Abstract
In adult bone marrow, mature erythroblasts are produced within structures called erythroblastic islands and then cross the endothelial barrier to reach circulation. Erythroblastic islands are composed of a central macrophage surrounded by maturing erythroblasts. In this study, it is shown that erythroid cells, but not the other mature hematopoietic cells, coexpress 2 angiogenic factors, vascular endothelial growth factor A (VEGF-A) and placenta growth factor (PlGF). Secretion of both VEGF-A and PlGF increases during in vitro erythroid differentiation. Erythroblast-conditioned medium can induce both migration of monocytes and endothelial cells and the permeability of endothelial cells. These effects are inhibited by anti-PlGF and/or anti-VEGF antibodies. Finally, it is shown that VEGF-A and PlGF proteins are expressed by bone marrow erythroblasts in vivo. Angiogenic factors secreted by erythroblasts may promote interactions either with macrophages in erythroblastic islands or with endothelial cells that would facilitate the passage of erythroid cells through the endothelial barrier.
Introduction
Erythropoiesis is a multistep process involving the differentiation of pluripotent hematopoietic stem cells through the lineage-committed burst-forming unit–erythroid (BFU-E) and colony-forming unit–erythroid (CFU-E) progenitor cells. These progenitors give rise to a series of early and late erythroblasts, leading to the formation of reticulocytes and finally mature erythrocytes.1 Interactions between hematopoietic precursor cells and their surrounding bone marrow environment are essential for erythroid differentiation as described in the model of the Sl/Sld mouse, whose anemia results from bone marrow stromal defects.2 In the bone marrow of mammals, erythropoiesis occurs in specific anatomic units called erythroblastic islands composed of a central macrophage surrounded by a ring of developing erythroblasts.3-5 Under normal circumstances, only mature cells cross the endothelial barrier into peripheral blood; immature cells are retained within the bone marrow.6Little is known about the initial events leading to the formation of erythroblastic islands and about interactions between erythroid cells and endothelial cells.
We have recently reported that neuropilin-1 is expressed on bone marrow stromal cells and that immature hematopoietic cells express several neuropilin-1 ligands.7 Neuropilin-1 was first characterized as a receptor for the semaphorin/collapsin protein family, which plays an important role in the guidance of growing axons.8,9 Recently, vascular endothelial growth factor A (VEGF-A)10 and placenta growth factor (PlGF)11were also identified as neuropilin-1 ligands. VEGF-A is a homodimeric protein that contains 1 of the 5 VEGF isoforms composed of 121, 145, 165, 189, or 206 amino acids (VEGF-121, VEGF-145, VEGF-165, VEGF 189, or VEGF-206).12 These various isoforms differ in their affinity for heparin and extracellular matrix components and are important regulators of angiogenesis and blood vessel permeability.13 They all bind the tyrosine kinase receptors, fetal liver kinase 1 (flk-1)14 and fms-like tyrosine kinase 1 (flt-1).15 Activation of flk-1 is correlated with the induction of angiogenesis and endothelial cell proliferation,16,17 and activation of flt-1 enhances endothelial cell17 and monocyte migration.18,19 PlGF is a homodimeric protein that shares substantial structural similarity with VEGF-A.20 Three PlGF isoforms have been described (PlGF-1, PlGF-2, and PlGF-3).21,22 PlGF-2 differs from the other 2 as it contains a heparin-binding domain.23 PlGFs do not interact with flk-1, but through binding to flt-1,24 they can induce the migration of endothelial cells25 and monocytes.18,26 Their function in blood vessel permeability remains uncertain.24,25,27 Finally, PlGF-1 can form active heterodimers with VEGF-A.28
In a search for expression of neuropilin-1 ligands in hematopoietic cells, we discovered that erythroid cells, but not other mature hematopoietic cells, produce both VEGF-A and PlGF. We analyzed VEGF-A and PlGF secretion during in vitro erythroid differentiation and studied the effects of this production on monocyte chemotaxis and on endothelial cell migration and permeability. Finally, we showed that normal bone marrow erythroblasts are a major source of these angiogenic factors in vivo.
Materials and methods
Reverse transcription–polymerase chain reaction analysis
Reverse transcription–polymerase chain reactions (RT-PCRs) were performed as previously described.7 Primer sequences were the following: Human VEGF forward primer 5′ATG AAC TTT CTG TCT TGG G3′, and reverse primer 5′CAC CGC CTC GGC TTG TCA CAT3′ (32 cycles, annealing at 60°C). Human PlGF forward primer 5′CGA GTA CCC CAG CGA GGT G3′, and reverse primer 5′GGA GTC ACT GAA GAG TGT GAC GG3′ (32 cycles, annealing at 60°C). S14 forward primer 5′GGC AGA CCG AGA TGA ATC CTC A3′, and reverse primer 5′CAG GTC CAG GGG TCT TGG TCC3′ (28 cycles, annealing at 64°C).
Cell cultures
Human erythroid cells were cultured as previously described.29 CD34+ cells were cultured in serum-free conditions for 7 days. This allows amplification of CD36+ erythroid progenitors, which were purified on day 7. These CD36+ cells were cultured for 7 days in the presence of erythropoietin. In these conditions, cells were composed of 97% CFU-E and late BFU-E progenitors at day 1 of the second stage of culture. Until day 3, cells were composed of immature blasts (late CFU-E progenitors and proerythroblasts). From day 4, most cells were identifiable as erythroblasts, and from day 6, all cells were erythroblasts, some of them displaying terminal differentiation.
