Platelet responses to collagen are mediated by the combined actions of the integrin α2β1, which serves as a major collagen-binding receptor, and the GPVI/FcRγ-chain complex, which transmits collagen-specific activation signals into the cell interior through the action of an immunoreceptor tyrosine-based activation motif within the cytoplasmic domain of the FcRγ-chain. Despite much progress in identifying components of the signaling pathway responsible for collagen-induced platelet activation, virtually nothing is known about the regulatory elements that modulate this important hemostatic event. PECAM-1, a recently recognized member of the inhibitory receptor family, contains a functional immunoreceptor tyrosine-based inhibitory motif within its cytoplasmic domain that, when tyrosine phosphorylated, recruits and activates the protein–tyrosine phosphatase, SHP-2. To test the hypothesis that PECAM-1 functions to regulate GPVI/FcRγ-chain–mediated platelet activation, the responses of wild-type versus PECAM-1–deficient murine platelets to GPVI-specific agonists were compared. Four distinct GPVI/FcRγ-chain–dependent responses were found to be significantly exaggerated in platelets derived from PECAM-1–deficient mice, including Mg++-independent adhesion to immobilized fibrillar collagen, collagen-induced platelet aggregation, platelet aggregation induced by the GPVI-specific agonist collagen-related peptide, and GPVI/FcRγ-chain–induced dense granule secretion. Together, these data provide compelling evidence that PECAM-1 modulates platelet responses to collagen, and they implicate this novel member of the inhibitory receptor family in the regulation of primary hemostasis.
Introduction
Platelet endothelial cell adhesion molecule-1 (PECAM-1, CD31) is a 130-kd member of the immunoglobulin (Ig) superfamily that is expressed on the surface of circulating platelets, endothelial cells, neutrophils, monocytes, and certain T-lymphocyte subsets. The extracellular domain of PECAM-1 is composed of 6 extracellular Ig-like homology units,1 the amino-terminal 2 of which mediates PECAM-1–PECAM-1 homophilic interactions.2,3 Antibodies to the extracellular domain have been shown to have profound physiologic and cell biologic effects, including delaying leukocyte transendothelial migration4-6and inhibiting angiogenesis.7 The extracellular domain of PECAM-1 also serves as a portal for entry into endothelial cells of certain strains of Plasmodium falciparum–infected erythrocytes.8 9
The PECAM-1 cytoplasmic domain also plays a key biologic role because a large number of extracellular stimuli (reviewed in Newman10) have been shown to result in the phosphorylation of 2 key tyrosine residues, located at positions 663 and 686 of the cytoplasmic domain.11 The sequence surrounding each of these 2 tyrosine residues conforms to an immunoreceptor tyrosine-based inhibitory motif (ITIM) that, when phosphorylated, provides a major docking site for the src homology 2 (SH2) domain–containing protein tyrosine phosphatase (PTP), SHP-2.11-14 Based on the presence of its highly conserved ITIM and the similarity in its genomic organization to that of other ITIM-bearing transmembrane receptors, we have recently proposed10 that PECAM-1 is a member of a growing family of transmembrane PTP-binding proteins collectively known as inhibitory receptors.
There are good examples at the cell biological level that support a role for inhibitory receptors in attenuating the action of agonist receptors that contain one or more immunoreceptor tyrosine-based activation motifs (ITAMs).15,16Human platelets express 2 such ITAM-bearing receptors—FcγRIIa,17 which serves as a low-affinity receptor for IgG,18 and the GPVI-FcRγ–chain complex, which transmits collagen-specific activation signals into the cell interior through the action of an ITAM present within the cytoplasmic domain of the FcRγ-chain.19 Although a role forITIM-bearing PECAM-1 in regulating the actions of either of these 2 ITAM-bearing agonist receptors in platelets has not been examined, we have recently established a regulatory role for PECAM-1 in attenuating the action of the ITAM-bearing T-cell antigen receptor in Jurkat T cells.20 These studies suggested to us that PECAM-1 may similarly function to negatively regulate protein tyrosine kinase (PTK)-dependent signal transduction pathways initiated by ITAM-containing stimulatory receptors in other cell types. The recent production of PECAM-1–deficient mice21 has enabled us to use a genetic approach to evaluate the effect of PECAM-1 on platelet responses to collagen. The purpose of the current investigation, therefore, was to examine the ability of PECAM-1 to modulate GPVI/FcRγ-chain–mediated platelet activation by comparing the responses of wild-type versus PECAM-1–deficient murine platelets to GPVI-specific agonists. Our findings provide compelling evidence that PECAM-1 modulates platelet responses to collagen and implicate this novel member of the inhibitory receptor family in the regulation of primary hemostasis.
