During maturation of the red blood cell (RBC) from the nucleated normoblast stage to the mature biconcave discocyte, both the structure and mechanical properties of the cell undergo radical changes. The development of the mechanical stability of the membrane reflects underlying changes in the organization of membrane-associated cytoskeletal proteins, and so provides an assessment of the time course of the development of membrane structural organization. Membrane stability in maturing erythrocytes was assessed by measuring forces required to form thin, tubular, lipid strands (tethers) from the surfaces of mononuclear cells obtained from fresh human marrow samples, marrow reticulocytes, circulating reticulocytes, and mature erythrocytes. Cells were biotinylated and manipulated with a micropipette to form an adhesive contact with a glass microcantilever, which gave a measure of the tethering force. The cell was withdrawn at controlled velocity and aspiration pressure to form a tether from the cell surface. The mean force required to form tethers from marrow reticulocytes and normoblasts was 27 ± 9 pN, compared to 54 ± 14 pN for mature cells. The energy of dissociation of the bilayer from the underlying skeleton increases 4-fold between the marrow reticulocyte stage and the mature cell, demonstrating that the mechanical stability of the membrane is not completely established until the very last stages of RBC maturation.
Introduction
During the last stages of maturation of red blood cells (RBCs), dramatic changes occur in the structure and organization within the cell. The cell loses its nucleus, surface molecules are shed in small vesicles, and the final surface-to-volume ratio of the cell is established.1-3During this time, proteins that will eventually form the membrane-associated cytoskeleton (membrane skeleton) are synthesized and assembled at the intracellular surface of the plasma membrane.4 The time course over which these protein assemblies become functionally viable is of interest, particularly with regard to hemolytic anemia and the early release of cells during hemorrhagic crisis, and could be important in designing methods for production of erythrocytes in vitro.
The function of the assembled membrane skeleton is fundamentally mechanical, and therefore, studies of membrane mechanical properties in maturing cells provide the most direct assessment of the development of the functional viability of the skeleton during maturation. Early studies of membrane properties of both murine and human reticulocytes indicated increased membrane stiffness (shear rigidity) in those membranes.5 This increased rigidity has been confirmed subsequently both by micropipette6 and cell deformation in shear (ektacytometry).7 In the latter study, evidence was also obtained that, despite increased mechanical stiffness, membranes of immature cells were less mechanically stable than their mature counterparts, as indicated by fragmentation of cells in fluid shear and in micropipette aspiration studies. The structural events associated with membrane fragmentation may involve either “tearing” of the membrane skeleton, or separation of the membrane bilayer from the underlying skeleton. Early fragmentation studies using ektacytometry showed a correlation between decreased membrane stability and chemical abnormalities related to lateral associations within the membrane skeleton, suggesting that the fragmentation involved mechanical failure of the skeleton itself.8 More recent studies using fluorescence imaging of micropipette-deformed cells showed that fragmentation in micropipettes involves lateral segregation of membrane components and separation of the lipid bilayer from the underlying skeleton.9Thus, fragmentation may occur as a result of different underlying mechanisms. An alternative to fragmentation methods for assessing membrane instability is the formation of membrane strands (tethers) from the surfaces of cells.10-12 This approach provides a direct and quantitative measure of the energy required to separate the membrane bilayer from the underlying membrane-associated cytoskeleton.13 14 In the present report we have applied this approach to demonstrate that the instability observed in immature RBCs lies in the tightness of the association between membrane bilayer and the underlying membrane skeleton, and we provide a quantitative measure of the degree of instability in terms of the work (energy) required to separate lipid bilayer from the surfaces of cells at different stages of maturation.
Materials and methods
Cell source and separation procedure
Circulating cells.
Normal mature human erythrocytes were obtained by venipuncture from healthy donors after informed consent according to the University of Rochester's Research Subjects Review Board. Heparin was used as anticoagulant. The reticulocytes were isolated by positive selection using magnetic beads coated with antibodies against transferrin receptor (CD71-M450, Dynal, Lake Success, NY. See Brun and coworkers.15). White blood cells were first removed by filtration through a cellulose column (Sigma Cell type-50, Sigma Chemical, St Louis, MO). A gentle negative pressure was applied to facilitate the filtration. A volume of 250 μL filtered whole blood was mixed with 25 μL magnetic beads on a rocker at room temperature for 1 hour. The remaining filtered whole blood was spun at 2500 rpm for 10 minutes to isolate plasma (centrifuge model HN-SII, International Equipment, Needham Heights, MA). The reticulocyte-bead mixture was washed 2 times in HEPES buffered saline (130 mM NaCl, 10 mM HEPES, plus 2.0 g/L bovine serum albumin [BSA], pH 7.3 ± 0.1) using a magnet to separate cells adhering to beads from the rest of the population. After washing, the beads were separated from the cells by incubation in plasma (which contains soluble transferrin receptor that competes with the cells for binding sites on the beads) on a rocker at room temperature for 1 hour.
