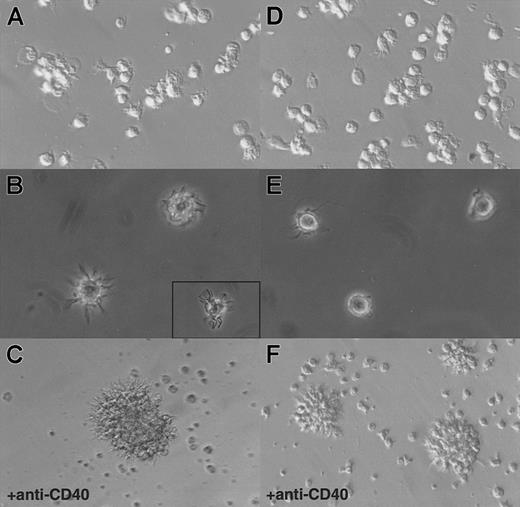
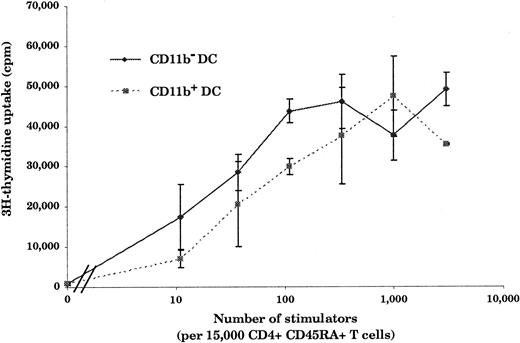
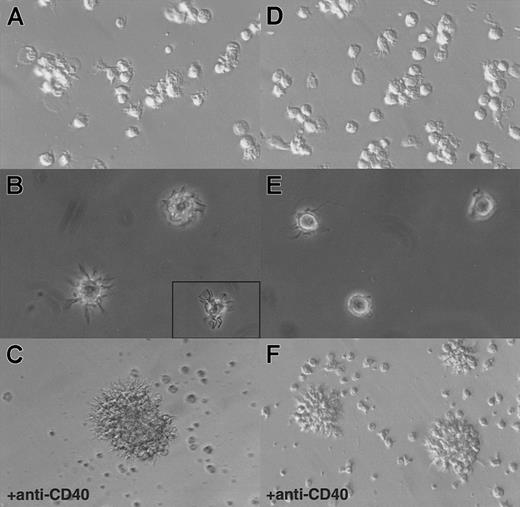
Hallmarks of DCs are, first, their unique ability to present antigens and stimulate naive T cells and, second, the exhibition of dendrites.1 As shown in Figure3, in an allogenic–mixed-leukocyte reaction (MLR) assay, both CD11b− and CD11b+DCs, sorted as shown in Figure 2A, proved to be potent stimulators of naive CD4 T cells. As shown in Figure 4A and B, CD11b− DCs just after sorting displayed a stellate morphology, typical of fully mature DCs. The CD11b+fraction (Figure 4D-E) comprised cells of dendritic morphology as well as “hairy” cells, which were not adherent to glass or plastic. We observed a rapid clustering (1 to 2 hours) of CD11b− DCs in culture, and after CD40 activation for 24 hours, we observed large clusters of DCs exhibiting long dendrites (Figure 4C). Freshly sorted CD11b+ DCs clustered slowly (3 to 5 hours) and, after CD40 activation, formed small clusters of cells displaying long dendrites (Figure 4F). The morphological features before and after activation, coupled with the fundamental property of activating naive T cells, indicated that both CD11b− and CD11b+fractions were DCs, albeit with some differences in form.
Stimulation of naive CD4+ T cells by CD11b− and CD11b+ DCs.
Both CD11b− and CD11b+ DCs are potent stimulators of naive CD4+ T cells. Freshly isolated DCs (10 to 3 × 103) were cultured for 5 days with 15 × 103 allogeneic CD45RA+CD4+T cells. As few as 10 CD11b− or CD11b+ DCs were able to induce naive T-cell proliferation. This figure is representative of 3 experiments.
Stimulation of naive CD4+ T cells by CD11b− and CD11b+ DCs.
Both CD11b− and CD11b+ DCs are potent stimulators of naive CD4+ T cells. Freshly isolated DCs (10 to 3 × 103) were cultured for 5 days with 15 × 103 allogeneic CD45RA+CD4+T cells. As few as 10 CD11b− or CD11b+ DCs were able to induce naive T-cell proliferation. This figure is representative of 3 experiments.
Morphology of CD11b− and CD11b+DCs freshly after sorting or after 24 hours of CD40 activation in culture.
(A) CD11b− DCs exhibit dendrites immediately on isolation and rapidly cluster. (B) A closer view shows that CD11b−DCs display a typical dendritic morphology. (C) After CD40 activation, CD11b− DCs form large, tight clusters of cells displaying long dendrites. (D) (E) Freshly isolated CD11b+ DCs do not adhere to glass or plastic and display a “hairy” morphology. (F) After CD40 activation, CD11b+ DCs form small clusters of cells exhibiting dendrites. Original magnification: 20 × (panels A, D, C, and F); 40 × (panels B and E).