For megakaryocytes and granulocytes, CD34+ cells were cultured in serum-free Iscove's Dulbecco's modified Eagle's medium (DMEM) (Life Technologies, Cergy Pontoise, France) in the presence of 15% of a commercial mixture of bovine serum albumin, insulin, and transferrin (BIT 9500) (Stem Cell Technologies, Vancouver, BC, Canada). To obtain megakaryocytes, 100 ng/mL thrombopoietin (Kirin Brewery, Tokyo, Japan) and 5 ng/mL stem cell factor (SCF) (Amgen; Thousand Oaks, CA) were added. In these conditions, more than 95% of the cells displayed megakaryocyte-lineage–specific CD41+/CD61+ staining by day 12. To obtain granulocytes, 10 ng/mL SCF, 10 ng/mL interleukin-3, 50 ng/mL flt-3–ligand, and 10 ng/mL granulocyte colony-stimulating factor were added. In these conditions, all the cells displayed a granulocytic phenotype by day 15, as determined both by direct observation after May-Grünwald Giemsa staining and by CD33 immunophenotyping.
Human umbilical artery endothelial cells (HUAECs) were cultured in Endothelial SFM Medium (Life Technologies) containing 20% fetal calf serum (FCS) and complemented with 1 ng/mL VEGF every other day. Culture of bovine corneal endothelial (BCE) cells was previously described.30
Enzyme-linked immunosorbent assays
Secretion of human VEGF-A (all isoforms) and PlGF (all isoforms) by erythropoietic cells was detected by Quantikine human VEGF and PlGF immunoassays (R&D Systems; Abingdon, United Kingdom) according to the manufacturer's protocol. From day 1 to day 7 of the second stage of culture, erythroid cells were maintained at a concentration of 500 000 cells per milliliter. Every day, 106 cells were harvested and replaced in fresh medium, and collected erythroblast-conditioned medium (ECM) was subjected to a quantitative sandwich immunoassay technique. For PlGF immunoassays, ECM was concentrated 5-fold by means of a Centricon-10 concentrator (Millipore; Bedford, MA).
Monocyte migration assay
Monocytes were isolated from peripheral blood mononuclear cells of healthy volunteers by means of a monocyte isolation kit (Miltenyi Biotech; Bergish Gladbach, Germany). Monocyte purity evaluated by CD14 staining was greater than 90%. Migration assays were performed by means of the Transwell system (Polylabo; Strasbourg, France).31 The upper and lower wells were separated by a 5-μm pore size polycarbonate filter. In the upper chamber, 2.105 monocytes were placed in a volume of 100 μL. VEGF-16530 and/or PlGF-1 (provided by D. Maglione, Naples, Italy) were added to the lower wells under a final volume of 600 μL in RPMI-1640 plus 1% FCS. ECM was concentrated by means of a Centricon-10 concentrator and added to the lower chamber under a final volume of 600 μL in RPMI-1640 plus 1% FCS. Negative controls were RPMI-1640 plus 1% FCS and cell-free erythroblast culture medium. Blocking rabbit antibodies directed against either human VEGF30 or human PlGF25were added at a final concentration of 50 μg/mL and 1 μg/mL, respectively. After 5 hours at 37°C, cells present in the lower chamber were numbered.
For control analysis, ECM was added in the lower compartment and/or in the upper compartment. ECM a contained 50 pg/mL PlGF and 1.2 μg/mL VEGF-A (30 pg PlGF and 700 pg VEGF-A in 600 μL), and ECM f contained 750 pg/mL PlGF and 18 μg/mL VEGF-A.
Endothelial cell migration and permeability assays
Migration wound assays were performed on HUAECs as previously described.30 Paracellular permeability assays were performed by measuring tritiated inulin (Amersham, Orsay, France) efflux through BCE cell monolayers (Ortéga et al, manuscript in preparation). Briefly, 105 BCE cells were seeded on 1-cm2 gelatinized filters mounted in diffusion chambers (Nunc, Roskilde, Denmark) in DMEM (Life Technologies) plus 10% FCS. At 4 to 6 days after confluence, cell monolayers were transferred into serum-free medium, and the modulators were added in the upper tank under a final volume of 500 μL in the presence of 2 μCi tritiated inulin. Aliquots of 10 μL were collected from lower chambers at different time intervals during a period of 120 minutes and counted in a scintillation counter. Blocking rabbit antibodies directed against human VEGF30 and against human PlGF25 were added at a final concentration of 50 μg/mL and 1 μg/mL, respectively. Negative controls consisted of cell-free erythroblast culture medium in either the absence or presence of 50 μg/mL rabbit preimmune serum.30
Immunohistochemistry
We examined 7 bone marrow biopsy samples with normal histological features. The bone marrow biopsies had been performed in the clinical staging of lymphomas. Bone marrow specimens were fixed in Bouin's fixative and then paraffin-embedded. Paraffin-embedded bone marrow sections were evaluated for VEGF-A, PlGF, and CD68 antigens by means of the alkaline phosphatase anti–alkaline phosphatase (APAAP) method.32 For better detection, sections were pretreated with microwave oven heating (3 cycles of 750 W for 5 minutes in 0.01 M citrate buffer, pH 6). Dilutions were 1:50 for anti–VEGF-A (rabbit anti–human VEGF-A [A-20]–G) (Santa Cruz Biotechnology; Santa Cruz, CA); 10 μg/mL for anti-PlGF (rabbit anti–human PlGF-125); and 1:75 for anti-CD68 (PG-M1) (Dako, Trappes, France). For detection of polyclonal antibodies, an incubation step with a mouse antirabbit immunoglobulin was added before proceeding with the APAAP technique. Rabbit antimouse immunoglobulins and APAAP complexes were obtained from Dako. Controls included the omission of the primary antibody and the use of an irrelevant antithyroglobuline polyclonal antibody (Dako). We used 2 combinations of antibodies in the double immuno-enzymatic staining procedure33: VEGF-A/CD68 and PlGF/CD68. Nuclei were counterstained with hematoxylin.