Marrow cells.
Human bone marrow cells were aspirated from the iliac crest of healthy donors after informed consent according to the University of Rochester's Research Subjects Review Board. The total marrow cells were diluted 1:1 with McCoy 5A medium (Gibco, Grand Island, NY) and layered onto Ficoll–Paque (1.077 g/mL; Pharmacia, Piscataway, NJ). Mononuclear cells (MNCs) from the interface band were collected after centrifugation at 300g for 30 minutes at room temperature. The MNCs were washed twice in McCoy 5A medium and then resuspended at a cell density of 1 × 106 cells/mL in filter-sterilized phosphate-buffered saline (PBS; Gibco) supplemented with 5% vol/vol fetal bovine serum (FBS; Gibco), 4.5 g/L d-glucose (Sigma), 0.2 mM l-glutamine (Gibco), 50 U/mL penicillin (Gibco), and 50 μg/mL streptomycin (Gibco). A fraction of the MNC was set aside for cytospin slide preparation. Briefly, 20 000 MNCs/slide were centrifuged in cytospin funnels at 500 rpm for 5 minutes using a cytospin centrifuge (Shandon, Sewickley, PA).
Biotinylation of cells
Circulating cells.
Mature erythrocytes and circulating reticulocytes were biotinylated with N-Hydroxysuccinimidobiotin (NHS-biotin; Pierce Chemical, Rockford, IL). Biotin was dissolved at 10 mg/mL in dimethyl sulfoxide then diluted into whole blood 1:1000 to make a concentration of 10 μg/mL. After mixing in the dark for 5 minutes, cells were centrifuged and washed twice in PBS (160 mM NaCl, 6.2 mM KH2PO4, 25 mM Na2HPO4, 290 mOsm) and twice in hypotonic PBS, made by diluting PBS with deionized water to the desired osmolarity (155 mOsm).
Marrow cells.
Bone marrow MNCs were surface labeled with biotin by incubation in low endotoxin phosphate-buffered saline (LE-PBS, Biowhittaker, Walkersville, MD) plus 5% FBS (Hyclone, Logan, UT) with water-soluble, reactive biotin (10 μg/mL EZ-Link sulfo NHS-LC-biotin, Pierce Chemical) for 5 minutes and stirred at room temperature in the dark. Prior to use, the serum was incubated overnight with beads coated with streptavidin (Dynal, M280 streptavidin beads) to remove biotin. Cells were separated by centrifugation and washed 3 times in LE-PBS, then suspended in filtered LE-PBS plus 5% FBS.
Mechanical measurements
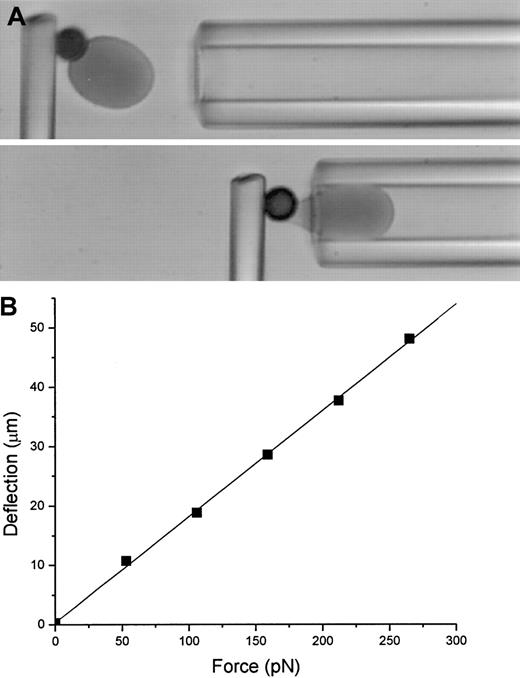
In preparation for mechanical testing, thin glass fibers (MO-SCI, Rolla, MO) were cemented (optical adhesive no. 68, Norland Products, New Brunswick, NJ) into the tapered tips of glass capillary tubes. A 3% gelatin solution (Sigma) was prepared in Na2HPO4 (pH 7.2-7.4) and mixed vigorously with 5 mg NHS-biotin (Pierce Chemical) dissolved in 250 μL dimethyl sulfoxide to form an emulsion. This solution was drawn into a glass capillary and painted onto the tips of the fibers under a dissection microscope. The gelatin was vapor fixed by suspending the painted fiber in a test tube above concentrated formaldehyde (Sigma). On the day before cells were tested, the fiber was mounted on the stage of an inverted microscope (Nikon Diaphot, 60 × objective, with monochromatic, 436-nm illumination, Nikon, Melville, NY) in a measuring chamber filled with hypotonic PBS (∼ 155 mOsm) containing BSA (3.0 mg/mL). Biotinylated RBCs were suspended at low concentration in 155 mOsm PBS plus BSA (3.0 mg/mL) and placed in the holding chamber adjacent to the measuring chamber on the microscope stage. Streptavidin-coated beads were introduced into the chamber and allowed to adhere to the cells. Cell-bead pairs were selected and aspirated into a large diameter (10 μm) transfer pipette. The pipette was withdrawn from the holding chamber and introduced into the measuring chamber and the cells were expelled. The transfer pipette was removed, and a calibration pipette, with an inside radius (Rp) of 2.0 to 2.3 μm, was introduced. (The RBCs suspended in the hypotonic buffer fit snugly into the pipette.) Cell-bead pairs were manipulated into contact with the biotinylated fiber. The cell was aspirated into the pipette and acted as a piston to transfer the force of the aspiration to the fiber (Figure1A). A series of known suction pressures (ΔP) were applied to deflect the fiber. The force on the fiber (f) was f = π Rp2Δp. Typically, 3 to 5 different calibration sequences were performed, each with a different cell, and the average slope of the force deflection curves was used to convert deflection to force. A typical calibration curve is shown in Figure 1B. After calibration, the pipette was withdrawn, the chamber was cleaned and filled with distilled water containing sodium azide (40 μM, Sigma) to retard bacterial growth, and the fluid level was maintained via a connection to a fluid reservoir overnight.