Morphology of CD11b− and CD11b+DCs freshly after sorting or after 24 hours of CD40 activation in culture.
(A) CD11b− DCs exhibit dendrites immediately on isolation and rapidly cluster. (B) A closer view shows that CD11b−DCs display a typical dendritic morphology. (C) After CD40 activation, CD11b− DCs form large, tight clusters of cells displaying long dendrites. (D) (E) Freshly isolated CD11b+ DCs do not adhere to glass or plastic and display a “hairy” morphology. (F) After CD40 activation, CD11b+ DCs form small clusters of cells exhibiting dendrites. Original magnification: 20 × (panels A, D, C, and F); 40 × (panels B and E).
CD11b− and CD11b+ thymic DC populations are distinct
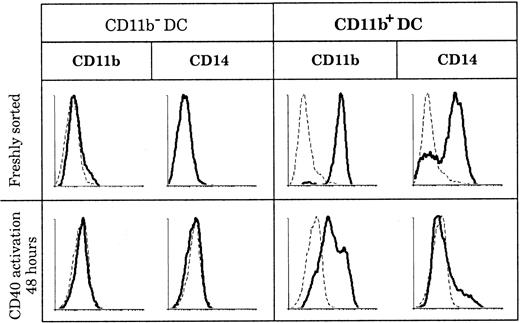
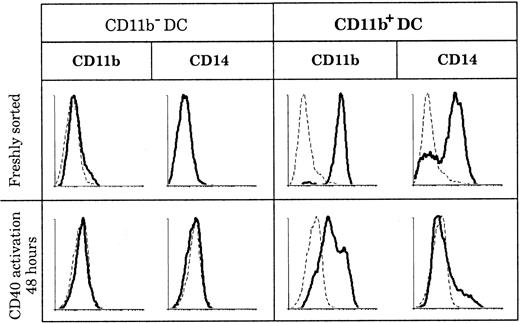
CD11b expression by DCs has been described in both humans and mice as having the ability to be transient and as depending on the state of activation. In mice, CD8+ DCs were shown to acquire CD11b expression after activation,8 and in humans “fraction 1” DCs in blood up-regulated CD11b following maturation.39 In order to determine if the discrepancies in CD11b expression that we observed among human thymic DCs reflected different activation states of the same DC population, we activated both thymic DC populations and measured CD11b expression. As shown in Figure 5, CD11b− DCs did not express CD11b or CD14, either when freshly isolated or after 2 days of CD40 activation by means of an agonistic anti-CD40 antibody. In parallel, CD11b+ DCs slowly down-regulated CD11b after 2 days of CD40 activation, but they still retained low levels of CD11b even after 3 days. Importantly, CD14 and CD64 expression was lost early in culture (less than 24 hours) by the immature CD11b+fraction, suggesting that these immature DCs needed only CD40 signaling to mature.
Marker persistence or loss in culture.
CD11b− and CD11b+ DCs were stained for CD11b and CD14 expression immediately after sorting and after 2 days of CD40 activation. Whereas CD11b and CD14 expression is negative before and after CD40 activation of CD11b− DCs, all CD11b+ DCs lose CD14 expression and slightly down-regulate but do not lose CD11b expression following CD40 activation. This figure is representative of 3 experiments.
Marker persistence or loss in culture.
CD11b− and CD11b+ DCs were stained for CD11b and CD14 expression immediately after sorting and after 2 days of CD40 activation. Whereas CD11b and CD14 expression is negative before and after CD40 activation of CD11b− DCs, all CD11b+ DCs lose CD14 expression and slightly down-regulate but do not lose CD11b expression following CD40 activation. This figure is representative of 3 experiments.