Materials and methods
Materials
A suspension of acid-insoluble equine tendon type I collagen fibrils was purchased from Chrono-Log (Havertown, PA). Collagen-related peptide (CRP:GKP*[GPP*]10GKP*G; single amino acid code; P* represents hydroxyproline) was synthesized and cross-linked as previously described.22 An adenosine triphosphate (ATP) determination kit and calcine am were purchased from Molecular Probes (Eugene, OR). PECAM-1−/−mice21 were obtained from Dr Tak W. Mak (Amgen Institute, Toronto, ON, Canada) and were bred at the Animal Resource Facility of the Medical College of Wisconsin. Immulon-2 96-well microtiter plates were purchased from Dynex Technologies (Chantilly, VA). All other chemicals were purchased from Sigma Chemical (St Louis, MO).
Preparation of mouse platelets
Blood (1.0-1.5 mL) from the inferior vena cava of tribromoethanol-anesthetized mice was drawn into 0.13 M sodium-citrate solution (0.1 mL). Blood pooled from 3 to 4 animals was diluted with an equal volume of modified Tyrode–HEPES buffer (134 mM NaCl, 0.36 mM NaH2PO4, 13.8 mM NaHCO3, 2.5 mM KCl, 20 mM HEPES, 5.5 mM glucose, 2.5 mg/mL bovine serum albumin, pH 7.4) and centrifuged at 200g for 10 minutes at room temperature. Platelets were collected by centrifuging the platelet-rich plasma at 500g for 20 minutes in the presence of 10 mM EDTA and 1 μmol/L prostaglandin E1. The sedimented platelets were resuspended in modified Tyrode–HEPES buffer to a density of 2.5 × 108 platelets/mL, supplemented with 1 mM CaCl2, and allowed to rest for 30 minutes at room temperature before experimentation.
Adhesion assays
Immulon-2 microtiter 96-well plates were coated with varying concentrations of fibrillar collagen without Mg2+ or with a constant concentration of poly L-lysine (0.01%) overnight at 4°C. Washed wild-type and PECAM-1−/− platelets, prepared as described above, were loaded with calcine am (5 μmol/L) for 30 minutes at 37°C. The loaded wild-type and PECAM-1−/− platelets were rewashed in modified Tyrode–HEPES buffer and suspended at a concentration of 1.2 × 108 platelets/mL. Calcine am-loaded platelets (100 μL) were added to the matrix-coated wells and incubated for the indicated amount of time at 37°C. Nonadherent platelets were removed by washing, and the extent of platelet adherence was determined by measuring the fluorescence emitted by adhered platelets at 520 nm after excitation at 490 nm. The assay was performed in triplicate, and each data point represented the mean ± SD of triplicate measurements.
Platelet aggregation and secretion
Platelets were suspended in 200 μL at a final concentration of 2.5 × 108 platelets/mL, and aggregometry was performed at 37°C in a Bio/Data PAP-4 channel aggregometer (Horsham, PA) with continuous stirring (1000 rpm). ATP release was determined by removing 10-μL aliquots at selected time points, centrifuging them at 500g for 1 minute at room temperature in the presence of 1 mM EDTA to prevent further platelet activation, and adding the supernatant to a 100-μL reaction mixture containing luciferase (0.5 mM) and luciferin (1.25 μg/mL). The luminescence generated in the presence of platelet-released ATP was read in a luminometer (TD-20e; Turner, Sunnyvale, CA) and compared to an ATP standard curve to determine the amount of ATP released.