To evaluate the coexpression of glycophorin-A and VEGF-A or PlGF in erythroblasts, a double immunofluorescence method34 was used on paraffin-embedded bone marrow sections. VEGF-A and PlGF antibody dilutions were the same as used in APAAP method. Anti–glycophorin-A (Dako) was diluted 1:100. The secondary antibodies, goat F(ab′)2 immunoglobulin (Ig)–G (heavy [H] and light [L]) fluorescein isothiocyanate conjugate and goat anti–mouse IgG (H and L) Cy3 were incubated with tissue sections at final concentrations of 20 and 4 μg per milliliter, respectively. Controls included the omission of the primary antibody. Tissue sections were analyzed under a microscope (Leica, Rueil-Malmaison, France) equipped with an epifluorescent system for the detection of fluorescein isothiocyanate, Texas red, or a combination of both.
Results
VEGF-A and PlGF transcripts are coexpressed during in vitro erythroid differentiation
We had previously shown that neuropilin-1 was expressed on bone marrow stromal cells and that neuropilin-1 ligand messenger RNAs (mRNAs) were expressed by immature hematopoietic cells.7In this study, we investigated whether mature hematopoietic cells expressed ligands for neuropilin-1 by RT-PCR analysis. Pure populations of human erythrocytes, granulocytes, or megakaryocytes were obtained after differentiation of CD34+ cells (see “Materials and methods”). VEGF-A transcripts were detected in the 3 hematopoietic cell types (Figure 1A, VEGF-A), but interestingly PlGF mRNAs (PlGF-1 and PlGF-2) were found only in erythroblasts (Figure 1A, PlGF). We therefore focused our attention on erythroid differentiation. Mature erythroblasts expressed 3 VEGF-A mRNAs encoding the VEGF-121, VEGF-145, and VEGF-165 isoforms (Figure1A, VEGF-A). No expression of the VEGF-189 and VEGF-206 isoforms could be detected. To confirm these results, we performed RNase protection assays. In these experiments, VEGF-145 mRNA was weakly expressed: VEGF-165 mRNA level was 3 times higher than VEGF-145 mRNA, and VEGF-121 mRNA level was 1.5 times higher than VEGF-165 mRNA (data not shown).
Expresssion of VEGF-A and PlGF.
(A) Expression of VEGF-A and PlGF mRNAs in human mature hematopoietic cells. RT-PCR experiments were performed with the use of total RNA isolated from human erythroblasts, granulocytes, or megakaryocytes. We designed primers in exons 1 and 8 of the VEGF-A gene and in exons 2 and 7 of the PlGF gene in order to amplify the different splice variants. In these conditions, expected PCR products are as follows: 442 base pair (bp) for VEGF-121, 514 bp for VEGF-145, 574 bp for VEGF-165, 646 bp for VEGF-189, and 697 bp for VEGF-206 (lane VEGF-A); 396 bp for PlGF-1, 333 bp for PlGF-2, and 509 bp for PlGF-3 (lane PlGF). As a control, we used amplification of RT products with S14 primers. (B) Expression of VEGF-A and PlGF mRNAs during in vitro erythroid differentiation. Day 1 (corresponding to a majority of CFU-E and late BFU-E progenitors) to day 7 (corresponding to differentiated erythroblasts) are the various times of the second stage of culture. The 2 major VEGF-A transcripts, VEGF-121 (442 bp) and VEGF-165 (574 bp), and the PlGF-1 transcript (333 bp) are shown. As a control, we used amplification of RT products with S14 primers.
Expresssion of VEGF-A and PlGF.
(A) Expression of VEGF-A and PlGF mRNAs in human mature hematopoietic cells. RT-PCR experiments were performed with the use of total RNA isolated from human erythroblasts, granulocytes, or megakaryocytes. We designed primers in exons 1 and 8 of the VEGF-A gene and in exons 2 and 7 of the PlGF gene in order to amplify the different splice variants. In these conditions, expected PCR products are as follows: 442 base pair (bp) for VEGF-121, 514 bp for VEGF-145, 574 bp for VEGF-165, 646 bp for VEGF-189, and 697 bp for VEGF-206 (lane VEGF-A); 396 bp for PlGF-1, 333 bp for PlGF-2, and 509 bp for PlGF-3 (lane PlGF). As a control, we used amplification of RT products with S14 primers. (B) Expression of VEGF-A and PlGF mRNAs during in vitro erythroid differentiation. Day 1 (corresponding to a majority of CFU-E and late BFU-E progenitors) to day 7 (corresponding to differentiated erythroblasts) are the various times of the second stage of culture. The 2 major VEGF-A transcripts, VEGF-121 (442 bp) and VEGF-165 (574 bp), and the PlGF-1 transcript (333 bp) are shown. As a control, we used amplification of RT products with S14 primers.
During erythroid differentiation, the amounts of the 2 major VEGF-A mRNAs, ie, VEGF-121 and VEGF-165, were roughly invariant from day 1 (corresponding to a majority of CFU-E and late BFU-E progenitors) to day 7 (corresponding to erythroblasts) of the second stage of culture (Figure 1B, VEGF). The major PlGF mRNA, ie, PlGF-1, was detected from day 2 (corresponding to a majority of CFU-E progenitors), and its level gradually increased during erythroid differentiation (Figure 1B, PlGF-1).