Differences in messenger RNA expression between CD11b+ and CD11b− DCs
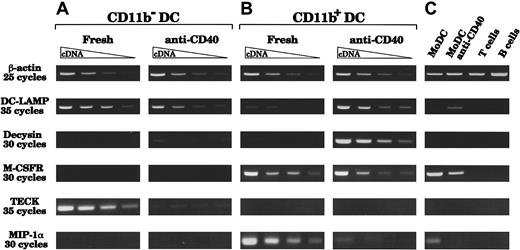
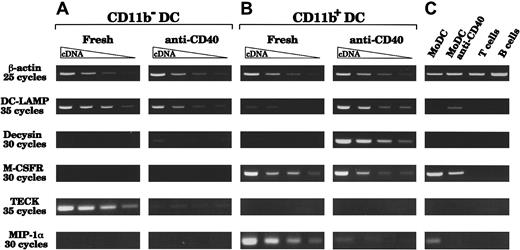
As another test for basic differences, we searched by reverse transcriptase PCR (RT-PCR) for potential markers that would segregate DC populations (Figure 6). We analyzed freshly sorted or CD40-activated CD11b− and CD11b+ thymic DCs. To ensure we were working with similar amounts of cDNA from each population, we made dilutions of cDNA samples to obtain similar expression of the housekeeping gene β-actin. We then compared expression of the maturation marker DC-LAMP,37 the disintegrin proteinase decysin (which was identified from CD40-activated GCDCs),40 the TECK (which was initially described in mouse thymic DCs),41 42the MIP-1α and the M-CSFR. As shown in Figure 6, DC-LAMP was strongly expressed by freshly sorted CD11b− DCs. In contrast, freshly sorted CD11b+ DCs expressed little DC-LAMP, consistent with the fact that this fraction contained a majority of immature DCs. However, after CD40 activation, CD11b+ DCs strongly expressed DC-LAMP. Thus, after CD40 activation, both populations were considered at a similar state of maturation/activation and were not segregated by this marker. Differences between these 2 DC populations, even after a similar state of activation was attained, were observed after analysis of decysin and M-CSFR mRNA expression. Indeed, CD11b+ DCs, but not CD11b− DCs, expressed decysin and the M-CSFR. As expected, decysin expression was detected only after CD40 activation, when CD11b+ DCs displayed high expression, whereas only a faint band was observed from the highest concentration of cDNA from activated CD11b−DCs. The M-CSFR was strongly expressed by freshly isolated CD11b+ DCs and was still expressed after CD40 activation, but it was never detected in cDNA from either freshly isolated or activated CD11b− DCs. The chemokine TECK was expressed by freshly isolated CD11b−, but not CD11b+, DCs although it was down-regulated upon CD40 activation. In contrast, MIP-1α expression was detected only in cDNA from freshly isolated CD11b+ DCs, and not in cDNA from CD11b− DCs. The same differences were observed at higher numbers of PCR cycles (data not shown). These results strongly suggested that CD11b− and CD11b+ DCs represented 2 distinct thymic DC populations.
Messenger RNA expression before and after CD40 activation.
CD11b+ DCs, but not CD11b− DCs, express the M-CSFR, MIP-1α, and decysin after CD40 activation; CD11b− DCs express TECK. Two-fold dilutions of cDNA were prepared from RNA purified from CD11b− and CD11b+ thymic DCs, freshly after sorting or after 24 hours of CD40 activation by means of an agonistic anti-CD40 antibody. (A) CD11b− DCs express high levels of DC-LAMP and TECK freshly after isolation. After 24 hours of CD40 activation, DC-LAMP is still expressed, but only very little decysin is expressed and TECK expression is down-regulated. MIP-1α and M-CSFR mRNA were never detected, before or after CD40 activation. (B) After CD40 activation, CD11b+ DCs up-regulate DC-LAMP and express very high amounts of decysin. CD11b+ DCs express MIP-1α and the M-CSFR freshly after sorting and retain some M-CSFR expression after CD40 activation. However, CD11b+ DCs do not express TECK, either freshly or after CD40 activation. (C) Controls from MoDCs (fresh or CD40-activated), tonsillar CD3+ T cells, and CD20+ B cells show that DC-LAMP and decysin are expressed only in mature DCs. The analysis is representative of 4 separate experiments using 2 separate thymus samples.
Messenger RNA expression before and after CD40 activation.
CD11b+ DCs, but not CD11b− DCs, express the M-CSFR, MIP-1α, and decysin after CD40 activation; CD11b− DCs express TECK. Two-fold dilutions of cDNA were prepared from RNA purified from CD11b− and CD11b+ thymic DCs, freshly after sorting or after 24 hours of CD40 activation by means of an agonistic anti-CD40 antibody. (A) CD11b− DCs express high levels of DC-LAMP and TECK freshly after isolation. After 24 hours of CD40 activation, DC-LAMP is still expressed, but only very little decysin is expressed and TECK expression is down-regulated. MIP-1α and M-CSFR mRNA were never detected, before or after CD40 activation. (B) After CD40 activation, CD11b+ DCs up-regulate DC-LAMP and express very high amounts of decysin. CD11b+ DCs express MIP-1α and the M-CSFR freshly after sorting and retain some M-CSFR expression after CD40 activation. However, CD11b+ DCs do not express TECK, either freshly or after CD40 activation. (C) Controls from MoDCs (fresh or CD40-activated), tonsillar CD3+ T cells, and CD20+ B cells show that DC-LAMP and decysin are expressed only in mature DCs. The analysis is representative of 4 separate experiments using 2 separate thymus samples.