Results
Platelets derived from PECAM-1–deficient mice exhibit exaggerated GPVI-mediated adhesion and aggregation responses
Platelet activation by collagen is thought to proceed by a 2-step mechanism involving the initial attachment of collagen fibrils to surface-expressed integrin α2β1, followed by an obligatory signal transduction cascade mediated, at least in part, by the GPVI/FcRγ-chain complex.19 The latter operates through tyrosine-phosphorylated ITAMs within the cytoplasmic domain of the FcRγ-chain dimer.23 Despite much progress in identifying components of the signaling pathway responsible for initiating and sustaining collagen-induced platelet activation,24-30 virtually nothing is known about the regulatory elements that modulate this important hemostatic event. Others and we have recently found that PECAM-1 becomes strongly tyrosine phosphorylated on its ITIM after exposure of platelets to collagen,31 32 leading us to speculate that agonist induction simultaneously activates a feedback inhibitory pathway mediated by PECAM-1.
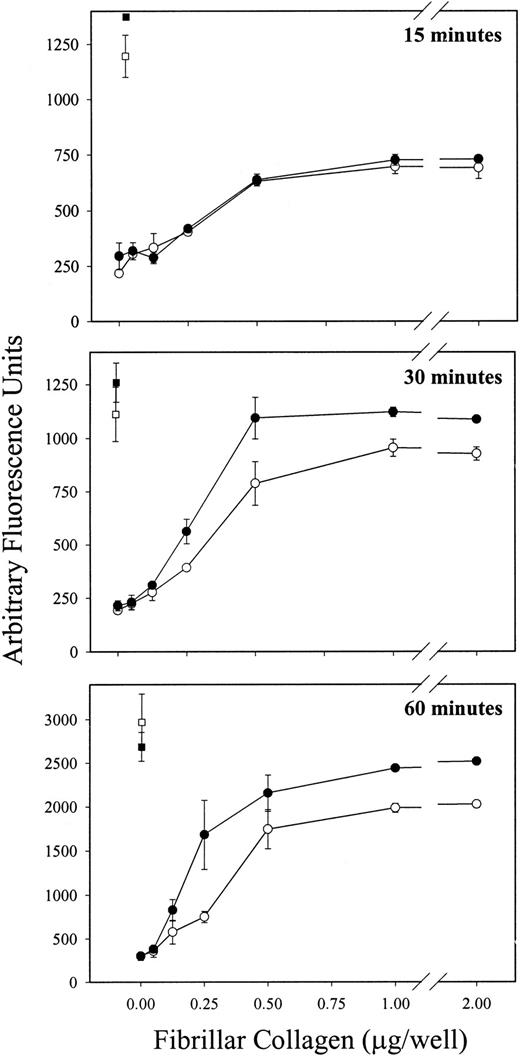
To test the hypothesis that PECAM-1 normally functions to attenuate GPVI-mediated platelet activation, we first compared the ability of platelets derived from wild-type versus PECAM-1–deficient mice to bind to immobilized fibrillar collagen in the absence of Mg2+—conditions that have previously been shown to result in GPVI-specific adhesion, independent of α2β1.24 33 As shown in Figure1, though wild-type and PECAM-1−/− platelets exhibited similar levels of adhesion after 15 minutes of exposure (Figure 1, top panel), PECAM-1−/− platelets bound significantly and reproducibly better at 30 (Figure 1, middle panel) and 60 (Figure 1, lower panel) minutes than did wild-type platelets. This effect was specific; adhesion to poly-L-lysine–coated wells was statistically indistinguishable between the 2 experimental groups.
PECAM-1−/− platelets demonstrate increased adhesion to immobilized fibrillar collagen in the absence of Mg2+.