Calibration of a microcantilever.
(A). Video micrograph of the calibration of a microcantilever. The biotinylated RBC adheres to the streptavidin-coated bead and serves as a piston to transmit the force of the pipette suction pressure to the fiber. The fiber deflection is measured as a function of the suction pressure, and the force is calculated from the pressure as described in the text. (B) An example of the force-deflection relationship for a microcantilever. The force constant for this cantilever was 5.6 pN/μm.
Calibration of a microcantilever.
(A). Video micrograph of the calibration of a microcantilever. The biotinylated RBC adheres to the streptavidin-coated bead and serves as a piston to transmit the force of the pipette suction pressure to the fiber. The fiber deflection is measured as a function of the suction pressure, and the force is calculated from the pressure as described in the text. (B) An example of the force-deflection relationship for a microcantilever. The force constant for this cantilever was 5.6 pN/μm.
On the morning of the actual experiment, the chamber was flushed thoroughly with physiologic saline and filled with LE-PBS plus 5% FBS (vol/vol) for measurements. (The serum was preincubated with streptavidin beads to remove biotin.) Biotinylated cells were suspended at low density and placed in a holding chamber on the stage of the microscope. Selected cells were transferred to the measurement chamber and placed where they could be retrieved later. In experiments on marrow cells, cells were selected based on their hemoglobin density and morphology, and streptavidin beads were introduced into the chamber after cells were transferred. In mature cell experiments, cells were selected with beads already attached, and the cell-bead pairs were transferred to the measurement chamber. When the appropriate number of cells and beads had been moved into the measurement chamber, the transfer pipette was replaced with a measurement pipette having an inside diameter of 1.8 to 2.2 μm. Cells were attached to the calibrated glass microcantilever via a streptavidin-coated bead, and a portion of the cell was aspirated into the micropipette and withdrawn, forming a tether (thin cylinder of bilayer) from the cell surface (Figure 2). The deflection of the fiber was used to determine the force required to form and maintain the tether. In some cases, the dependence of the tethering force on aspiration pressure was determined by applying a series of aspiration pressures. After the formation of the tether and after each change in pressure, the force was allowed to relax for 5 to 10 minutes to reach a steady-state value.
Tether formation.
Video micrographs show tether formation from a marrow reticulocyte (left column) and a normoblast (right column). The top images show the cell morphology and the resting position of the cantilever. As the cells are withdrawn, the cantilever deflects, providing a measure of the force (middle panels). When the force gets big enough, the surface yields, and a strand of membrane is pulled out between the cell and its attachment site on the bead. After a length of 10 to 30 μm is reached, the cell is held stationary and the force on the tether is allowed to relax to a steady value (bottom panels).
Tether formation.
Video micrographs show tether formation from a marrow reticulocyte (left column) and a normoblast (right column). The top images show the cell morphology and the resting position of the cantilever. As the cells are withdrawn, the cantilever deflects, providing a measure of the force (middle panels). When the force gets big enough, the surface yields, and a strand of membrane is pulled out between the cell and its attachment site on the bead. After a length of 10 to 30 μm is reached, the cell is held stationary and the force on the tether is allowed to relax to a steady value (bottom panels).
Statistics
Statistical significance was assessed by applying the Student t test at a 95% confidence level.