Plasmacytoid cells are localized in both the thymic cortex and medulla; mature DCs are localized in the medulla
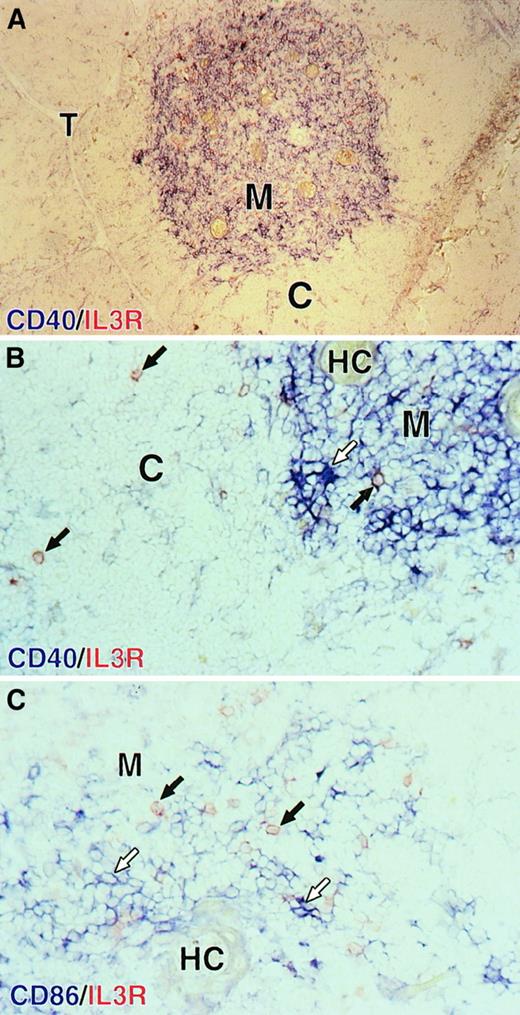
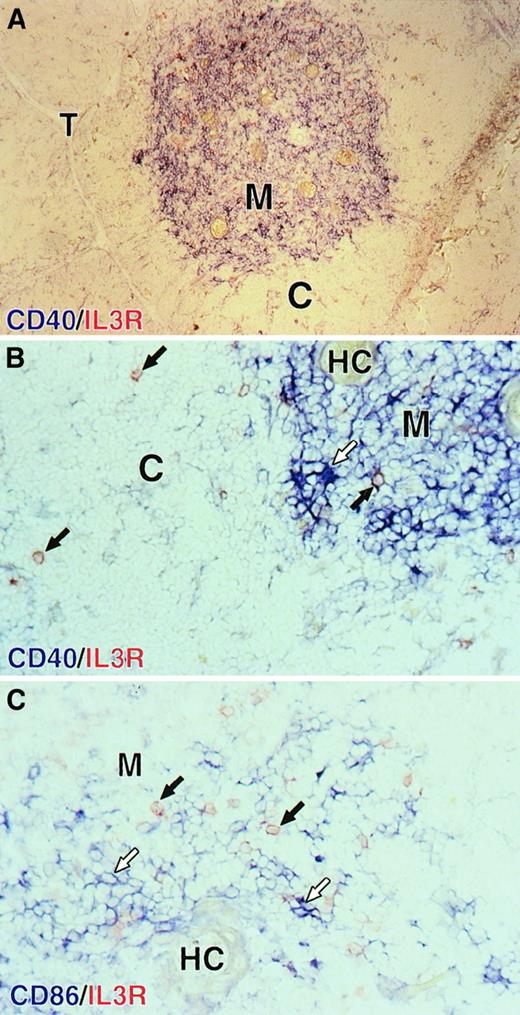
Res et al28 recently showed that in phenotype, thymic plasmacytoid cells resemble their tonsillar and blood counterparts23 24 and that they developed into DCs upon culture with IL-3 and CD40L. Consequently, we tried to determine if one of our thymic DC populations corresponded to the mature dendritic form of plasmacytoid cells. As a first approach, we rechecked the localization of mature DCs and plasmacytoid cells on thymic tissue sections. As shown in Figure 7A, mature DCs expressing high levels of CD40 in blue were found close to Hassall corpuscles, which identify the thymic medulla. Round plasmacytoid cells, identified by high expression of the IL-3Rα in red, were found in both the cortex and the medulla, near blue CD40highDCs displaying typical dendrites (Figure 7B). In contrast to medullary B cells and epithelial cells, which can express CD40, only DCs expressed detectable levels of CD86 in blue. As shown in Figure 7C, blue CD86high DCs were surrounded by round red IL-3Rαhigh plasmacytoid cells in the medulla. Consistent with our observations by means of flow cytometry, no blue CD86high DC was found to coexpress the IL-3Rα.
Localization of plasmacytoid cells and DCs in the human thymus.
Plasmacytoid cells are both in the cortex and the medulla; mature DCs are localized in the thymic medulla. (A) Mature DCs expressing high levels of CD40 (in blue) are localized in the medulla (M) identified by the presence of Hassall corpuscles (HCs). (B) A closer view of the corticomedullary junction shows that round red IL-3Rα+plasmacytoid cells (black arrows) are found in the cortex and are found in the medulla near blue dendritic CD40highmature DCs (empty arrows). (C) In the medulla, blue CD86high DCs (empty arrows) are surrounded by red IL-3Rα+ plasmacytoid cells (black arrows). No blue CD86high DCs was found to coexpress the IL-3Rα. M indicates medulla; C, cortex; T, trabeculae; HC, Hassall corpuscles. Original magnification: 100 × (panel A); 400 × (panels B and C).
Localization of plasmacytoid cells and DCs in the human thymus.
Plasmacytoid cells are both in the cortex and the medulla; mature DCs are localized in the thymic medulla. (A) Mature DCs expressing high levels of CD40 (in blue) are localized in the medulla (M) identified by the presence of Hassall corpuscles (HCs). (B) A closer view of the corticomedullary junction shows that round red IL-3Rα+plasmacytoid cells (black arrows) are found in the cortex and are found in the medulla near blue dendritic CD40highmature DCs (empty arrows). (C) In the medulla, blue CD86high DCs (empty arrows) are surrounded by red IL-3Rα+ plasmacytoid cells (black arrows). No blue CD86high DCs was found to coexpress the IL-3Rα. M indicates medulla; C, cortex; T, trabeculae; HC, Hassall corpuscles. Original magnification: 100 × (panel A); 400 × (panels B and C).
Thymic plasmacytoid cells can, upon culture, adopt a phenotype similar to CD11b− DCs
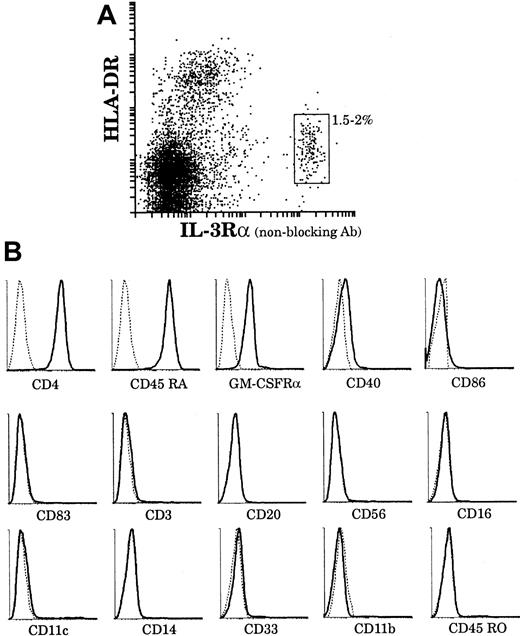
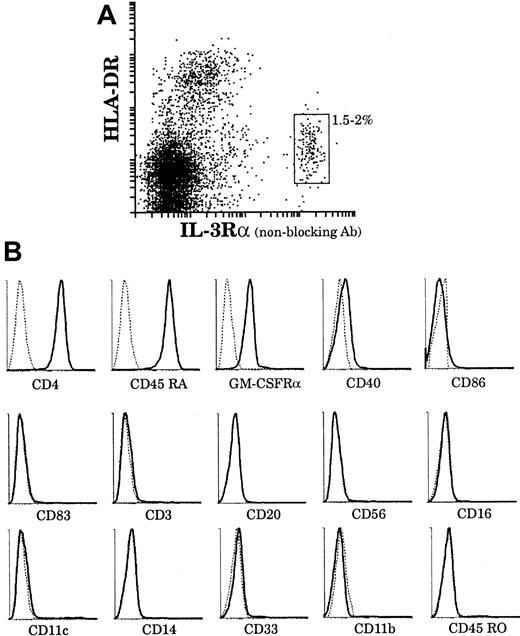
As a second approach to assess the relationship between plasmacytoid cells and our mature thymic DCs, we analyzed the phenotype of plasmacytoid cells using 4-color immunofluorescence staining. Plasmacytoid cells represented 1.5% to 2% of the thymic light-density cell fraction as gated on the basis of IL-3Rαhigh and HLA-DRlow surface expressions (Figure8A). As shown in Figure 8B, IL-3Rαhigh cells were CD4+, CD3−, CD11c−, CD11b−, CD14−, CD33−, CD20−, CD45RO−, CD56−, CD16−, CD45RA+, GM-CSFRα+, HLA-DR+, and CD40low and did not express CD86 or CD83. Thus, IL-3Rαhigh thymic cells corresponded in phenotype to plasmacytoid cells described in tonsils and blood.23,24 We then sorted thymic plasmacytoid cells using a nonblocking anti–IL-3Rα antibody and assessed their further development in culture with IL-3. As previously described,28 upon culture with IL-3 and soluble CD40L, plasmacytoid cells clustered after 6 to 8 hours and, after 2 to 3 days, displayed long dendrites (data not shown). However, it was only after 6 days of culture that plasmacytoid-derived DCs had matured and acquired CD83 expression (Figure 9A). Moreover, they then expressed high levels of HLA-DR and CD86 and still expressed CD45RA, but did not express CD45RO and CD11c. Thus these IL-3–derived culture-matured DCs did not correspond to either of our freshly isolated mature DC populations.
The surface phenotype of thymic plasmacytoid cells.
(A) Plasmacytoid cells were released from a thymus tissue sample and light-density cells selected. The enriched preparation was immunofluorescent stained for IL-3Rα (PE) and HLA-DR (Cy5). HLA-DRlowIL-3Rαhigh plasmacytoid cells represented 1.5% to 2% of the preparation. HLA-DRhighIL-3Rαlow cells were composed of DCs and B cells. (B) By means of 4-color immunofluorescence staining, the phenotype of HLA-DRlow(Cy5)IL-3Rαhigh(Texas-Red) plasmacytoid cells was analyzed with the use of FITC and PE-labeled antibodies. This analysis is representative of 3 separate thymus samples.
The surface phenotype of thymic plasmacytoid cells.
(A) Plasmacytoid cells were released from a thymus tissue sample and light-density cells selected. The enriched preparation was immunofluorescent stained for IL-3Rα (PE) and HLA-DR (Cy5). HLA-DRlowIL-3Rαhigh plasmacytoid cells represented 1.5% to 2% of the preparation. HLA-DRhighIL-3Rαlow cells were composed of DCs and B cells. (B) By means of 4-color immunofluorescence staining, the phenotype of HLA-DRlow(Cy5)IL-3Rαhigh(Texas-Red) plasmacytoid cells was analyzed with the use of FITC and PE-labeled antibodies. This analysis is representative of 3 separate thymus samples.
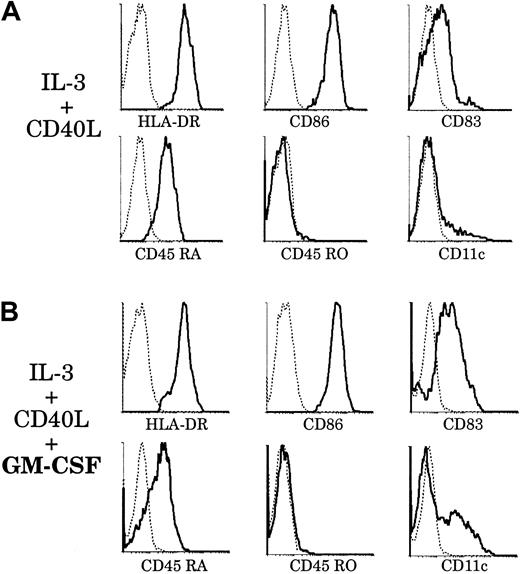
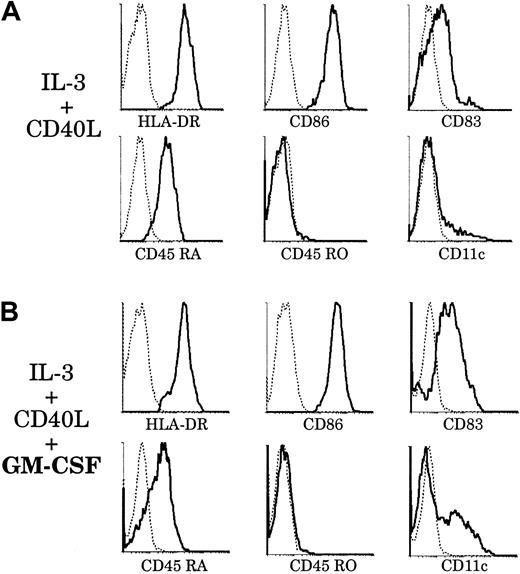
Effect of GM-CSF on thymic plasmacytoid cells.
Upon culture with GM-CSF, thymic plasmacytoid cells acquire a phenotype resembling that of thymic CD11b− DCs. Thymic plasmacytoid cells were sorted as shown in Figure 8A and cultured for 6 days, with (panel B) or without (panel A) GM-CSF. (A) After 6 days of culture with IL-3 and soluble CD40L, plasmacytoid cells differentiated into DCs and up-regulated HLA-DR, CD86, and CD83. They retained CD45RA expression and were still negative for CD45RO and CD11c. (B) When cultured with IL-3, soluble CD40L, and GM-CSF, plasmacytoid-derived DCs expressed higher levels of CD86 and CD83. A fraction down-regulated CD45RA and acquired CD11c expression. This figure is representative of 3 experiments.
Effect of GM-CSF on thymic plasmacytoid cells.
Upon culture with GM-CSF, thymic plasmacytoid cells acquire a phenotype resembling that of thymic CD11b− DCs. Thymic plasmacytoid cells were sorted as shown in Figure 8A and cultured for 6 days, with (panel B) or without (panel A) GM-CSF. (A) After 6 days of culture with IL-3 and soluble CD40L, plasmacytoid cells differentiated into DCs and up-regulated HLA-DR, CD86, and CD83. They retained CD45RA expression and were still negative for CD45RO and CD11c. (B) When cultured with IL-3, soluble CD40L, and GM-CSF, plasmacytoid-derived DCs expressed higher levels of CD86 and CD83. A fraction down-regulated CD45RA and acquired CD11c expression. This figure is representative of 3 experiments.