Calcine am-loaded platelets derived from wild-type (open symbols) and PECAM-1−/− (closed symbols) mice were allowed to adhere, in the absence of Mg2+, to plates coated with increasing concentrations of fibrillar collagen (circles) or to plates coated with a constant concentration of polyL-lysine (squares) for 15, 30, or 60 minutes at 37°C. Wild-type and PECAM-1−/− platelets were labeled to a similar extent with calcine am (not shown). After removal of nonadherent platelets, fluorescence associated with adherent platelets was measured as described in “Materials and methods.” Each assay was performed in triplicate, and each data point represents the mean of triplicate measurements ± SD. Similar results were obtained from 3 independent experiments. Note that wild-type and PECAM-1−/− platelets bound to a similar extent to polyL-lysine–coated plates, whereas at later time points, higher levels of adhesion to fibrillar collagen, in the absence of Mg2+, was observed in PECAM-1−/− platelets relative to the wild-type platelets.
PECAM-1−/− platelets demonstrate increased adhesion to immobilized fibrillar collagen in the absence of Mg2+.
Calcine am-loaded platelets derived from wild-type (open symbols) and PECAM-1−/− (closed symbols) mice were allowed to adhere, in the absence of Mg2+, to plates coated with increasing concentrations of fibrillar collagen (circles) or to plates coated with a constant concentration of polyL-lysine (squares) for 15, 30, or 60 minutes at 37°C. Wild-type and PECAM-1−/− platelets were labeled to a similar extent with calcine am (not shown). After removal of nonadherent platelets, fluorescence associated with adherent platelets was measured as described in “Materials and methods.” Each assay was performed in triplicate, and each data point represents the mean of triplicate measurements ± SD. Similar results were obtained from 3 independent experiments. Note that wild-type and PECAM-1−/− platelets bound to a similar extent to polyL-lysine–coated plates, whereas at later time points, higher levels of adhesion to fibrillar collagen, in the absence of Mg2+, was observed in PECAM-1−/− platelets relative to the wild-type platelets.
There is growing support for the notion that ITAM- and ITIM-bearing receptors act antagonistically when expressed in the same cell, possibly because of the potential for ITIM-associated PTPs to dephosphorylate ITAM-bearing receptors or their tyrosine-phosphorylated substrates.16,34 To determine whether the observed exaggerated platelet adhesion to immobilized collagen was specific for ITAM-bearing agonist receptors, we compared platelet aggregation responses to GPVI-specific agonists22with those obtained using thrombin, which acts through a heterotrimeric G-protein–coupled receptor.35
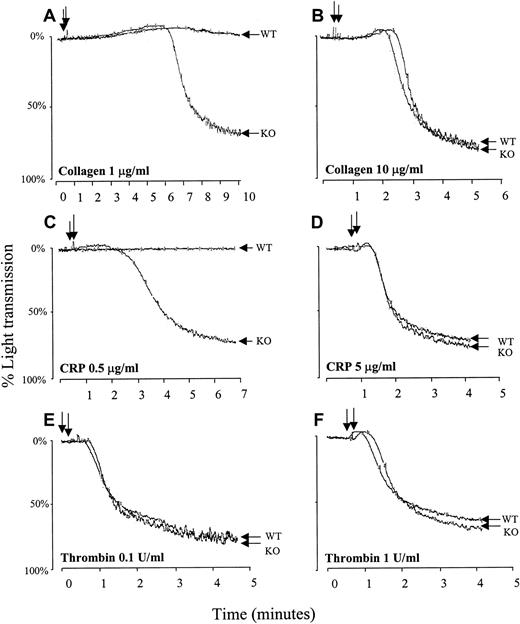
Relative to wild-type platelets, PECAM-1–deficient platelets exhibited strikingly hyper-aggregation responses to subthreshold doses of fibrillar collagen (Figure 2A) or CRP (Figure 2C), though the aggregation responses were equivalent at higher concentrations of either agonist (Figure 2B,D). In contrast, platelet activation by either low-dose (Figure 2E) or high-dose (Figure 2F) thrombin was identical in wild-type and PECAM-1−/−platelets, indicating that PECAM-1 has no detectable effect on this G-protein–coupled signal transduction pathway, as predicted. These results demonstrate that, like platelet adhesion, platelet aggregation mediated by the ITAM-bearing GPVI/FcRγ-chain complex is enhanced in the absence of PECAM-1. The observation that higher concentrations of either GPVI-specific agonist can overcome the difference in aggregation between PECAM-1−/− and wild-type platelets suggests that PECAM-1 deficiency lowers the threshold for the induction of platelet aggregation after collagen binding to GPVI. Interestingly, mice heterozygous for PECAM-1 expression exhibited near-normal responses to low-dose collagen or low-dose CRP, suggesting that, like integrin αIIbβ3 defects, 50% receptor expression is sufficient to maintain normal function.