Theory
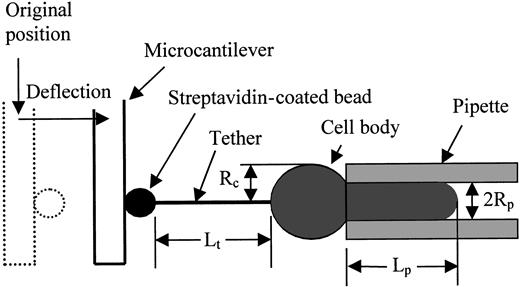
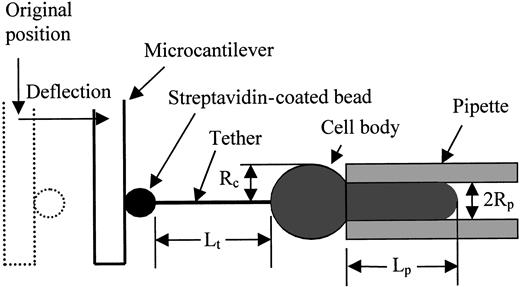
For smooth-surfaced bilayer membrane capsules, the mechanics of tether formation have been delineated.14,16,17 For phospholipid vesicles, equilibrium equations relating the pipette aspiration pressure, tether force, cell dimensions, and material constants were obtained using either force balance17 or energy methods.18 Energy methods have been applied to obtain equilibrium relationships for tethers formed from cells having linkages between the bilayer and the underlying cytoskeleton.13 14 The dimensions of the system are shown schematically in Figure 3. The cell is held in a pipette with inside radius Rpand the length of the cell projection into the pipette isLp. The pressure difference between the inside of the pipette and the surrounding buffer is ΔP, and the force on the tether is f. The length of the tether isLt and its radius is Rt. The form of the energy function for the system is:
In this equation the first term corresponds to the energy required to bend the membrane into a cylinder of radiusRt. The energy is characterized by the bending stiffness of membrane kc, which has units of energy. The second and third terms represent the work of external forces, and the fourth term corresponds to the energy needed to separate the membrane bilayer from the underlying membrane skeleton and associated proteins. This energy is represented byWsk, which has units of energy per unit area. Contributions to the bending energy due to the relative stretching of the adjacent leaflets of the membrane as a result of tether formation are neglected because previous studies have shown that this energy is not significant in RBCs for tethers up to 100 μm in length. (For more detail on this energy term, see references 13 and 19.) To obtain equations of equilibrium we take the variation of this function subject to the constraints that the membrane area and cell volume are constant. Under these conditions, 2 independent equilibrium equations are obtained:
where Rc is the radial dimension of the spherical portion of the cell outside the pipette. These are written as approximations because terms on the order of the tether radius are neglected in comparison with terms on the order of the cell radius. These can be combined to eliminate the radius of the tether and obtain an expression for the tethering force as a function of the work of separating bilayer from skeleton and the holding pressure in the micropipette
where the membrane tension is given by:
Thus, the square of the tethering force is predicted to increase linearly with the aspiration pressure, and the intercept of the line is proportional to the energy cost of separating bilayer and skeleton (Wsk).
Schematic illustration of the tether formation experiment showing the critical dimensions of the system.
The streptavidin-coated bead adheres to both the biotinylated gelatin-coated fiber tip and the biotinylated cell surface. As the cell is withdrawn a tether (cylindrical membrane strand) forms between the cell and the bead. The deflection of the fiber provides a measure of the force on the tether.
Schematic illustration of the tether formation experiment showing the critical dimensions of the system.
The streptavidin-coated bead adheres to both the biotinylated gelatin-coated fiber tip and the biotinylated cell surface. As the cell is withdrawn a tether (cylindrical membrane strand) forms between the cell and the bead. The deflection of the fiber provides a measure of the force on the tether.
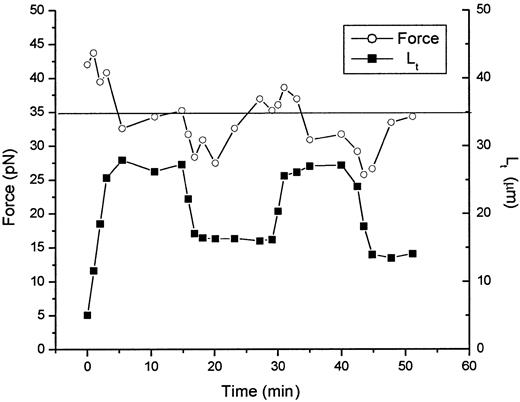
The development of equation 4 involves 2 important assumptions about the tether formation process. The first of these is that the system can be treated as being in thermodynamic equilibrium. This condition is verified by noting that the same equilibrium condition is reached whether it is approached from increasing or decreasing tether length. This is illustrated in Figure 4, in which the tethering force and tether length are plotted as a function of time for a typical tether formed from a marrow reticulocyte. When the tether length is changed, the system is temporarily in disequilibrium, but relaxes to an equilibrium state over a period of 5 to 10 minutes. The source of this disequilibrium is not known, but it appears to be related to the velocity of tether formation and the magnitude of the change in tether length. When the difference between the instantaneous tether force and the equilibrium tethering force is large, longer times are required for the relaxation to equilibrium to occur.
Time course of force relaxation after lengthening and shortening of the tether.
Both the tether length (lower curve, filled symbols) and tether force (upper curve, open symbols) are shown as functions of time. The equilibrium force for this tether is between 34 and 35 pN. This value is approached after the initial formation of the tether and again after successive lengthening and shortening of the tether length. This behavior is characteristic of a system in thermodynamic equilibrium.