VEGF-A and PlGF proteins are both secreted by erythroblasts during in vitro differentiation
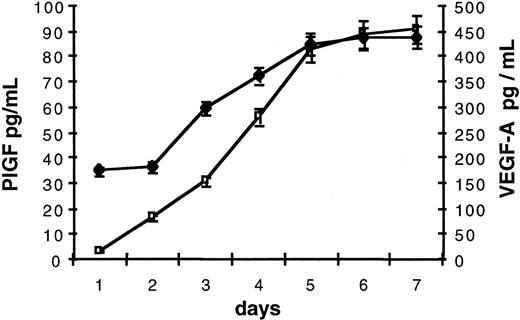
As VEGF-A and PlGF transcripts were detected in erythroblasts, we examined whether these factors were secreted during in vitro erythroid differentiation. From days 1 to 7 of culture, cells were harvested every day and replaced in fresh culture medium. The concentration of VEGF-A and PlGF in collected ECM was evaluated by means of enzyme-linked immunosorbent assays (ELISAs) that take all VEGF-A and PlGF isoforms into account. As PlGF levels in ECM were close to the minimum detectable dose, we measured PlGF levels in 5-fold concentrated ECM. Culture supernatants contained both VEGF-A and PlGF (Figure2). Secretion of these factors gradually increased during erythroid differentiation by 3-fold for VEGF-A and 100-fold for PlGF (Figure 2). VEGF-A and PlGF levels reached a plateau at day 6 of the second stage of culture (corresponding to differentiated erythroblasts) at 450 pg/mL per 106 cells for VEGF-A and 18 pg/mL per 106 cells (90 pg/mL in 5-fold concentrated ECM as indicated in Figure 2) for PlGF.
VEGF-A and PlGF secretion by erythroblasts.
VEGF-A (♦) and PlGF (■) contents of ECM were evaluated by means of ELISAs measuring all VEGF-A and PlGF isoforms. ECM was collected from 106 cells at days 1 to 7 of second stage of culture. For PlGF, values correspond to protein levels measured in 5-fold concentrated ECM. Each point represents the mean concentration (pg/mL) and standard deviation of 4 independent assays.
VEGF-A and PlGF secretion by erythroblasts.
VEGF-A (♦) and PlGF (■) contents of ECM were evaluated by means of ELISAs measuring all VEGF-A and PlGF isoforms. ECM was collected from 106 cells at days 1 to 7 of second stage of culture. For PlGF, values correspond to protein levels measured in 5-fold concentrated ECM. Each point represents the mean concentration (pg/mL) and standard deviation of 4 independent assays.
ECM induces migration of human monocytes
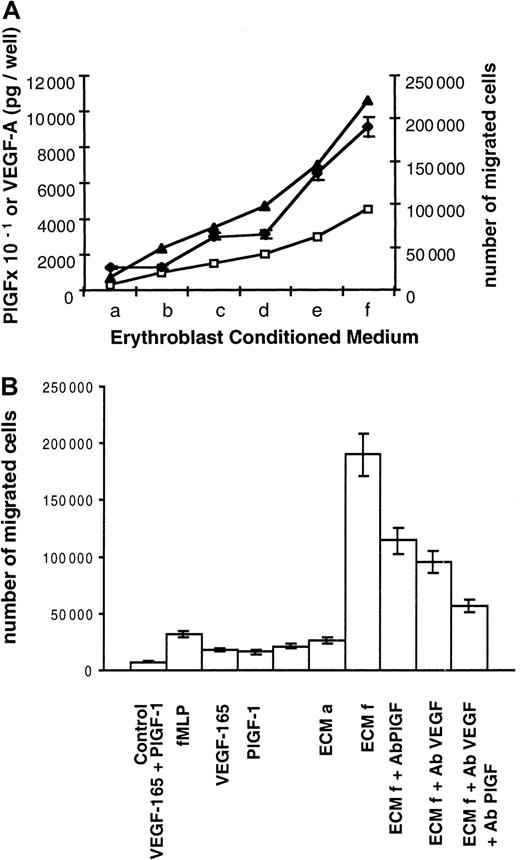
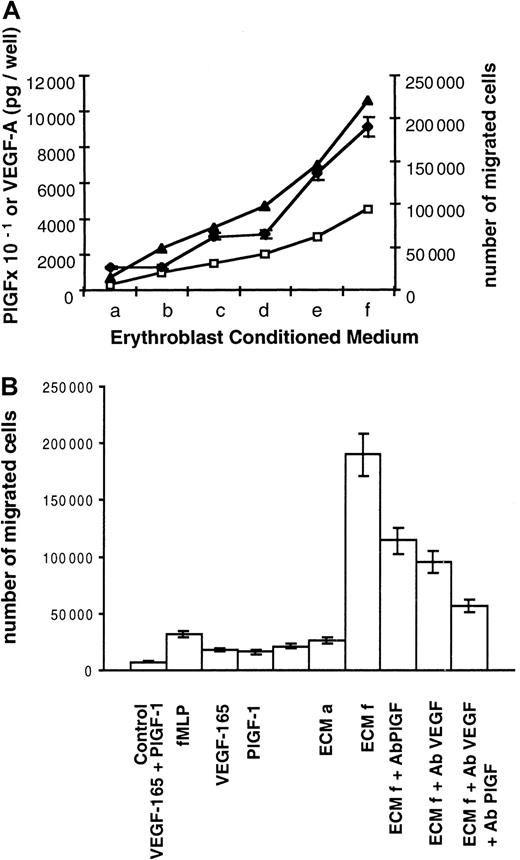
PlGF or VEGF-A receptors (flt-1, flk-1, and neuropilin-1) are not expressed in erythroblastic cells (data not shown). Therefore, we assumed that PlGF and VEGF-A may exclusively act as paracrine effectors. Macrophages are found in close association with erythroid cells within erythroblastic islands, and like their precursors, monocytes, they express the flt-1 receptor. Morever, monocytes are known to migrate in response to PlGF and VEGF-A. Therefore, these cells may constitute potential targets for erythroblasts. To test this hypothesis, we investigated whether ECM was able to induce migration of human monocytes. Monocyte migration was assayed across polycarbonate filters facing ECM, which was collected at day 6 of the second stage of culture. Different ECM volumes (a = 0.6 mL, b = 2 mL, c = 3 mL, d = 4 mL, e = 6 mL, f = 9 mL) were concentrated and used under a final volume of 600 μL so that concentration factors were 1 for a, 3.3 for b, 5 for c, 6.6 for d, 10 for e, and 15 for f. Using increasing ECM volumes, we showed that ECM induced monocyte migration and that the number of migrated cells was directly correlated with PlGF and VEGF-A quantities (Figure 3A). In addition and as shown in Table 1, migration of monocytes depended on the presence of an ECM-positive gradient between the lower and the upper compartments (higher concentration below the filter). Checkerboard analysis has previously revealed that the response of monocytes to VEGF and probably to PlGF was a result of chemotaxis and not of chemokinesis.18 These data and ours indicate that ECM is able to induce a chemotactic response in human monocytes with no appreciable chemokinetic response.