PECAM-1−/− platelets show hyper-aggregation in response to stimulation with GPVI-specific agonists.
Murine platelets were isolated from wild-type (WT) and PECAM-1−/− (KO) mice, washed, and resuspended at a concentration of 2.5 × 108 platelets/mL. Stirred platelets (200 μL) were exposed to fibrillar collagen in absence of Mg2+ at a concentration of 1 μg/mL (A) or 10 μg/mL (B), to CRP at a concentration of 0.5 μg/mL (C) or 5 μg/mL (D), or to thrombin at a concentration of 0.1 (E) or 1 (F) U/mL. Changes in light transmission of the stimulated platelet suspension were measured in an aggregometer. Similar results were obtained with 5 separate platelet preparations. Note that compared to wild-type platelets, PECAM-1−/− platelets showed hyper-aggregation in response to stimulation with low concentrations of GPVI-specific agonists but not in response to stimulation with high concentrations of the same agonists or thrombin.
PECAM-1−/− platelets show hyper-aggregation in response to stimulation with GPVI-specific agonists.
Murine platelets were isolated from wild-type (WT) and PECAM-1−/− (KO) mice, washed, and resuspended at a concentration of 2.5 × 108 platelets/mL. Stirred platelets (200 μL) were exposed to fibrillar collagen in absence of Mg2+ at a concentration of 1 μg/mL (A) or 10 μg/mL (B), to CRP at a concentration of 0.5 μg/mL (C) or 5 μg/mL (D), or to thrombin at a concentration of 0.1 (E) or 1 (F) U/mL. Changes in light transmission of the stimulated platelet suspension were measured in an aggregometer. Similar results were obtained with 5 separate platelet preparations. Note that compared to wild-type platelets, PECAM-1−/− platelets showed hyper-aggregation in response to stimulation with low concentrations of GPVI-specific agonists but not in response to stimulation with high concentrations of the same agonists or thrombin.
Effect of PECAM-1 deficiency on dense granule secretion
We have recently shown that co-ligation of PECAM-1 with the ITAM-bearing T-cell receptor on the surface of Jurkat T cells attenuates calcium mobilization from intracellular stores.20 The physiological consequences of this inhibition, however, have not been examined. Because platelet alpha and dense granule secretion also require the elevation of cytosolic calcium and because the GPVI-FcRγ-chain–mediated signaling acts through phospholipase Cγ2 (PLCγ2) to mobilize intracellular calcium ion stores,36 we hypothesized that, like adhesion and aggregation, the platelet release reaction should also be accentuated in PECAM-1–deficient platelets stimulated with GPVI-specific agonists.