However, in preliminary studies, we found that plasmacytoid cells derived from thymus and tonsils expressed the GM-CSFRα (data not shown), as did both CD11b− and CD11b+ DCs. This suggested that, in contrast to original descriptions,23 GM-CSF could be of importance for the fate of plasmacytoid cells. Accordingly, we introduced GM-CSF in our culture system and analyzed the phenotype of plasmacytoid-derived DCs after 6 days (Figure 9B). Plasmacytoid cells grown with GM-CSF, IL-3, and soluble CD40L expressed higher levels of CD83 than those grown with IL-3 and CD40L alone. More importantly, there was a marked down-regulation of CD45RA expression and up-regulation of CD11c by a fraction of plasmacytoid-derived DCs. These CD11c+CD86highCD83highCD45RAlowDCs did not seem to arise from a putative CD45RO+ monocyte contaminant, as no CD45RO expression was detected. However, we cannot exclude the possibility that this phenotype results from the selected outgrowth of a particular plasmacytoid cell subset and not from the homogeneous differentiation of these cells. Thus, with the help of GM-CSF, which appeared to facilitate maturation, a proportion of plamacytoid-derived DCs had acquired a phenotype resembling that of freshly isolated CD11b− thymic DCs. We could detect by RT-PCR the expression of DC-LAMP but not decysin mRNA by these plasmacytoid-derived DCs (data not shown).
CD11b− DCs, but not CD11b+ or plasmacytoid-derived DCs, secrete bioactive IL-12 following CD40 ligation
One major difference recorded between monocyte-derived and plasmacytoid-derived DCs was that the latter appear unable to produce any bioactive IL-12 after CD40 ligation.25 We used this functional characteristic as a tool to help determine if plasmacytoid cells would differentiate into thymic DCs in vivo. Using a set of cytokines described as being the most powerful inducer of bioactive IL-12 p70 secretion (including GM-CSF, IFN-γ, IL-4, and soluble CD40L),43 we stimulated freshly isolated CD11b− and CD11b+ DCs for 1 to 7 days and measured IL-12 p70 secretion by ELISA. As shown in Table1, CD11b− DCs secreted substantial amounts of IL-12 p70 after 2 days of stimulation, whereas CD11b+ DCs were very weak producers regardless of the time length of culture. Moreover, we tested the ability of thymic plasmacytoid cells and plasmacytoid-derived DCs to secrete IL-12 p70. We were not able, in our system, to detect any bioactive IL-12 from the supernatant of plasmacytoid cells cultured for 2 days with IL-3, GM-CSF, and CD40L or from the supernatant of plasmacytoid-derived DCs restimulated with GM-CSF, IFN-γ, IL-4, and CD40L on day 5 of culture.
Discussion
Our results show that the human thymus contains 2 populations of mature DCs, expressing different lineage markers and displaying different capacities for IL-12 secretion. The major thymic DC population (more than 65%) is most readily distinguished from other DCs by being clearly CD11b− and also CD33lowand CD45ROlow. Other key markers are CD11c+ and CD4+. This population expresses markers of fully mature DCs, being HLA-DRhigh, CD40+, CD86+, and CD83+; in addition, DC-LAMP mRNA could be detected by RT-PCR. Besides the absence of B- and T-cell markers, this DC population does not express myeloid-associated surface markers, being CD11b−, CD14−, and CD64− and expressing low levels of CD33. Moreover, M-CSFR gene expression could not be detected by RT-PCR before or after activation, and only low decysin expression was noted. This major thymic DC population, localized in the medulla together with the other mature thymic CD11b+ DCs, was fully able to activate naive CD4 T cells in an MLR and was characterized by a typical dendritic morphology when freshly isolated. Overall, this major population exhibited all the characteristics of mature DCs, but lacked those particular myeloid-associated markers found on MoDCs and on tonsillar GCDCs.