Induction of human monocyte migration by ECM.
(A) Correlation between monocyte migration and VEGF-A and PlGF levels contained in ECM. Two 105 monocytes obtained from peripheral blood were placed in the upper compartment of a modified Boyden chamber. ECM was collected at day 6 of culture and added to the lower compartment of the chamber. ECM a to ECM f corresponds to increasing ECM quantities. Different ECM volumes (a = 0.6 mL, b = 2 mL, c = 3 mL, d = 4 mL, e = 6 mL, f = 9 mL) were concentrated and used under a final volume of 600 μL so that concentration factors were 1 for a, 3.3 for b, 5 for c, 6.6 for d, 10 for e, and 15 for f. PlGF and VEGF-A contents were determined by ELISA. PlGF picograms per well, VEGF-A picograms per well: levels of PlGF × 10−1(■) and VEGF-A (▴) in the different ECMs. Number of migrated cells (♦) represents the mean number of migrated cells during 3 independent experiments ± SD. (B) Monocyte chemotactic activities of ECM and recombinant factors. Culture medium (control), chemotactic tripeptide fMLP (6 μmoles per well), VEGF-165 (10 ng per well), PlGF-1 (10 ng per well), and both VEGF-165 (10 ng per well) and PlGF-1 (10 ng per well) or ECM were added to the lower compartment. ECM a and f are described in Figure 3A. Anti-PlGF blocking antibodies (ECMf Ab PlGF), anti-VEGF blocking antibodies (ECMf Ab VEGF), or both (ECMf Ab PlGF Ab VEGF) were added to ECMf. Number of migrated cells represents the mean number of migrated cells during 3 independent experiments ± SD.
Induction of human monocyte migration by ECM.
(A) Correlation between monocyte migration and VEGF-A and PlGF levels contained in ECM. Two 105 monocytes obtained from peripheral blood were placed in the upper compartment of a modified Boyden chamber. ECM was collected at day 6 of culture and added to the lower compartment of the chamber. ECM a to ECM f corresponds to increasing ECM quantities. Different ECM volumes (a = 0.6 mL, b = 2 mL, c = 3 mL, d = 4 mL, e = 6 mL, f = 9 mL) were concentrated and used under a final volume of 600 μL so that concentration factors were 1 for a, 3.3 for b, 5 for c, 6.6 for d, 10 for e, and 15 for f. PlGF and VEGF-A contents were determined by ELISA. PlGF picograms per well, VEGF-A picograms per well: levels of PlGF × 10−1(■) and VEGF-A (▴) in the different ECMs. Number of migrated cells (♦) represents the mean number of migrated cells during 3 independent experiments ± SD. (B) Monocyte chemotactic activities of ECM and recombinant factors. Culture medium (control), chemotactic tripeptide fMLP (6 μmoles per well), VEGF-165 (10 ng per well), PlGF-1 (10 ng per well), and both VEGF-165 (10 ng per well) and PlGF-1 (10 ng per well) or ECM were added to the lower compartment. ECM a and f are described in Figure 3A. Anti-PlGF blocking antibodies (ECMf Ab PlGF), anti-VEGF blocking antibodies (ECMf Ab VEGF), or both (ECMf Ab PlGF Ab VEGF) were added to ECMf. Number of migrated cells represents the mean number of migrated cells during 3 independent experiments ± SD.
Then we compared the chemotactic activity of ECM with that of recombinant factors. As shown in Figure 3B, 10 ng recombinant PlGF-1 plus 10 ng VEGF-165 induced the migration of 10.5% of monocytes (21 000 migrated cells per well). Similar chemotactic activity (26 000 migrated cells per well) was obtained with ECM a, which contained only 30 pg PlGF and 700 pg VEGF-A. Furthermore, 95% of monocytes migrated (190 000 migrated cells per well) in response to ECM f, which contained 450 pg PlGF and 10 ng VEGF-A. Interestingly, such a migration rate was never reached with the tripeptide fMLP control chemoattractant18 or with higher quantities of recombinant VEGF-165 and/or PlGF-1.