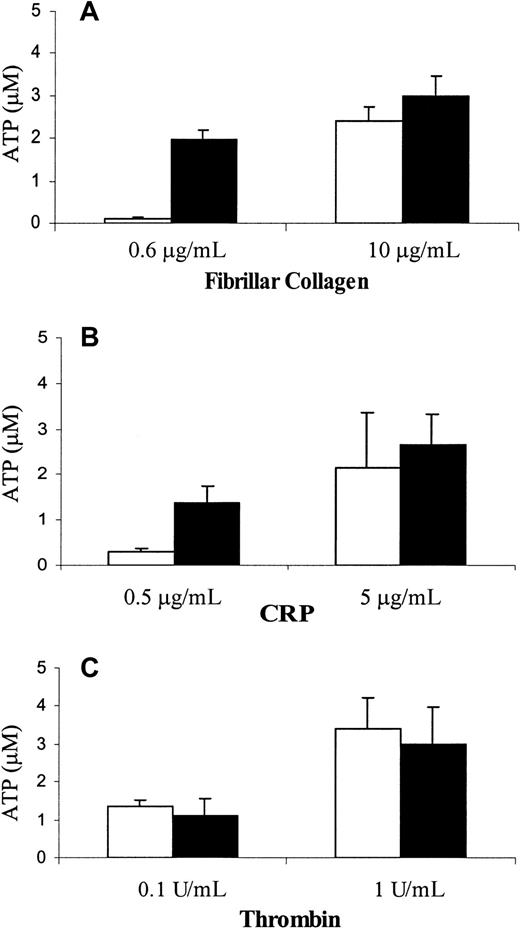
To determine whether PECAM-1 deficiency has an effect on granule release, we compared the ability of wild-type and PECAM-1−/− platelets to secrete dense granule-derived ATP on stimulation of GPVI. As shown in Figure3, PECAM-1–deficient platelets released their granules at subthreshold doses of fibrillar collagen in the absence of Mg2+ (Figure 3A, left) and CRP (Figure 3B, left). ATP secretion was indistinguishable in response to either high- or low-dose thrombin (Figure 3C) or after the addition of higher concentrations of fibrillar collagen in the absence of Mg2+(Figure 3A, right) or CRP (Figure 3B, right). These data are consistent with the notion that PECAM-1 normally acts as a negative regulator of GPVI/FcRγ-chain–mediated granule release and that, in its absence, responses to such agonists are enhanced.
PECAM-1−/− platelets have a lower threshold for release of dense granular contents in response to stimulation through GPVI collagen receptor.
Platelets isolated from wild-type (open bars) and PECAM-1−/− (closed bars) mice were stimulated with varying concentrations of GPVI-specific agonists, including fibrillar collagen in absence of Mg2+ (A) and CRP (B), or with thrombin (C). The amount of ATP released was measured using the luciferin–luciferase assay as described in “Materials and methods.” The assays were performed in triplicate, and the results are expressed as the mean of triplicate measurements ± SD. Similar results were obtained from 3 independent experiments. Note that the release of dense granular contents in response to stimulation with low concentrations of GPVI-specific agonists was elevated in PECAM-1−/− platelets compared to the wild-type platelets.
PECAM-1−/− platelets have a lower threshold for release of dense granular contents in response to stimulation through GPVI collagen receptor.
Platelets isolated from wild-type (open bars) and PECAM-1−/− (closed bars) mice were stimulated with varying concentrations of GPVI-specific agonists, including fibrillar collagen in absence of Mg2+ (A) and CRP (B), or with thrombin (C). The amount of ATP released was measured using the luciferin–luciferase assay as described in “Materials and methods.” The assays were performed in triplicate, and the results are expressed as the mean of triplicate measurements ± SD. Similar results were obtained from 3 independent experiments. Note that the release of dense granular contents in response to stimulation with low concentrations of GPVI-specific agonists was elevated in PECAM-1−/− platelets compared to the wild-type platelets.