Time course of force relaxation after lengthening and shortening of the tether.
Both the tether length (lower curve, filled symbols) and tether force (upper curve, open symbols) are shown as functions of time. The equilibrium force for this tether is between 34 and 35 pN. This value is approached after the initial formation of the tether and again after successive lengthening and shortening of the tether length. This behavior is characteristic of a system in thermodynamic equilibrium.
The second important assumption in developing equation 4 is that there is no significant change in the elastic energy stored as a result of the deformation of the cell when either the aspiration pressure was changed, or the tether length was altered. For tether lengths that are practical to achieve experimentally, the amount of material drawn into the tether is too small to produce measurable deformations of the cell body. Thus, the equilibrium tethering force is expected to be independent of tether length, and this is confirmed experimentally. On the other hand, elastic energy changes associated with cell deformation resulting from changes in aspiration pressure are important to consider, particularly in the present study because of the changes in cytoskeletal organization and cell deformability that accompany the maturation process. The effects of aspiration-induced cytoskeletal deformation are manifested in an altered dependence of the tethering force on aspiration pressure, which enters equation 4 via the term τ. From the perspective of force balance, cytoskeletal rigidity results in gradients in the membrane tension τ, such that the surface forces generated by the aspiration pressure are not “felt” in regions far from the pipette entrance. Thus, the degree to which the tethering force depends on membrane tensions generated by pipette aspiration (τ) depends on the rigidity of the membrane-associated cytoskeleton, or more specifically, how the elastic stiffness of the cytoskeleton compares with the magnitude of the applied pressure. For mature RBCs, the membrane rigidity is relatively small, and pressures can be applied that make its contribution small. For less mature cells, the cytoskeletal rigidity is larger, and this rigidity tends to reduce the dependence of the tethering force on the aspiration pressure. In the extreme case, the tethering force is independent of aspiration pressure, and the contribution from the aspiration pressure does not appear in the expression for force14:f2 = 8π2 kcWsk.
This is an important consideration in the present study because the changes in cytoskeletal organization during late-stage maturation have significant effects on the relationship between tethering force and aspiration pressure, as will be shown. Thus, differences in tethering force for cells of different maturity may reflect not only differences due to the intrinsic energy of association between bilayer and skeleton, but also differences due to the degree to which cytoskeletal rigidity influences the contribution of the holding pressure in the pipette. To avoid these complications, direct comparisons of the strength of the bilayer skeletal interactions should be made in the limit as the aspiration pressure (contained in τ) approaches zero.
Results
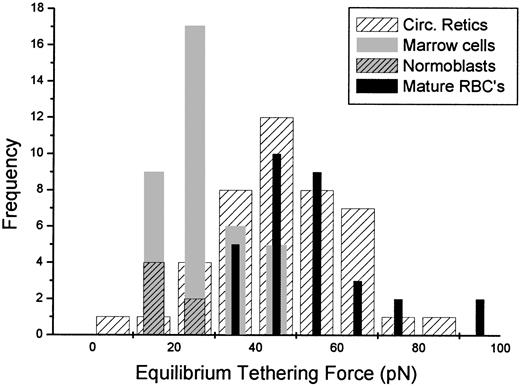
In an initial series of measurements, circulating reticulocytes were tested and the equilibrium tethering force was compared to that of control cells, that is, mature RBCs from unfractionated whole blood (Figure 5). Tethers approximately 30 μm in length were formed at a pipette holding pressure of approximately 98 Pa (1.0 cm H2O) and then the force was allowed to “relax” for approximately 20 minutes to ensure that a steady value was reached. (Note: The time required for the tether to reach an apparent equilibrium depended on the conditions of the measurement. In simple pulling experiments where the tether formation rate was high and tethers were long, relaxation of the tethering force could be detected up to 15 minutes after pulling stopped. For experiments in which tethers were held at shorter, constant lengths and only the aspiration pressure was changed, relaxation of the tethering force occurred more quickly, and the force appeared to reach equilibrium within 5 to 10 minutes of the change in pressure.) Forty-three circulating reticulocytes were tested and compared to tethering forces from 31 control cells. Although some cells in the reticulocyte population exhibited tethering forces below the range of forces observed for control cells, the difference between the means of the 2 populations was not statistically significant. Subsequently, similar tests were performed on hemoglobin-dense cells obtained directly from marrow. (Cells were selected from the mononuclear fraction based on their anucleate or normoblast-like appearance and on their dark color under blue illumination.) The force of tether formation from these cells was significantly less than the forces measured for mature RBCs. The mean value for the steady-state tethering force for marrow cells, including marrow reticulocytes and late-stage normoblasts, was 27 ± 9 pN (mean ± SD, n = 37). This is significantly smaller than tethering forces measured for mature red cells (54 ± 14 pN, n = 31).13 In addition to these quantitative differences, immature cells from marrow (unlike their mature counterparts) frequently exhibited irregular contours and often changed shape during testing. Interestingly, these changes in contour had very little effect, and in many cases no measurable effect at all, on the magnitude of the tether force.
Equilibrium tethering forces.
Histogram shows the distribution of equilibrium tethering forces for mature cells (black bars), circulating reticulocytes (hatched bars), marrow cells (including reticulocytes and normoblasts, gray bars), and normoblasts only (gray, hatched bars). All tethers were formed at membrane tensions ranging from 0.05 to 0.10 mN/m (aspiration pressures ranging from 1.0 to 2.0 cm H2O). Although several circulating reticulocytes exhibited tethering forces that fell below the range of values for the mature cells, the difference between the circulating reticulocyte population and the population of mature cells was not statistically significant. However, tethering forces for reticulocytes and normoblasts from marrow samples were significantly lower than for mature cells.
Equilibrium tethering forces.
Histogram shows the distribution of equilibrium tethering forces for mature cells (black bars), circulating reticulocytes (hatched bars), marrow cells (including reticulocytes and normoblasts, gray bars), and normoblasts only (gray, hatched bars). All tethers were formed at membrane tensions ranging from 0.05 to 0.10 mN/m (aspiration pressures ranging from 1.0 to 2.0 cm H2O). Although several circulating reticulocytes exhibited tethering forces that fell below the range of values for the mature cells, the difference between the circulating reticulocyte population and the population of mature cells was not statistically significant. However, tethering forces for reticulocytes and normoblasts from marrow samples were significantly lower than for mature cells.
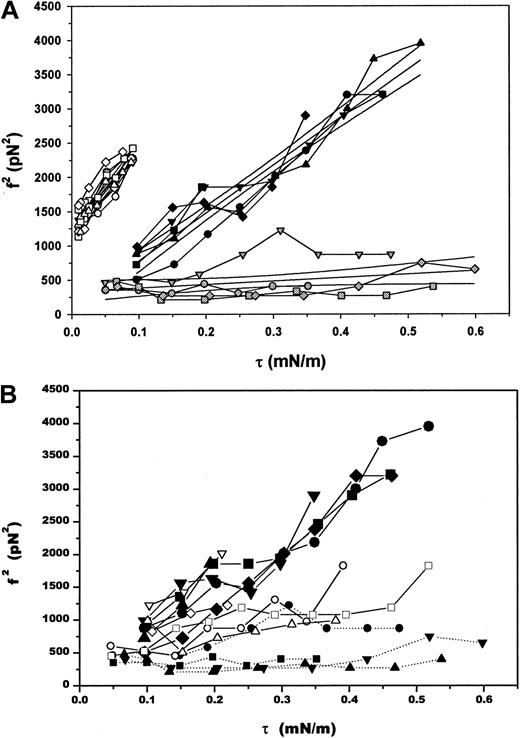
To quantify the changes in instability in terms of the separation energy Wsk, the tethering force was measured as a function of the pipette holding pressure. As expected (equation 4), a linear relationship between the force squared and the apparent membrane tension was observed for both reticulocytes and mature RBCs (Figure6A). The extrapolated intercept was used to calculate the bilayer-skeletal separation energyWsk. Assuming that the bending stiffness of the mature membranes and the reticulocytes was the same, and taking the extrapolated values of ∼ 1200 pN2 for mature cells and ∼300 pN2 for reticulocytes and normoblasts, we estimate that membrane modifications during the last stages of erythrocyte maturation result in a 4-fold increase in the energy required to separate bilayer from skeleton from 19 μJ/m2to 78 μJ/m2.
Tethering forces as a function of pipette holding pressure.
(A) The square of the equilibrium tethering force as a function of the membrane tension for selected cells. Open symbols represent measurements made on mature RBCs, dark symbols correspond to marrow reticulocytes, and gray symbols correspond to normoblasts. Linear regressions to each group of cells and 95% confidence intervals for the fits are shown as solid lines amid the data. (B) Square of the tethering force as a function of the membrane tension for a broader representation of cells from marrow. A continuous range of behavior was observed, from the force being independent of the membrane tension induced by the aspiration pressure to a linear dependence of the force squared on membrane tension. Intermediate behaviors shown here probably reflect changes in cytoskeletal rigidity and a transition from a highly wrinkled surface to the smooth contour of a nearly mature reticulocyte. Each group of connected symbols represents a different cell, all of which are marrow reticulocytes except the lowermost 4 curves (solid symbols: square, up-triangle, down-triangle and circle), which are normoblasts.
Tethering forces as a function of pipette holding pressure.
(A) The square of the equilibrium tethering force as a function of the membrane tension for selected cells. Open symbols represent measurements made on mature RBCs, dark symbols correspond to marrow reticulocytes, and gray symbols correspond to normoblasts. Linear regressions to each group of cells and 95% confidence intervals for the fits are shown as solid lines amid the data. (B) Square of the tethering force as a function of the membrane tension for a broader representation of cells from marrow. A continuous range of behavior was observed, from the force being independent of the membrane tension induced by the aspiration pressure to a linear dependence of the force squared on membrane tension. Intermediate behaviors shown here probably reflect changes in cytoskeletal rigidity and a transition from a highly wrinkled surface to the smooth contour of a nearly mature reticulocyte. Each group of connected symbols represents a different cell, all of which are marrow reticulocytes except the lowermost 4 curves (solid symbols: square, up-triangle, down-triangle and circle), which are normoblasts.
A range of behaviors was observed for the marrow population with regard to the dependence of the tethering force on aspiration pressure (Figure6B). Cells that were nonnucleated exhibited the greatest dependence of force on pressure, and cells that had the largest nuclei and the most ruffled surfaces exhibited almost no dependence. As discussed in the previous section, this observation is consistent with the higher mechanical stiffness of less mature cells.5-7 In addition, the presence of a reserve of membrane created by surface wrinkling in less mature cells makes it possible to form tethers from the cell surface without drawing membrane from the pipette, and so without doing work against the holding pressure. Thus, in addition to differences in stability between marrow cells and mature cells, we observe a progressive shift in behavior from that characteristic of a stiff, ruffled membrane with local reservoirs of membrane surface area from which tethers can be formed, to behavior characteristic of smooth membranes, in which cells are sufficiently deformable that the excess membrane area can be drawn completely into the micropipette, creating a smooth membrane contour. Thus, the observed changes in the dependence of the tethering force on aspiration pressure are consistent with the decrease in cytoskeletal rigidity that is known to occur during late-stage erythroid maturation.
Discussion
A great deal is known about the composition and organization of the membrane of mature RBCs, but surprisingly little is known about the processes of protein synthesis and assembly that lead to the formation of the mature cell. This is in part due to the difficulty of obtaining immature RBCs in sufficient quantity and uniformity to perform biochemical studies. RBCs are notoriously difficult to obtain in culture, and marrow samples include cells of multiple lineages at diverse stages of maturation. Consequently, most prior studies have relied on transformed, erythroleukemic cells as a model system for studying patterns of protein synthesis during erythropoiesis. Even in this case, however, it is rare to bring erythroid precursors to the fully mature form, and furthermore, interpretation of these studies may be problematic because of alterations to the natural maturation sequence that could result from transformation. Finally there is the additional consideration that biochemical and ultrastructural studies reveal information about structure and composition, but not about function, and so, although there are a few studies that provide information about the timing of the appearance of many of the membrane skeletal proteins found in the mature cell, studies in which the mechanical function of these assembled proteins is assessed are extremely rare. An important and notable exception is the study published by Chasis and coworkers7 examining changes in the mechanical rigidity and stability of maturing reticulocytes and assessing the importance of actin filaments and microtubules in different aspects of the maturation process. The present findings confirm many of the conclusions reached in that study and provide additional quantitative measures of the changes in membrane stability during the final stages of erythroid maturation.
The decrease in membrane stability that is documented here contrasts with the increased rigidity of immature RBCs that has been documented in a number of previous studies. In one of the first applications of micropipette manipulation of RBCs, it was shown that it is significantly more difficult to deform the surfaces of normoblasts and reticulocytes than it is to deform the membranes of mature cells.5 This finding has been confirmed subsequently by others.6,7 Chasis and coworkers7 also found evidence that immature RBCs are mechanically unstable, a conclusion that is confirmed in the present study. This seemingly paradoxical result shows that there are important fundamental differences in the mechanisms that account for resistance to membrane deformation, and mechanisms that determine the stability of the membrane bilayer and the strength of its association with the underlying cytoskeleton.8
The changes in membrane stability observed in the present study reflect the final stages of organization of the erythrocyte membrane skeleton (or membrane-associated cytoskeleton), which, in the mature cell, accounts for the elastic resistance of the membrane in extension (surface shear deformation) and also acts to stabilize the membrane bilayer against fragmentation.8,20,21 A number of studies have documented the appearance and assembly of RBC membrane proteins from the pronormoblast to the late normoblast. Decreased turnover of spectrin and actin, and changes in the expression of alternatively spliced forms of protein 4.1 are known to occur between the early and late stages of normoblast maturation.4,22 In the reticulocyte, reductions in lipid content and loss of surface receptors is known to occur during the final steps of maturation to the mature cell.1,2 Chasis and coworkers have shown that microtubles and microfilaments play an active role in enucleation and reticulocyte motility, respectively,7 but there is little information on the molecular reorganizations that occur in the membrane skeleton and associated proteins during these final stages of reticulocyte maturation. The findings of the present study confirm conclusions reached previously that important changes in membrane organization occur during the last 48 to 72 hours of reticulocyte development, and demonstrate that these changes are essential for establishing the stability and deformability of the mature cell.
For smooth vesicular membranes, the relationship between force and aspiration pressure can be used to calculate the bending stiffness of bilayer membranes.16 The mechanical analysis used to obtain the relationship for calculating kcincludes the assumption that the membrane tension is uniform over the cell surface. This assumption is valid for pure phospholipid bilayers and for mature RBCs under conditions in which the membrane tension generated by the aspiration pressure is large in comparison with variations in membrane tension arising from the elastic deformation of the membrane skeleton. The observation that the dependence of tethering force on aspiration pressure varies widely among normoblasts and marrow reticulocytes of different maturity demonstrates that this assumption is not valid for these cells. Thus, the apparent membrane bending stiffness for reticulocyte membranes calculated from the slope of the data obtained in the present study (0.886 × 10−19J) is unlikely to be a true reflection of the intrinsic bending stiffness of the membrane. The value of 1.55 × 10−19J for mature cells is also somewhat lower than what has been reported in previous studies. Based either on measurements of surface buckling, or on tether formation studies in which a different method of calculation was used, it was found that kc for mature cells falls in the range from 1.8 to 2.5 × 10−19J.12,13,23 Thus, refinements in the analytical framework that account for surface elasticity are clearly needed. Until such an analysis is completed, an alternative approach would be to determine the bending stiffness for the cells by a method in which the tether radius is calculated based on the displacement of material from the micropipette as the tether is formed.13 24Unfortunately, the irregular and dynamic shape of marrow reticulocytes makes this approach unreliable because it presumes that the cell surface contour has a stationary shape. Thus, the heterogeneity of properties and complex shapes of marrow cells make it appear unlikely that a satisfactory analytical framework can be developed for calculating kc from measurements of tether formation for these cells. This complicates the comparison of the energy costs for bilayer skeletal separation for different types of cells, because Wsk appears always as a product with the bending stiffness kc. (See equation 4.) Our inability to measure kc directly for these cells requires us to make comparisons between cell types assuming that the bending stiffnesses of the membranes are similar. Thus, although large differences in the bending stiffness of membranes of different maturity are not expected, the reader should keep in mind that part of the reported differences in Wsk may be attributable to differences in the bending stiffness of the different membranes.
Two other membrane systems have been tested by tether formation. In neuronal growth cone, the forces required to form tethers are on the order of 5.0 to 10.0 pN,14 considerably smaller than what is measured for even the relatively unstable RBC precursors we have examined here. These forces, assuming a membrane bending stiffness of 2.0 × 10−19 J, correspond to a separation energy of only 4.0 μJ/m2.14 This value approaches the behavior of a pure phospholipid bilayer. Neutrophils show a much higher resistance to surface loss. The minimum tethering force for neutrophils was measured at 45 pN, corresponding to a separation energy of 128 μJ/m2 25 of similar order to what we observe for mature RBCs.
Membrane instability could account for the difficulties that have been encountered in trying to produce viable mature RBCs from marrow precursors in culture. The instability that we have documented in maturing RBCs indicates that there are mechanisms at work within the marrow (eg, close-packing of cells) that stabilize membranes against surface loss during maturation. Indeed, it is well known that erythropoiesis in vivo occurs in discrete characteristic foci, erythoblastic islands, consisting of one or more central macrophages (CM) surrounded by a group of maturing erythrocytes.26 The cytoplasmic folds and processes of the CM interdigitate extensively within the erythoblastic island, covering 75% to 80% of the surface of each erythroblast and frequently penetrating deeply into cytoplasmic indentations in these cells.27 It has been postulated that the close apposition of cell surfaces within the erythoblastic island might serve to facilitate intercellular communication and transfer of chemical agents between the cells, and there is evidence that the CM may also facilitate erythroid enucleation and surface remodeling. The results of the present study lead us to speculate that these close intercellular contacts may also serve to stabilize the maturing membrane against premature loss of surface, thus enabling the RBC to maintain a high ratio of surface to volume during late-stage maturation.
In conclusion, micromechanical studies of the properties of RBC precursors document an instability in the membranes of immature cells compared with their mature counterparts. Marrow reticulocytes exhibit a resistance to surface area loss through bilayer skeletal separation that is comparable to what is measured for less mature normoblasts. In contrast, circulating reticulocytes exhibit membrane stability that is indistinguishable from the mature cell. These findings reveal that there are important changes in the organization of membrane skeletal proteins after enucleation and that the stability of the mature cell is attained only in the last days of cell maturation.
The authors thank Ms Donna Brooks for technical support and Ms Donna Phillips for help in preparing the manuscript.
Supported by the U.S. Public Health Service under National Institutes of Health grant no. PO1-HL18208. Additional support was obtained from the National Science Foundation (BES-9631670) and the National Aeronautic and Space Administration (NAG-8-1382). A. M. is grateful for a fellowship from the Alexander S. Onassis Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Richard E. Waugh, Department of Pharmacology and Physiology, 601 Elmwood Ave, Box 711, Rochester, NY 14642-8711; email:waugh@seas.rochester.edu.