In contrast, the minor thymic DC population (less than 35%) is clearly CD11b+, and also CD11chigh, CD33high, and CD45ROhigh. Like the major population, it is CD4+. To collect sufficient numbers of these cells, we included during sorting not only the mature form of these cells, but also an immature form expressing intermediate to high levels of HLA-DR; this immature form was also CD14+, CD64+, and CD83−. On activation with CD40, the less mature DCs rapidly lost CD14 and CD64 expression and dramatically increased both DC-LAMP and decysin gene expression. M-CSFR gene expression was readily detected by RT-PCR, both before and after CD40 signaling, in contrast to the major thymic DC population. Although isolated as a mix of fully mature and partially mature DCs, all cells in the CD11b+ fraction appeared to be committed to the DC lineage. Thus, they did not adhere to plastic, did not need signals such as IL-4 to differentiate into mature DCs in culture, and were very potent activators of naive CD4 T cells in an MLR. Their morphology was more “hairy,” with finer and less typical dendrites than the major thymic DC population. Overall, this minor thymic DC population showing markers of myeloid origin resembled tonsillar GCDCs.40 44
The production of IL-12 by DCs has become a major indicator of functional differences between DC subpopulations.10,13,25,43,45 In the thymus, negative selection of self-reactive T cells is performed by DCs6 and has been shown to be dependent on the presence of IL-12.46It is therefore of particular interest that we observed a difference between thymic DC populations in their ability to secrete IL-12. It should be noted that we found no significative difference by RT-PCR for TNF-α and IFN-α expression, which was low but positive for each DC population (data not shown). After CD40 ligation the CD11b−, major, thymic DC population was able to release substantial amounts of bioactive IL-12. In contrast, the CD11b+ thymic DCs release no, or very little, bioactive IL-12 after CD40 ligation. Nor was IL-12 produced by the thymic plasmacytoid DC precursor, in agreement with the lack of IL-12 production by the equivalent cells from blood.25
Another important difference is that only the CD11b− DC population expressed the chemokine TECK, whereas only the CD11b+ DC population expressed the inflammatory chemokine MIP-1α. TECK expression in DCs seems to be induced by the thymic microenvironment and to be important in early thymic development and directional migration of thymocyte subsets by signaling through its receptor CCR9.41,42 In contrast, MIP-1α signals through CCR1 and CCR5 and is considered an inflammatory chemokine38reported to be expressed in the thymic microenvironment.42,47 Thymocytes express different chemokine receptors depending on their stage of development.48 Accordingly, the CD11b− and CD11b+ DC populations, which differ in their ability to express TECK and MIP-1α, might attract thymocytes at different stages of T-cell maturation. Alternatively, if the CD11b+ DCs represent blood-derived DCs, they might be irrelevant for thymocyte development. Thus, as well as a range of surface-marker differences, the 2 thymic DC populations display different functional abilities.
The origin of these 2 human thymic DC populations and their relationship to the DC populations in the mouse thymus are issues we hoped to clarify. Our results provide some pointers, but some contradictions remain. The minor (and variable) CD11b+thymic DC population appears from its surface markers to be of immediate myeloid origin; such DCs may have entered the thymus directly from the bloodstream. Although the major CD11b− thymic DC population might also enter the thymus from the bloodstream, an endogenous origin seems more likely. Several potential DC precursors exist in the human thymus. An early CD34+ precursor population with limited ability to form myeloid cells has been shown to produce DCs in culture49-52; however, when generated in culture, such DCs express CD11b. Another possibility is that the CD11b− DCs represent the mature dendritic form of the thymic plasmacytoid cells. At first, this seemed unlikely since the DCs that develop in culture with IL-3 from these precursors exhibit a unique phenotype, lacking CD11c and retaining CD45RA. However, we found in our system that when GM-CSF is also added to the culture, thymic plasmacytoid cells then acquire some CD11c expression and down-regulate CD45RA expression while differentiating into DCs. In surface phenotype they now resemble the normal thymic CD11b− population. However, we cannot exclude the possibility that this observation results from the selected outgrowth of a subset of plasmacytoid cells, and not from the homogeneous differentiation of these cells.
One argument against a plasmacytoid origin for the thymic CD11b− DCs is that the latter are effective producers of IL-12, whereas neither the plasmacytoid cells themselves nor the DC progeny we produced in culture were effective at IL-12 secretion. However, it is possible that our culture system simply is deficient in the factors needed to mature DCs into effective IL-12 producers. Indeed, Cella et al45 recently reported that plasmacytoid-derived DCs were able to secrete similar amounts of bioactive IL-12 as blood CD11c+. It is important to note that we used a soluble trimeric CD40L in this study, and not CD40L-transfected mouse fibroblasts that were used in other studies25,28 45 and might secrete other growth factors.
The ability of thymic CD11b− DCs to secrete IL-12 does, however, line up well with the high IL-12–producing capacities of mouse CD11b−CD8+ DCs, which form the major DC type in the mouse thymus. Despite the absence of the CD8 marker on any human DC subpopulation,53CD11b−CD8+ mouse DCs and CD11b−human DCs share common features.
We thank Dr D. Tarlinton and Dr L. Wu for helpful discussions and F. Battye, D. Kaminaris, V. Lapatis, and J. Parker for assistance with flow cytometric sorting. This study is dedicated to the memory of the late Dr Jacques Chiller.
Supported in part by research funding from the University of Melbourne and the Cooperative Research Centre for Vaccine Technology, Queensland Institute of Medical Research, P.O. Royal Brisbane Hospital, Australia (S.V.); and by a Deutsche Krebshilfe fellowship (H.H.).
Preliminary results were presented at the 5th International Symposium on Dendritic Cells, Pittsburgh, PA, September 1998.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Stéphane Vandenabeele, The Walter and Eliza Hall Institute of Medical Research, P.O. Royal Melbourne Hospital, 3050 Melbourne, Victoria, Australia; e-mail: vandenabeele@wehi.edu.au.