Discussion
After injury to the vascular endothelium, platelets adhere to and become activated by collagen fibers in the exposed subendothelium. Among the numerous platelet receptors for collagen that have been reported, the integrin α2β1 appears to play a major role in the initial adhesion to collagen,37 a concept that has been supported by the finding that antibodies specific for α2β1 block platelet adhesion to collagen38,39 and by the observation that α2β1-deficient patients have a mild bleeding disorder associated with defective collagen-induced platelet aggregation.40,41 The platelet-specific receptor GPVI has also been implicated in platelet responses to collagen because collagen-induced platelet activation and aggregation are also defective in GPVI-deficient patients.42
GPVI is associated within the plane of the plasma membrane with the Fc-receptor γ-subunit,43 a 12-kd, disulfide-linked, ITAM-containing homodimer that normally functions as the signaling component of high-affinity cell surface receptors for IgG and IgE. In contrast to these immunoglobulin receptors, the GPVI–FcRγ-chain complex functions as an ITAM-containing adhesion receptor that supports platelet interactions with collagen and subsequently transduces signals that lead to platelet activation and aggregation.23,24,44-48 It has been proposed that the signaling pathways of PTK-binding, ITAM-containing activating receptors are regulated by ITIM-containing, PTP-binding inhibitory receptors.16 Because PECAM-1 is the only known ITIM-containing receptor in platelets,10 we used a genetic approach to investigate the possibility that PECAM-1 might regulate GPVI signaling. We observed that, in response to GPVI-specific interactions with collagen, platelets from PECAM-1−/−mice exhibited increased adhesion, hyper-aggregation, and a lowered threshold for dense granule release relative to wild-type platelets. These findings are consistent with the conclusion that the GPVI signal transduction pathway is under the control of an ITIM-containing inhibitory receptor and that the inhibitory receptor normally used is PECAM-1.
We could not, a priori, have predicted whether PECAM-1 deficiency would have a stimulatory or an inhibitory effect on collagen-induced, GPVI-mediated signal transduction. This is because the ability of an immunoglobulin–ITIM-bearing membrane receptor to have a positive rather than a negative regulatory effect is influenced by a number of factors, including the target specificity of the phosphatase recruited and the identity of the phosphatase substrates that are proximal to the receptor–PTP complex. The situation with PECAM-1 is complicated by the observations that, although the PECAM-1 ITIM clearly provides a docking site for the cytoplasmic PTP SHP-2,11-14,49-51tyrosine-phosphorylated PECAM-1 has also been reported to associate with SHP-113,49-51 and with the 5′-inositol phosphatase SHIP.51 The situation is further complicated by the observations that, depending on whether the pathway is regulated, SHP-1 and SHP-2 appear to be capable of conveying either positive or negative regulatory signals.20,52-59 Because the identity of the phosphatase recruited by PECAM-1 in response to GPVI signaling, the target substrates for SHP-1 and SHP-2 in general, and the subcellular location of these substrates relative to that of PECAM-1 are completely unknown, there was no way to predict the physiological consequences of PECAM-1 deficiency. Interestingly, Pasquet et al60 have recently found that SHP-1 becomes tyrosine phosphorylated after platelet activation by GPVI and associates with other tyrosine-phosphorylated proteins of 28, 32, 50, 70, and 130 kd, and they suggested that SHP-1 plays a functional role in platelet responses to collagen. The 130-kd co-precipitating band was not, however, identified as PECAM-1. The identity of the receptor(s) that recruit and activate SHP-1 under these conditions remains to be determined.