Finally, we evaluated the role of VEGF-A and PlGF present in ECM using blocking antibodies. As shown in Figure 3B, chemotaxis induced by ECM f (190 000 migrated monocytes) was inhibited 40% by blocking antibodies against PlGF (114 000 cells, lane ECM + Ab PlGF) and 50% by blocking antibodies against VEGF (95 000 cells, lane ECM + Ab VEGF). Chemotaxis of monocytes was inhibited 70% by both blocking antibodies (57 000 cells, ECM + Ab PlGF + Ab VEGF).
Taken together, these data demonstrate that ECM induces a chemotactic response of monocytes and that this activity is mediated in part by PlGF and VEGF-A. In addition, ECM is more potent than recombinant VEGF-A and PlGF.
ECM induces endothelial cell migration and permeability
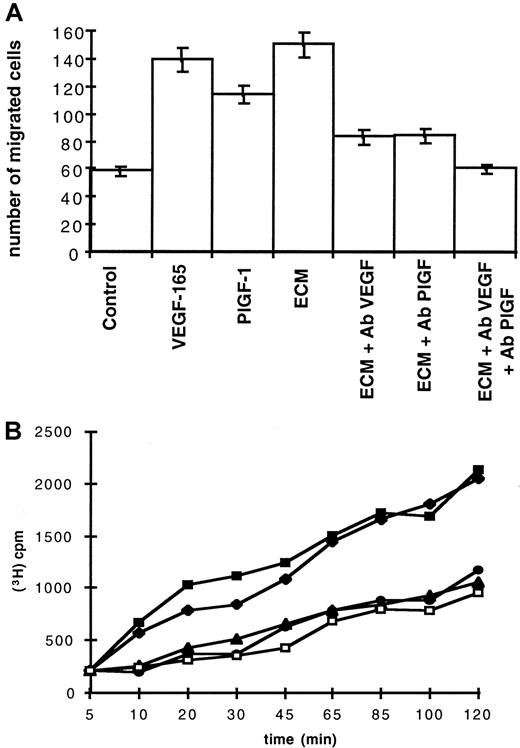
Since vascular endothelial cells express VEGF-A and PlGF receptors and interact with erythroid cells in bone marrow, they could also be target cells for VEGF-A and PlGF contained in ECM. Therefore, we investigated whether ECM could induce migration and permeabilization of endothelial cells. In these assays, ECM contained 38 pg/mL VEGF-A and 7 pg/mL PlGF. Figure 4A shows results of migration wound assays performed on HUAECs. ECM induced a significant migration (150 migrated cells per field), which was similar to the results obtained with either 50 ng/mL VEGF-165 (140 migrated cells per field) or 50 ng/mL PlGF-1 (115 migrated cells per field). This migration was inhibited 55% by anti-VEGF blocking antibodies (Figure4A, ECM + Ab VEGF) or by anti-PlGF blocking antibodies (Figure 4A, ECM + Ab PlGF). Addition of both blocking antibodies totally inhibited this migration (Figure 4A, ECM + Ab VEGF + Ab PlGF).
Migration and permeability of endothelial cells.
(A) Induction of endothelial cells migration by ECM. ECM contained 38 pg/mL VEGF-A and 7 pg/mL PlGF. Chemotaxis of HUAECs was evaluated in the presence of 50 ng/mL VEGF-165, 50 ng/mL PlGF-1, or ECM in the absence (ECM) or in the presence of either anti-VEGF antibodies (ECM + Ab VEGF), anti-PlGF antibodies (ECM + Ab PlGF), or both blocking antibodies (ECM + Ab VEGF + Ab PlGF). Control was cell-free erythroblast culture medium in the absence or presence of 50 μg/mL rabbit preimmune serum (control). Each point represents the mean number of migrated cells and SDs of 3 different experiments. (B) ECM induces permeability of endothelial cells. ECM contained 38 pg/mL VEGF-A and 7 pg/mL PlGF. Cell permeability was measured by inulin passage through the BCE monolayer and evaluated by measuring 3H-inulin efflux (indicated cpm) during a period of 120 minutes. Inulin efflux with 50 ng/mL VEGF-165 was measured in the absence (▪) or in the presence (●) of blocking anti-VEGF antibodies and with ECM in the absence (♦) or in the presence (▴) of blocking anti-VEGF antibodies. Control was cell-free culture medium in the absence or in the presence of 50 μg/mL rabbit preimmune serum (■).
Migration and permeability of endothelial cells.
(A) Induction of endothelial cells migration by ECM. ECM contained 38 pg/mL VEGF-A and 7 pg/mL PlGF. Chemotaxis of HUAECs was evaluated in the presence of 50 ng/mL VEGF-165, 50 ng/mL PlGF-1, or ECM in the absence (ECM) or in the presence of either anti-VEGF antibodies (ECM + Ab VEGF), anti-PlGF antibodies (ECM + Ab PlGF), or both blocking antibodies (ECM + Ab VEGF + Ab PlGF). Control was cell-free erythroblast culture medium in the absence or presence of 50 μg/mL rabbit preimmune serum (control). Each point represents the mean number of migrated cells and SDs of 3 different experiments. (B) ECM induces permeability of endothelial cells. ECM contained 38 pg/mL VEGF-A and 7 pg/mL PlGF. Cell permeability was measured by inulin passage through the BCE monolayer and evaluated by measuring 3H-inulin efflux (indicated cpm) during a period of 120 minutes. Inulin efflux with 50 ng/mL VEGF-165 was measured in the absence (▪) or in the presence (●) of blocking anti-VEGF antibodies and with ECM in the absence (♦) or in the presence (▴) of blocking anti-VEGF antibodies. Control was cell-free culture medium in the absence or in the presence of 50 μg/mL rabbit preimmune serum (■).
Permeability assays were performed on BCE monolayers. As shown in Figure 4B, ECM induced inulin efflux through BCE monolayers. The profile of this efflux was similar to that obtained with 50 ng/mL VEGF-165. Activities of ECM and recombinant VEGF-165 were both inhibited in the presence of anti-VEGF blocking antibodies. Anti-PlGF blocking antibodies had no significant effect (data not shown).
From these results, we conclude that ECM is able to induce migration of endothelial cells and that this activity is mediated by PlGF and VEGF-A. We also demonstrate that VEGF-A produced by erythroblasts is able to enhance cell permeability. In both experiments, ECM is more potent than recombinant VEGF-A and PlGF.
Normal human bone marrow erythroblasts express VEGF-A and PlGF
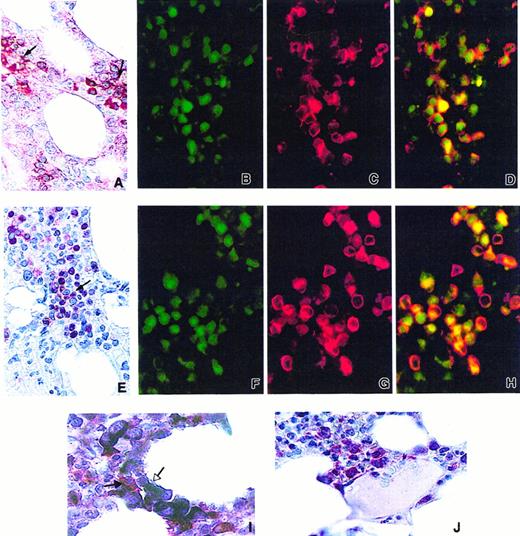
As VEGF-A and PlGF are expressed by erythroblasts in culture, we analyzed the expression of VEGF-A and PlGF in normal human bone marrow by immunohistochemistry. By means of the APAAP method on paraffin-embedded bone marrow tissue sections, many erythroblasts grouped in islands were shown to stain strongly with VEGF-A antibody (Figure 5A). As previously reported,35 36 we could also observe that megakaryocytes and myeloid cells disclosed cytoplasmic staining (data not shown). Using a double immunofluorescence method performed with VEGF-A antibody (Figure 5B) and a specific erythroblastic marker, the glycophorin-A antibody (Figure 5C), we confirmed that most glycophorin-A–positive cells expressed VEGF-A (Figure 5D). Using the APAAP method, we also observed that many erythroid cells grouped in islands displayed a PlGF cytoplasmic staining weaker than VEGF-A staining (Figure 5E). No staining was observed in megakaryocytes and granulocytes (data not shown). A double immunofluorescence technique showed that most PlGF-positive cells (Figure 5F) were also stained with glycophorin-A antibody (Figure 5G), thus confirming the erythroid nature of the PlGF-expressing cells (Figure 5H).
Expression of VEGF-A and PlGF in human normal bone marrow.
(A) Expression of VEGF-A in the cytoplasm of erythroblastic island cells (APAAP technique, naphtol fast red) (black arrows). (B,C) Expression of VEGF-A (green, panel B) and glycophorin-A (red, panel C) examined by immunofluorescence. (D) Coexpression in erythroblasts is demonstrated by double immunofluorescence (yellow). (E) Expression of PlGF in the cytoplasm of erythoblastic island cells (APAAP technique, naphtol fast red) (black arrow). (F,G) Expression of PlGF (green, panel F) and glycophorin-A (red, panel G) analyzed by immunofluorescence. (H) Coexpression in erythroblastic cells is demonstrated by double immunofluorescence (yellow). (I) Double-immunoenzymatic staining: few macrophages stained in red with CD68 (PG-M1) (APAAP method, naphtol fast red) (black arrow). Macrophages are surrounded by VEGF-A–positive erythroblasts (APAAP method, NBT/BCIP revelation) (white arrow). (J) Erythroblasts showing cytoplasmic staining for VEGF-A (APAAP technique, naphtol fast red) are located in the vicinity of a sinusoid.
Expression of VEGF-A and PlGF in human normal bone marrow.
(A) Expression of VEGF-A in the cytoplasm of erythroblastic island cells (APAAP technique, naphtol fast red) (black arrows). (B,C) Expression of VEGF-A (green, panel B) and glycophorin-A (red, panel C) examined by immunofluorescence. (D) Coexpression in erythroblasts is demonstrated by double immunofluorescence (yellow). (E) Expression of PlGF in the cytoplasm of erythoblastic island cells (APAAP technique, naphtol fast red) (black arrow). (F,G) Expression of PlGF (green, panel F) and glycophorin-A (red, panel G) analyzed by immunofluorescence. (H) Coexpression in erythroblastic cells is demonstrated by double immunofluorescence (yellow). (I) Double-immunoenzymatic staining: few macrophages stained in red with CD68 (PG-M1) (APAAP method, naphtol fast red) (black arrow). Macrophages are surrounded by VEGF-A–positive erythroblasts (APAAP method, NBT/BCIP revelation) (white arrow). (J) Erythroblasts showing cytoplasmic staining for VEGF-A (APAAP technique, naphtol fast red) are located in the vicinity of a sinusoid.
Most of the VEGF-positive erythroblasts were grouped in islands around CD68+ macrophages, as shown by double VEGF-A/CD68 immuno-enzymatic staining (Figure 5I). VEGF-A–positive erythroid cells were also found in the vicinity of sinusoids (Figure 5J). Similar results were obtained by using PlGF antibody (data not shown).
These results demonstrate that erythroblastic cells express both VEGF-A and PlGF in normal human bone marrow.
Discussion
In this study, we show that 2 angiogenic factors, VEGF-A and PlGF, are secreted by human erythroid cells during in vitro differentiation and in vivo in normal human bone marrow. Few data about factors secreted by erythroid cells have been reported so far. Early erythroid progenitors BFU-Es produce Epo.37,38 Rauscher murine erythroleukemia cells and splenic erythroid cells of phenylhydrazine-treated mice produce platelet-derived growth factor.39,40 In this study, we detected VEGF-A expression in erythroblasts, megakaryocytes, and granulocytes; PlGF was expressed only in erythroblasts. Our results are in accordance with previous reports that described VEGF-A expression in normal human hematopoietic cells.41 On the other hand, PlGF was found to be expressed in placenta, cultured endothelial cells, and thyroid,20,23,42 43 but no data about its expression in hematopoietic cells have been reported so far.
Interestingly, erythroblasts express no receptor for PlGF and VEGF-A (flt-1, flk-1, or neuropilin-1). This suggests that VEGF-A and PlGF may act as paracrine effectors within the bone marrow. Monocytes and endothelial cells that express VEGF-A and PlGF receptors may be target cells.
The ability of ECM to induce migration of human monocytes was tested in a Boyden chamber assay. We show that ECM is able to induce chemotaxis of monocytes in a dose-dependent manner and that the maximal response is obtained with a sample that contains 10 ng VEGF-A and 450 pg PlGF. In addition, we show that monocyte chemotaxis is mediated by VEGF-A and PlGF because both blocking antibodies inhibited it by 70%. Whether VEGF-A and PlGF are sufficient to mediate this function is not clear. We show that 10 ng recombinant VEGF-165 plus 10 ng recombinant PlGF-1 actually induce the same response as an ECM sample containing only 700 pg VEGF-A and 30 pg PlGF. These results clearly indicate that ECM is more potent than recombinant factors and that there may be another component in ECM that is needed for enhancing VEGF-A and PlGF activities.
We also demonstrate that ECM is able to induce endothelial cell migration in wound assays. This activity is inhibited 55% by anti-PlGF or by anti–VEGF-A antibodies and is totally inhibited by both antibodies. These data suggest that endothelial cell migration induced by ECM is mediated by PlGF and VEGF-A. Again, ECM is more potent than recombinant factors. Finally, we demonstrate that ECM induces the permeability of a BCE cell monolayer. As this activity is inhibited by anti–VEGF-A antibodies but not by anti-PlGF antibodies, we conclude that permeability may be relayed by VEGF-A. It is interesting to note that in addition to erythrocytes, granulocytes and megacaryocytes also express VEGF-A transcripts and are able to cross the bone marrow endothelial barrier. Therefore, VEGF-A may be the mediator of transendothelial migration of hematopoietic cells through the bone marrow barrier.
The roles of different VEGF-A isoforms in mediating VEGF-A activities are poorly understood. In ECM, the major 2 VEGF-A transcripts are VEGF-121 and VEGF-165; VEGF-145 is weakly expressed. Unfortunately, specific reagents to differentiate the relative amounts of the different protein isoforms are not available. Monocytes express the flt-1 receptor, but do not express either the KDR or the neuropilin-1 receptor. In addition, VEGF-121 and VEGF-165, but not VEGF-145, can bind to flt-1. Finally, VEGF-121 and VEGF-165 are chemotactic at equivalent doses for human monocytes, and their properties are mediated by the flt-1 receptor.18,19 Therefore, VEGF-121 and VEGF-165 present in ECM may be equivalent for inducing chemotaxis of monocytes. The roles of VEGF-121, VEGF-165, and VEGF-145 in inducing migration and permeability of endothelial cells remain difficult to define. Neuropilin-1 binding is restricted to VEGF-165, and KDR chemotactic activity is known to be enhanced in the presence of neuropilin-1 by 4 times.10 Therefore, presence of both neuropilin-1 and KDR receptors on endothelial cells may relay VEGF-165 activity on these cells.
In conclusion, we show that bone marrow erythroblasts are a source of VEGF-A and PlGF, which may act as paracrine factors on monocytes/macrophages and/or endothelial cells. Our results suggest that PlGF and VEGF-A secreted by erythroblasts may be mediators of a cross-talk between erythroblasts and macrophages to organize or maintain the structure of the erythroblastic island. In addition, our data suggest that angiogenic factors may induce interactions between erythroblasts and endothelial cells that may help mature erythrocytes to cross the bone marrow barrier and reach the peripheral blood.
We thank Drs J. Alain, A. Angelillo-Scherrer, M. L. Boulland, L. Croisille, J. M. Freyssinier, H. Jouault, C. Lacombe, J. Marquet, N. Martin-Garcia, S. Montrereau, and P. Rincé for their advice.
Supported by the Institut National de la Santé et de la Recherche Médicale (INSERM), the Centre National de la Recherche Scientifique (CNRS), and the Ligue contre le Cancer and the Association pour la Recherche contre le Cancer (ARC grant 5715 to V.L.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Valérie Lemarchandel, INSERM U474, Maternité Port-Royal, 123 Bd de Port-Royal, 75014 Paris, France; e-mail: lemarchandel@cochin.inserm.fr.