The observation that PECAM-1−/− platelets are hyperresponsive to collagen stimulation represents the first demonstration of an in vivo cellular phenotype associated with PECAM-1 deficiency. Initial characterization of PECAM-1−/− mice by Duncan et al21 was carried out before the realization that PECAM-1 contained a functional ITIM and focused on its role as an adhesion rather than a signaling molecule. In models of leukocyte transendothelial migration, they found an accumulation of neutrophils at the perivascular basement membrane; however, the extent of leukocyte infiltration into the peritoneal cavity in response to an inflammatory stimulus was normal, as was T-cell homing to lymphoid organs and to the site of cutaneous hypersensitivity. Relevant to the current investigation, these authors found that platelet responses to adenosine diphosphate (ADP) are indistinguishable in PECAM-1 knockout versus wild-type mice. However, because ADP-induced platelet activation is mediated by G–protein-coupled rather than ITAM-coupled receptors,61,62 platelet responses to ADP would not be expected to be regulated by the PECAM-1/SHP-2 complex. The current study expands on the initial observations by showing that PECAM-1 deficiency affects platelet responses to the ITAM-containing GPVI-FcRγ-chain collagen receptor complex, but not to G–protein-coupled receptors for agonists such as thrombin or ADP. These findings are consistent with our recent observation that in vitro co-ligation of PECAM-1 with the ITAM-bearing T-cell antigen receptor (TCR) inhibits TCR-induced calcium-mobilization from intracellular stores.20
Our finding that platelets from PECAM-1−/− mice show hyper-aggregation to collagen stimulation might lead one to predict that bleeding times in PECAM-1–deficient mice would be shorter than in wild-type mice. Interestingly, we have recently shown that tail vein bleeding times in PECAM-1−/− mice are mildlyprolonged.63 These apparently paradoxic findings were resolved by bone marrow transplantation studies showing that the prolonged tail vein bleeding time in PECAM-1−/−mice is caused by a vascular rather than a platelet function abnormality.63 It is likely that skin template bleeding time, which, unlike the tail vein bleeding time commonly studied in the mouse, solely measures platelet function, would be shorter in PECAM-1−/− mice than in wild-type mice.
Despite the rapid formation of the PECAM-1/SHP-2 signaling complex during platelet aggregation,12 virtually nothing is known about the physiologic consequences of this association. One potential mechanism by which a PECAM-1/PTP signaling complex might interfere with GPVI-dependent platelet activation may involve dephosphorylation of PTKs and PTK substrates that become recruited to the nearby ITAM-bearing GPVI-FcRγ-chain collagen receptor complex. Consistent with this potential mechanism, it is notable that the same members of the Src family of PTKs that become activated on GPVI stimulation are also responsible for tyrosine phosphorylation of PECAM-1.32 Signaling molecules that might represent candidate substrates might include those that are shared by GPVI and TCR signaling pathways; we have recently shown that PECAM-1 becomes tyrosine phosphorylated, forms a complex with SHP-2, and attenuates calcium release from intracellular stores in T cells on co-ligation of the TCR with PECAM-1.20 The SH2 domain–containing leukocyte protein of 76 kd (SLP-76), originally identified as a component of the TCR signal transduction pathway,64 is one such candidate substrate. It has been shown to be an essential intermediate between Syk phosphorylation and PLCγ2 activation in platelet responses to collagen.65,66 Similarly, the linker for activation of T cells (LAT), a 36- to 38-kd adaptor molecule in TCR signaling,67 also seems to be involved in signal transduction mediated by the GPVI-FcRγ-chain complex because platelets from LAT−/− mice exhibit decreased PLCγ2 tyrosine phosphorylation and are impaired in their ability to expose P-selectin and to bind fibrinogen in response to stimulation through the GPVI collagen receptor.68 Studies are in progress to determine whether components common to the GPVI and TCR signal transduction pathways serve as substrates for dephosphorylation by the PECAM-1/SHP-2 signaling complex. Elucidation of the signaling pathways affected by PECAM-1 is likely to be important in furthering our understanding of the role played by this novel member of the immunoglobulin–ITIM family in vascular cell biology.
We thank Drs Gordon S. Duncan and Tak W. Mak (Amgen Institute, Toronto, ON, Canada) for providing PECAM-1–deficient mice.
Supported by National Institutes of Health grant P01 HL44612 (D.K.N., P.J.N.). S.P. is a recipient of a Northwestern Mutual Life Fellowship in Biomedicine awarded to the Medical College of Wisconsin.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Peter J. Newman, Blood Research Institute, The Blood Center of Southeastern Wisconsin, 638 N. 18th St, PO Box 2178, Milwaukee, WI 53233; e-mail: pjnewman@bcsew.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal