Abstract
Chemokines constitute a large family of chemotactic cytokines that selectively attract different blood cell types. Although most inflammatory chemoattractants are only induced and released in the circulation during acute infection, a restricted number of CXC and CC chemokines are constitutively present in normal plasma at high concentrations. Here, such a chemotactic protein was purified to homogeneity from serum and fully identified as a novel CC chemokine by mass spectrometry and amino acid sequence analysis. The protein, tentatively designated Regakine-1, shows less than 50% sequence identity with any known chemokine. This novel CC chemokine chemoattracts both neutrophils and lymphocytes but not monocytes or eosinophils. Its modest chemotactic potency but high blood concentration is similar to that of other chemokines present in the circulation, such as hemofiltrate CC chemokine-1, platelet factor-4, and β-thromboglobulin. Regakine-1 did not induce neutrophil chemokinesis. However, it synergized with the CXC chemokines interleukin-8 and granulocyte chemotactic protein-2, and the CC chemokine monocyte chemotactic protein-3, resulting in an at least a 2-fold increase of the neutrophil and lymphocyte chemotactic response, respectively. The biologic effects of homogeneous natural Regakine-1 were confirmed with chemically synthesized chemokine. Like other plasma chemokines, it is expected that Regakine-1 plays a unique role in the circulation during normal or pathologic conditions.
Introduction
Serum is a rich source of leukocyte chemotactic factors that influence the migration of different leukocytic cell types to and from the blood circulation. For example, anaphylatoxin, or C5a, a cleavage product formed during complement activation, chemoattracts both polymorphonuclear and mononuclear blood cells. Other serum proteins such as platelet factor-4 and neutrophil-activating protein-2 are thrombocyte-derived chemotactic cytokines belonging to the chemokine family.1-4 In contrast to C5a, these and other chemokines are each selectively attracting a defined set of leukocytic cell types. The chemokine family is subdivided into 2 major classes, ie, CXC and CC chemokines depending on the positioning of conserved cysteine residues.1-5 The spectrum of target cells for each chemokine depends on the expression of one or more specific receptors on the different leukocyte subtypes. The receptors of all chemokines as well as those of C5a and other chemoattractants such as leukotriene B4 and bacterialN-formylmethionyl–containing peptides belong to the family of G protein–coupled 7-transmembrane domain receptors.6
Addition of bovine or human serum is often essential for the growth or maintenance of continuous and primary cell cultures. For example, we and others have used in the past low serum concentrations to preserve high viability of freshly isolated human leukocytes or to support the growth of hematopoietic progenitor cells in well-defined media.7 Conflicting or variable experimental results have often been related to the presence or absence of serum or partially purified plasma proteins in the test system, eg, in chemotaxis assays.8-12 Furthermore, even after transfer of cells to serum-free conditions, the biologic responses can still be influenced by serum components sticking to the cultured cells. Indeed, because chemokines have high affinity for heparin-like glycosaminoglycan molecules, serum-derived chemokines are candidates to interfere in migration assays.13 This is likely the case if more than one cell type (cultured in serum) is implicated in the test system, eg, to measure transendothelial migration of leukocytes.14
In an attempt to identify additional serum-derived chemotactic factors that might influence standard chemotaxis assays, we have purified such molecules from commercially available bovine serum routinely used to grow or maintain cells in vitro. This study revealed the existence of an unknown bovine CC chemokine for which no human homolog has yet been described. Furthermore, this CC chemokine did not only attract lymphocytes, but also neutrophilic granulocytes. The relatively high abundancy of this chemokine compared with other CC chemokines indicates a different physiologic role for this molecule.
Materials and methods
Chemokine purification
Chemotactic activity present in bovine serum was first concentrated and partially purified by adsorption to silicic acid (Matrex Silica, particle size 35-70 μm, pore size 10 nm; Millipore, Bedford, MA) as previously described for chemokines.15Tissue culture–grade newborn or fetal calf serum (Life Technologies, Paisley, United Kingdom) was diluted 1:5 in Eagle's minimum essential medium with Earle's salts (Life Technologies) and stirred with 10 g/L silicic acid at 4°C for 2 hours. The silicic acid was sedimented by centrifugation and washed with phosphate-buffered saline (PBS) containing 1 M NaCl. Adsorbed proteins were eluted at neutral pH in cold PBS containing 1.4 M NaCl and 50% ethylene glycol. Subsequently, the silicic acid eluate was dialyzed against equilibration/loading buffer (50 mM Tris-HCl, 50 mM NaCl, pH 7.4) before fractionation by heparin-Sepharose chromatography (Amersham Pharmacia Biotech, Uppsala, Sweden). Proteins were eluted from the column in a linear NaCl gradient (0.05-2 M NaCl in the loading buffer; 5 mL fractions). For all fractions, the protein concentration was determined by a Coomassie blue G-250 binding assay using the Bio-Rad commercial kit (Bio-Rad Laboratories, Hercules, CA). For further purification, fractions containing chemotactic activity were prepared for Mono S (Amersham Pharmacia Biotech) cation-exchange fast protein liquid chromatography by dialysis against 50 mM formate, pH 4.0. A linear NaCl (0-1 M) gradient in 50 mM formate, pH 4.0, was used to elute proteins (1 mL fractions). Finally, the chemokine was purified to homogeneity by reverse-phase high performance liquid chromatography (RP-HPLC). Samples were injected on a 220 × 2.1 mm C-8 Aquapore RP-300 column (Applied Biosystems, Foster City, CA), equilibrated with 0.1% trifluoroacetic acid (TFA) in water, and the proteins were eluted with an acetonitrile gradient (0%-80% in equilibration buffer; 0.4 mL fractions).
After each purification step, fractions were analyzed for purity by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions on Tris/tricine gels.15 Proteins were visualized by silver staining. The relative molecular mass markers included in the gels were carbonic anhydrase (Mr 31 000), soybean trypsin inhibitor (Mr 21 500), and lysozyme (Mr 14 400) (Bio-Rad) and the low molecular weight marker aprotinin (Mr 6500) (Pierce Chemical, Rockford, IL).
Chemokine identification by amino acid sequence analysis and mass spectrometry
For internal sequence analysis, pure protein was enzymatically digested by incubation for 18 hours at 37°C with the endoproteinases lysine-C (25 mM Tris-HCl buffer, 1 mM ethylenediaminetetraacetic acid [EDTA], pH 8.5; Boehringer Mannheim, Mannheim, Germany) or asparagine-C (20 mM sodium acetate buffer, 10 mM dithiothreitol, 1 mM EDTA, pH 5.5; Pierce Chemical) at an enzyme:substrate ratio of 1:20. Proteolytic fragments were separated by RP-HPLC on a 50 × 1 mm C-8 Aquapore RP-300 column (Applied Biosystems) and eluted with an acetonitrile gradient (0%-80%) in water containing 0.1% TFA (0.2 mL fractions). To detect cysteine residues, proteins were reduced for 2 hours at 70°C in 0.2 M Tris, pH 8.4, containing 100 mM dithiothreitol and 1% SDS. The solution was diluted 5 times, and cysteines were alkylated with acrylamide (final concentration of 2 M) for 45 minutes at 37°C. Subsequently, salts were removed on Prospin cartridges (Applied Biosystems).
The NH2-terminal amino acid sequences of homogeneous intact or fragmented peptides were determined by Edman degradation using a pulsed liquid phase 477A/120A protein sequencer (Applied Biosystems). Extended sequences were obtained by removing the background on the sequencer with o-phtalaldehyde. Briefly, when a proline was present at the NH2-terminal position during the Edman degradation, the peptides without NH2-terminal proline (derived from incomplete chemical reactions) were NH2-terminally blocked by incubation for 10 minutes at 43°C in o-phtalaldehyde solution (20 mgo-phtalaldehyde and 50 μL β-mercaptoethanol in 10 mL acetonitrile) on the sequencer in a basic N-methylpiperidine atmosphere. Subsequently, Edman degradation was proceeded with a double cleavage time for the following proline. The COOH-terminal sequence of 2 nM of intact natural chemokine was determined on a Procise COOH-terminal sequencer (Model 492C, Applied Biosystems).
For mass spectrometry, RP-HPLC–purified proteins were diluted in 50% acetonitrile/50% water/0.1% acetic acid to a concentration of 0.5 to 5 nM and injected at 5 μL/min (dry gas flow 3 L/min, dry temperature 300°C, nebulizer gas pressure of 7 psi, skimmer 1 voltage of 31 V, octopole lens at 3 V, and trap drive at 75.3) on an Esquire ion trap mass spectrometer (Bruker/Daltonic, Bremen, Germany). Relative molecular masses of peptides or proteins were calculated from 100 or more averaged spectra (accumulation time of ± 0.1 msec) to increase the accuracy of the mass/charge measurements.
Chemical synthesis of Regakine-1
Regakine-1 was chemically synthesized (0.1 mM scale) using standard Fmoc programs on a solid-phase peptide synthesizer (Model 433A, Applied Biosystems) as described in detail elsewhere.15 16 Final deprotection and cleavage of the peptide from the resin was performed with TFA, and the synthetic chemokine was separated from the resin over a glass filter. Crude synthetic Regakine-1 was separated from incomplete fragments by RP-HPLC on a Resource RPC column (Amersham Pharmacia Biotech). After purification, disulfide bridges were formed by incubation (90 minutes, 20°C) of unfolded peptide in 150 mM Tris, pH 8.6; 2 M ureum, 3 mM EDTA, 0.3 mM oxidized glutathione, and 3 mM reduced glutathione. The folded peptide was purified by RP-HPLC. The molecular mass of unfolded and folded peptide was confirmed by mass spectrometry on an Esquire ion trap mass spectrometer.
Isolation of peripheral blood cells and chemotaxis assay
Polymorphonuclear and mononuclear cells from human peripheral blood were separated by density gradient centrifugation (30 minutes, 400g) on Ficoll-sodium diatrizoate (Lymphoprep, Nycomed Pharma, Oslo, Norway). The total mononuclear cell fraction (2 × 106 cells/mL) was used for chemotaxis as a source for monocytes. Lymphocytes were further enriched by magnetic cell sorting (MACS, Miltenyi Biotec, Bergisch Gladbach, Germany) after labeling with magnetic microbeads coated with monoclonal antibody against CD3 and used at 107 cells/mL in migration assays. Neutrophilic and eosinophilic granulocytes were isolated from the polymorphonuclear cell pellet obtained by density gradient centrifugation. This pellet was first suspended in hydroxyethyl starch (Plasmasteril, Fresenius, Bad Homburg, Germany) for 30 minutes to remove most erythrocytes by sedimentation. Residual erythrocytes were then lysed in bidistilled water (30 seconds). The total granulocytic cell fraction was used at 106 cells/mL in neutrophil chemotaxis tests. Finally, after tagging the neutrophils with anti-CD16 beads (Miltenyi Biotec), eosinophils were isolated by MACS as the negatively selected cell fraction. Eosinophils were seeded at a final concentration of 2 × 106 cells/mL for migration tests.
For the isolation of bovine neutrophils, whole peripheral blood of adult cows was collected, diluted in PBS, and fractionated by density gradient centrifugation on Lymphoprep (Nycomed Pharma). The granulocyte pellet was resuspended and washed, and residual erythrocytes were lysed by hypotonic shock. Chemotaxis with bovine neutrophilic granulocytes was performed as described for human neutrophils.
The chemotactic potency of chemokines was determined in the Boyden microchamber (Neuro Probe, Gaithersburg, MD). Cell fractions and samples were diluted in Hank's balanced salt solution (Life Technologies) supplemented with human serum albumin (Belgian Red Cross) at 1 mg/mL (dilution buffer) and tested in triplicate. For granulocytes, migration through 5-μm pore-size polycarbonate membranes (Nuclepore, Corning Costar, Acton, MA) was measured after 45 minutes at 37°C for neutrophils and after 1 hour for eosinophils. Lymphocyte chemotaxis (4 hours, 37°C) was performed using fibronectin-coated (25 μg/mL; 12 hours, 4°C) polycarbonate membranes (5-μm pore-size), and for monocyte chemotaxis (2 hours, 37°C) polyvinylpyrrolidone-treated polycarbonate membranes (5- μm pore-size) were used. In each chemotaxis experiment eitherN-formyl-methionyl-leucyl-phenylalanine (fMLP; Sigma, St Louis, MO), purified natural interleukin 8 (IL-8) (neutrophils), or synthetic monocyte chemotactic protein–3 (MCP-3) (monocytes, lymphocytes, eosinophils) was included as a positive control.12 16 After incubation, the cells were fixed and stained using Hemacolor solutions (Merck, Darmstadt, Germany). Migrated cells were counted microscopically in 10 oil immersion fields at a 500 × magnification. The chemotactic potency of a sample was expressed as the chemotactic index (CI), ie, the number of cells migrated to the chemoattractant divided by the number of cells migrated to dilution buffer. Chemokinesis was measured by adding the chemokine to the cells at the time of transfer to the upper wells of the microchamber or by preincubation of the test cells with chemokine for 10 minutes at 37°C prior to transfer to the microchamber. The latter conditions were also used in experiments measuring the combined effect of Regakine-1 and the CXC chemokines IL-8 or granulocyte chemotactic protein–2 (GCP-2) in the migration assay; ie, neutrophils were preincubated with different concentrations of Regakine-1 (10 minutes, 37°C) and then added, without washing, to the upper compartment of the microchamber. Alternatively, Regakine-1 was added simultaneously with IL-8 to the lower wells of the microchamber to measure a synergistic effect in the chemotaxis assay. Statistical analysis of chemotaxis data was performed using the Mann-Whitney test.
Results
Isolation and identification of a novel CC chemokine from bovine serum
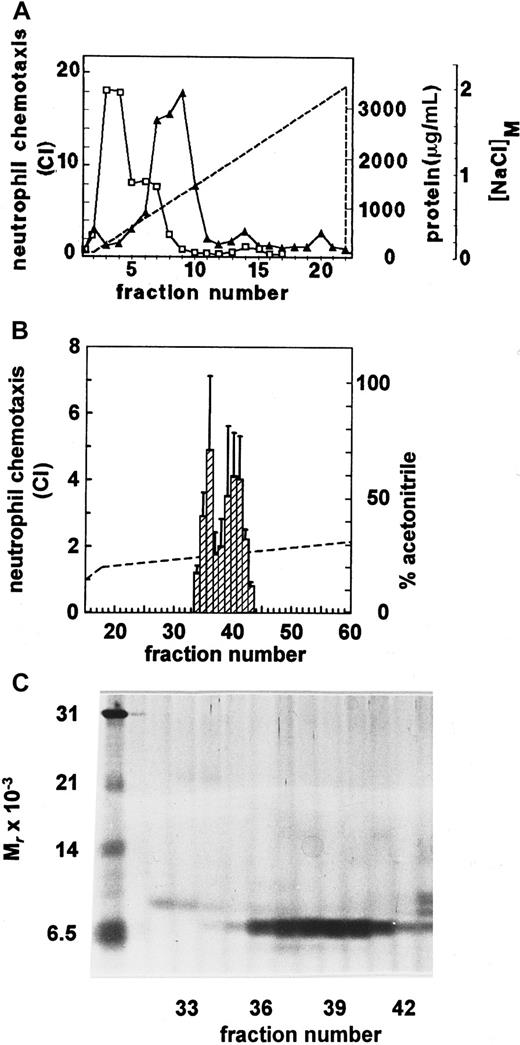
Tissue culture–grade newborn calf serum was processed according to our standard procedure for the isolation of chemokines from conditioned medium of in vitro–stimulated cell cultures.8,12,15 Due to its high protein content (about 50 mg/mL), the serum (2 L) was diluted 1:5 prior to adsorption of proteins to silicic acid. Because protein binding to this substrate was rather selective (99% unadsorbed), only 1 g of the initial amount of protein was recovered by elution from silicic acid. Subsequent heparin-Sepharose affinity chromatography allowed further enrichment of serum-derived chemotactic activity for neutrophils, which eluted at 0.5 M NaCl, after the bulk of protein showing low or no affinity for heparin (Figure 1A). Further purification to homogeneity of the biologic entity was achieved by cation-exchange chromatography (elution at 0.3 to 0.4 M NaCl) and finally by RP-HPLC. The neutrophil chemotactic activity was recovered from the RP-HPLC column (Figure 1B) over a rather broad range in the elution gradient (from 25% to 30% acetonitrile). However, SDS-PAGE analysis showed the presence of a single protein band of 7.5 kd corresponding to the neutrophil chemotactic activity (Figure 1C). None of the fractions containing the chemotactic protein corresponded to known bovine CXC chemokines. Indeed, IL-8 and GCP-2 derived from stimulated MDBK cells eluted at different positions upon cation-exchange chromatography and RP-HPLC.17 Surprisingly, NH2-terminal sequence analysis of this pure protein revealed the presence of a novel CC chemokine, whereas the CXC hallmark is typical for neutrophil chemoattractants. In view of the unusual source (serum) and target cell (neutrophils) for this CC chemokine, the molecule was tentatively designated Regakine-1.
Purification of neutrophil chemotactic activity from serum.
Chemotactic factors isolated from newborn calf serum were first fractionated in a NaCl (0-2 M) gradient (––) by heparin-Sepharose affinity chromatography (A). The protein concentration (■-■) in the fractions was evaluated by the Coomassie blue binding assay. The neutrophil chemotactic activity (▴-▴), expressed as chemotactic indexes (CI), was determined in the Boyden microchamber assay at 1/10 dilution. (B) The final purification step of the neutrophil chemotactic activity by RP-HPLC is shown. Proteins were recovered from the HPLC column in an acetonitrile (0-80%) gradient (––). Neutrophil chemotactic potencies (▨) were determined at dilution 1/50 and represent the mean CI ± SEM of 5 independent experiments. HPLC fractions containing neutrophil chemotactic activity were analyzed by SDS-PAGE (C) under reducing conditions (fractions 32 to 43, 4 μL/lane). The proteins were visualized by silver staining. The left lane shows Mrmarkers (see “Materials and methods”).
Purification of neutrophil chemotactic activity from serum.
Chemotactic factors isolated from newborn calf serum were first fractionated in a NaCl (0-2 M) gradient (––) by heparin-Sepharose affinity chromatography (A). The protein concentration (■-■) in the fractions was evaluated by the Coomassie blue binding assay. The neutrophil chemotactic activity (▴-▴), expressed as chemotactic indexes (CI), was determined in the Boyden microchamber assay at 1/10 dilution. (B) The final purification step of the neutrophil chemotactic activity by RP-HPLC is shown. Proteins were recovered from the HPLC column in an acetonitrile (0-80%) gradient (––). Neutrophil chemotactic potencies (▨) were determined at dilution 1/50 and represent the mean CI ± SEM of 5 independent experiments. HPLC fractions containing neutrophil chemotactic activity were analyzed by SDS-PAGE (C) under reducing conditions (fractions 32 to 43, 4 μL/lane). The proteins were visualized by silver staining. The left lane shows Mrmarkers (see “Materials and methods”).
The complete primary structure (70 residues) of Regakine-1 was obtained by NH2- and COOH-terminal sequence analysis and by sequencing internal fragments obtained by proteolytic digestion with the endoproteinases asparagine-C and lysine-C (Figure2). In addition, mass spectrometry allowed for the identification of the COOH-terminal serine that was undetectable during the COOH-terminal sequence analysis. Both the origin and the primary structure of Regakine-1 were confirmed by an independent purification and sequencing run using fetal calf serum instead of newborn calf serum. Furthermore, this same CC chemokine was isolated and identified from serum obtained through coagulation of blood from adult cows collected in a local slaughterhouse. This confirmed the true bovine nature of this molecule and excluded possible artifacts due to industrial processing of commercially available fetal or newborn serum, ie, the admixture with serum from other species. Furthermore, it demonstrated that the presence of this chemokine in serum is not restricted to young animals. On average, 100 μg Regakine-1 was isolated from 1 L bovine serum. This amount is comparable to the production of IL-8 by in vitro–stimulated leukocytes from 1 L human blood.12
Identification of the complete primary structure of Regakine-1 by amino acid sequence and by mass spectrometry analysis.
Natural Regakine-1 purified to homogeneity from serum (Figure 1) was subjected to NH2-terminal amino acid sequence analysis. Internal sequences were obtained by Edman degradation after proteolytic digestion and RP-HPLC purification. The COOH-terminal part of the sequence was evidenced by experimental COOH-terminal sequencing and mass spectrometry. X indicates unidentified residues. The numbers along the top of the figure indicate amino acid renumbering. The Regakine-1 Swiss-Prot protein database18 accession number isP82943.
Identification of the complete primary structure of Regakine-1 by amino acid sequence and by mass spectrometry analysis.
Natural Regakine-1 purified to homogeneity from serum (Figure 1) was subjected to NH2-terminal amino acid sequence analysis. Internal sequences were obtained by Edman degradation after proteolytic digestion and RP-HPLC purification. The COOH-terminal part of the sequence was evidenced by experimental COOH-terminal sequencing and mass spectrometry. X indicates unidentified residues. The numbers along the top of the figure indicate amino acid renumbering. The Regakine-1 Swiss-Prot protein database18 accession number isP82943.
The sequence of Regakine-1 was not picked up by a search in the SWISS-PROT/TrEMBL protein database.18 Alignment of the sequence of Regakine-1 with those of known human and bovine chemokines (Figure 3) and other proteins did not reveal a high structural similarity (< 50%). However, residues other than the 4 cysteines that are conserved in most CC chemokines are also selectively present in the amino acid sequence of Regakine-1, such as Ile20, Pro21, Tyr28, Val40, Phe42, Ala52, Pro54, Trp58, and Val59 (Figure 3). Regakine-1 was found to be most homologous to human eotaxin (49% identical residues). However, murine, guinea pig, rat, and human eotaxin share residues that are not present in the sequence of Regakine-1. Because for other known bovine chemokines the structural homology with their human counterparts is evidenced by more than 65% identical residues (eg, 67% for GCP-217), the human homolog of Regakine-1 remains to be identified.
Sequence alignment and homology of Regakine-1 with other CC chemokines.
Residues of Regakine-1 conserved in one of the other CC chemokines listed are shaded. The percentage identical residues between Regakine-1 and the other chemokines is indicated.
Sequence alignment and homology of Regakine-1 with other CC chemokines.
Residues of Regakine-1 conserved in one of the other CC chemokines listed are shaded. The percentage identical residues between Regakine-1 and the other chemokines is indicated.
Neutrophil and lymphocyte chemotactic potency of natural Regakine-1
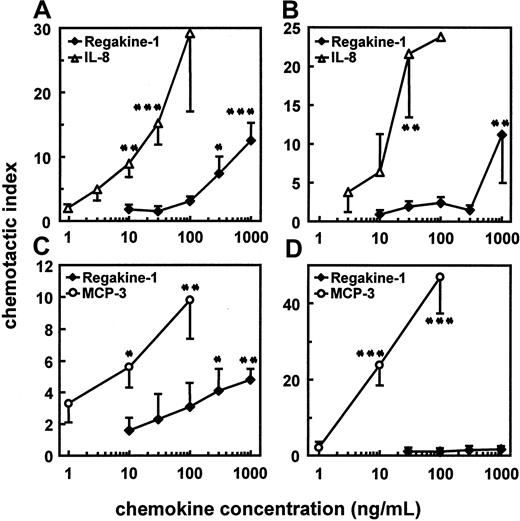
The bovine serum–derived CC chemokine (purified from different serum batches) was compared with human leukocyte-derived IL-8 in the standard microchamber migration assay using human and bovine neutrophils. On human neutrophils, IL-8 was still chemotactic at 10 ng/mL, whereas for Regakine-1, 300 ng/mL was necessary to obtain a significant chemotactic effect (Figure4A). In addition to its lower potency (minimal effective concentration), the efficacy (maximal CI) of Regakine-1 was on average weaker than that of human IL-8 (Figure 4A and data not shown). Furthermore, Regakine-1 was tested on bovine neutrophils to confirm the chemotactic potency in the homologous species. Figure 4B shows that on bovine neutrophils comparable results were obtained, human IL-8 being a more potent chemoattractant than Regakine-1. The chemotactic effect of Regakine-1 on granulocytes remained restricted to neutrophils, because human eosinophils, responsive to MCP-3 at 30 ng/mL, were not attracted by this chemokine at 1000 ng/mL (data not shown). This indicates that the relatively higher structural identity of Regakine-1 with human eotaxin is probably not biologically relevant.
Chemotactic potency and efficacy of natural Regakine-1 on various leukocyte cell types.
Pure natural Regakine-1 (Figure 1) was dose-dependently evaluated for chemotactic activity in the microchamber assay on human (A) and bovine (B) neutrophils, in parallel with pure natural human IL-8.12 For lymphocyte (C) and monocyte (D) chemotactic activity, natural Regakine-1 was compared with synthetic human MCP-3.16 The chemotactic response is expressed as the mean CI (± SEM) derived from at least 3 independent experiments. Significant differences from controls, determined by the Mann-Whitney test, are indicated by asterisks (*P < .1, **P < .01, ***P < .001).
Chemotactic potency and efficacy of natural Regakine-1 on various leukocyte cell types.
Pure natural Regakine-1 (Figure 1) was dose-dependently evaluated for chemotactic activity in the microchamber assay on human (A) and bovine (B) neutrophils, in parallel with pure natural human IL-8.12 For lymphocyte (C) and monocyte (D) chemotactic activity, natural Regakine-1 was compared with synthetic human MCP-3.16 The chemotactic response is expressed as the mean CI (± SEM) derived from at least 3 independent experiments. Significant differences from controls, determined by the Mann-Whitney test, are indicated by asterisks (*P < .1, **P < .01, ***P < .001).
In view of the modest and unexpected chemotactic activity of Regakine-1 on neutrophils, this CC chemokine was further investigated on mononuclear cells. In contrast to MCP-3, which induced monocyte migration from 10 ng/mL onward, up to 1000 ng/mL natural Regakine-1 had no significant chemotactic effect on freshly isolated peripheral blood monocytes (Figure 4D). However, natural Regakine-1 was chemotactic for CD3+ lymphocytes at 300 ng/mL, whereas MCP-3 was active on these cells at 10 ng/mL (Figure 4C). These biologic data demonstrate that Regakine-1 has a modest but significant chemotactic activity for both neutrophils and lymphocytes.
Chemotactic activity of synthetic Regakine-1
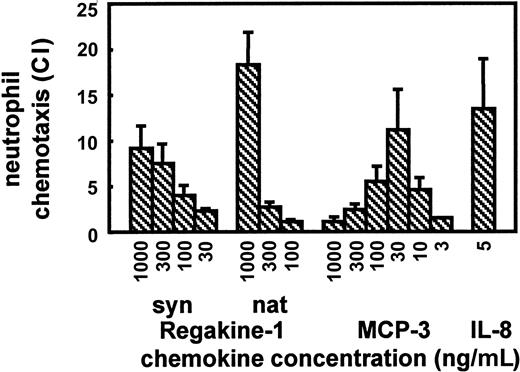
To exclude that the chemotactic effect of Regakine-1 was due to a minor contamination of this CC chemokine with other more potent chemokines, Regakine-1 was chemically synthesized by Fmoc chemistry. The synthetic protein was deprotected, folded, and purified to homogeneity according to a standard procedure used in our laboratory.15,16 Synthetic Regakine-1 was found to be biochemically and biologically identical to the natural product, as shown by mass spectrometry, amino acid sequence analysis, SDS-PAGE, and chemotaxis assays. The neutrophil chemotactic potency of both synthetic and natural Regakine-1 was inferior to that of human IL-8 and MCP-3 (Figure 5), another CC chemokine to which weak neutrophil chemotactic activity has been ascribed.19However, Regakine-1 was equally efficacious on neutrophils when compared with MCP-3, as can be deduced from the maximal CIs (Figure 5). Notably, the concentration of MCP-3 required to maximally attract neutrophils (30 ng/mL) can only be reached in serum during pathologic conditions, eg, viral infection,20 whereas 100 ng/mL Regakine-1 is a physiologic plasma concentration. Additionally, the lymphocyte chemotactic activity of Regakine-1 was also confirmed with the synthetic protein (data not shown). Taken together, these data with synthetic Regakine-1 confirm the authentic chemotactic activity of the natural chemokine.
Neutrophil chemotactic activity of synthetic Regakine-1.
Pure natural Regakine-1 (Figure 1) was compared with chemically synthesized and purified Regakine-1, synthetic human MCP-3,16 and human IL-812 for chemotactic activity on human peripheral blood neutrophils. Results represent the mean CI (± SEM) of at least 3 independent experiments in the microchamber migration assay.
Neutrophil chemotactic activity of synthetic Regakine-1.
Pure natural Regakine-1 (Figure 1) was compared with chemically synthesized and purified Regakine-1, synthetic human MCP-3,16 and human IL-812 for chemotactic activity on human peripheral blood neutrophils. Results represent the mean CI (± SEM) of at least 3 independent experiments in the microchamber migration assay.
Regakine-1 enhances the chemotactic potency of IL-8 and GCP-2
In an attempt to further define the role of Regakine-1 in leukocyte migration, it was verified whether this chemokine exerts chemokinetic effects. When applied with the cells in the upper compartment of the microchamber, different concentrations of IL-8 and MCP-3 as well as synthetic or natural Regakine-1 failed to induce neutrophil chemokinesis (Table 1). Furthermore, preincubation of the neutrophils for 10 minutes with either MCP-3 or Regakine-1 did not induce chemokinesis (Table 1). However, under the same conditions neutrophils responded chemotactically to IL-8 (at 15 and 50 ng/mL) when added in the lower compartment of the chamber (data not shown).
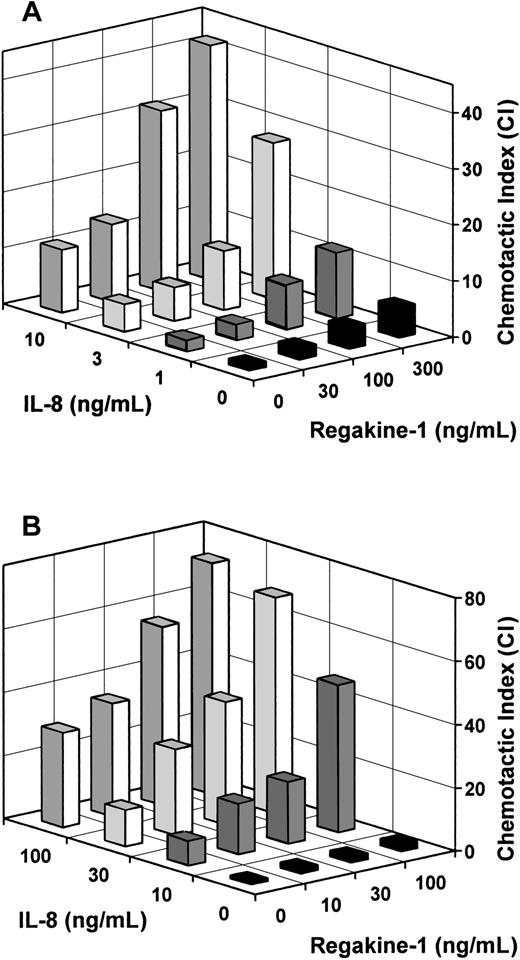
In the next experimental setting, Regakine-1 and IL-8 were verified for their cooperative effect in the chemotaxis assay (Figure6). When applied together in the lower compartment of the microchamber, the chemotactic response toward suboptimal doses of IL-8 (1-10 ng/mL) was further enhanced by physiologic concentrations (100-300 ng/mL) of Regakine-1 (Figure 6A). For example, when 300 ng/mL Regakine-1 was combined with 3 or 10 ng/mL IL-8, a 3-fold increase in chemotactic response was observed, ie, a 3-fold enhancement in the number of migrated cells above the additive effect of both chemokines when tested separately (P < .05). Similarly, a combination of 100 ng/mL Regakine-1 and fMLP at 10−9 M synergized in the neutrophil chemotaxis assay, yielding a 5-fold increase in CI (89 ± 29, n = 4, P < .05) compared with the additive effect of Regakine-1 (5.6 ± 2.0, n = 4) and fMLP (11.3 ± 3.1, n = 4).
Synergistic effect of Regakine-1 and IL-8 on neutrophil chemotaxis.
Different concentrations of IL-8 (0-10 ng/mL) and pure natural Regakine-1 (0-300 ng/mL) were combined in the lower compartment of the microchamber to measure neutrophil chemotaxis (A). Alternatively (B), human neutrophils were incubated with different concentrations (0-100 ng/mL) of natural Regakine-1 (10 minutes, 37°C) before transfer to the upper compartment of the microchamber to measure the chemotactic response to various concentrations of IL-8 (0-100 ng/mL). The mean CIs are derived from 3 independent experiments. The SEM did not exceed 20% of the mean CI and is omitted for clarity.
Synergistic effect of Regakine-1 and IL-8 on neutrophil chemotaxis.
Different concentrations of IL-8 (0-10 ng/mL) and pure natural Regakine-1 (0-300 ng/mL) were combined in the lower compartment of the microchamber to measure neutrophil chemotaxis (A). Alternatively (B), human neutrophils were incubated with different concentrations (0-100 ng/mL) of natural Regakine-1 (10 minutes, 37°C) before transfer to the upper compartment of the microchamber to measure the chemotactic response to various concentrations of IL-8 (0-100 ng/mL). The mean CIs are derived from 3 independent experiments. The SEM did not exceed 20% of the mean CI and is omitted for clarity.
Furthermore, an enhanced chemotactic response toward IL-8 was obtained when Regakine-1 was added together with the cells in the upper compartment of the chamber. Indeed, when neutrophils were preincubated for 10 minutes with 100 ng/mL Regakine-1 before transfer to the chamber, the chemotactic response toward 10 or 30 ng/mL IL-8 was increased 3-fold (Figure 6B). Comparable data (Table2) were obtained when neutrophils were treated with synthetic (300 ng/mL) instead of natural Regakine-1 to enhance the chemotactic effect of IL-8 (5 or 15 ng/mL) as well as of GCP-2 (15 or 50 ng/mL). On average, a 2.5-fold enhancement of the CI of both GCP-2 and IL-8 was observed by preincubation of neutrophils with Regakine-1 (Table 2). Addition of Regakine-1 to the cells did not induce cell migration (CI 1.3 ± 0.2) toward dilution buffer in the lower microchamber compartment. This indicates that, irrespective of the presence of a chemotactic gradient for Regakine-1, this chemokine is capable of enhancing the migration capacity of neutrophils toward CXC chemokines.
Synergy between Regakine-1 and MCP-3 in lymphocyte chemotaxis
To further explore the cooperation between Regakine-1 and other chemoattractants, the former CC chemokine was combined in the lower wells of the microchamber with MCP-3 to stimulate lymphocyte chemotaxis. Figure 7 illustrates that 100 to 300 ng/mL Regakine-1 together with a suboptimal concentration of MCP-3 (3 ng/mL) resulted in a 2-fold increase in lymphocyte chemotactic response above the additive effect of the individual chemokines. However, at an optimal concentration of MCP-3 (30 ng/mL), Regakine-1 failed to further enhance the efficacy of MCP-3 as a lymphocyte chemoattractant. Furthermore, at an inactive concentration (30 ng/mL), Regakine-1 failed to synergize with MCP-3. Taken together, these data demonstrate that the synergistic action of Regakine-1 is not restricted to neutrophil chemotaxis but is also effective between members of the same CC chemokine subfamily in lymphocyte chemotaxis. This indicates that different receptors or signal transduction pathways are implicated.
Regakine-1 enhances the lymphocyte chemotactic response toward MCP-3.
Different concentrations of MCP-3 (0-30 ng/mL) and pure natural Regakine-1 (0-300 ng/mL) were combined in the lower compartment of the microchamber to induce lymphocyte chemotaxis. The mean CIs are derived from 4 to 9 independent experiments. Statistically significant increases above the additive effect of the individual chemokines, determined by the Mann-Whitney test, are indicated by asterisks (*P < .1).
Regakine-1 enhances the lymphocyte chemotactic response toward MCP-3.
Different concentrations of MCP-3 (0-30 ng/mL) and pure natural Regakine-1 (0-300 ng/mL) were combined in the lower compartment of the microchamber to induce lymphocyte chemotaxis. The mean CIs are derived from 4 to 9 independent experiments. Statistically significant increases above the additive effect of the individual chemokines, determined by the Mann-Whitney test, are indicated by asterisks (*P < .1).
Discussion
Chemotactic cytokines or chemokines form a large family of selective leukocyte chemoattractants. CXC chemokines predominantly stimulate the migration of neutrophils or lymphocytes, whereas CC chemokines attract one or more leukocytic cell types, including monocytes, dendritic cells, lymphocytes, natural killer cells, eosinophils, and basophils.1-5 For most chemokines their biologic selectivity can be explained by binding and signaling through cell-specific G protein–coupled 7-transmembrane domain receptors.6 This study describes the isolation and identification of a novel CC chemokine derived from serum, often used to support cell viability or proliferation. The 7.5-kd protein was purified to homogeneity from fetal and newborn calf serum, and its primary structure was elucidated by mass spectrometry and NH2- and COOH-terminal amino acid sequence analysis on peptide fragments. Because its amino acid sequence did not show more than 50% identity with any known human or bovine chemokine, this CC chemokine was tentatively designated Regakine-1. Natural Regakine-1 exerted chemotactic activity for neutrophils and lymphocytes, 300 ng/mL being the minimal effective concentration. However, Regakine-1 was found to be abundantly present (about 100 ng/mL) in fetal, newborn, and adult bovine serum. Contamination of natural Regakine-1 preparations with other neutrophil- or lymphocyte-attracting chemokines is excluded because the chemotactic activity of natural Regakine-1 was confirmed with chemically synthesized protein. Regakine-1 did not show chemotactic activity for monocytes or eosinophils at concentrations up to 1 μg/mL. The CC chemokine did not exert chemokinetic activity but enhanced the neutrophil and lymphocyte chemotactic response to CXC chemokines (IL-8 and GCP-2) and CC chemokines (MCP-3), respectively. Indeed, when Regakine-1 was combined with IL-8 or MCP-3, the number of migrated cells increased at least 2-fold compared with the cumulative effect of these individual chemokines.
Other human plasma proteins such as platelet factor-4 (PF-4) and β-thromboglobulin (β-TG), derived from α-granules of activated blood platelets, were structurally identified more than 20 years ago.21,22 Although many activities have been ascribed to PF-4,1,4,23 a G protein–coupled receptor for this oldest CXC chemokine has still to be identified. In view of its high affinity for heparin, PF-4 was shown to activate neutrophils through binding to chondroitin sulfate–type glycosaminoglycans.24 The β-TG is an NH2-terminal cleavage product of platelet basic protein and connective tissue–activating protein III, to which biologic effects other than chemotactic activity have been attributed.1,4,23 Further proteolytic processing of β-TG into neutrophil-activating protein-2 generates25 a functional chemokine that binds to the CXC chemokine receptor 2 (CXCR2).1 Like PF-4, β-TG, and Regakine-1, the more recently discovered hemofiltrate CC chemokine–1 (HCC-1) was found at high concentrations in serum.26 HCC-1 is reported to be rather weak in activating monocytes and in enhancing the proliferation of CD34+progenitor cells (1 μg/mL minimal effective concentration) and has been shown to bind CCR1 with low affinity.26 27
The rather weak chemotactic activity of Regakine-1, a characteristic feature shared with PF-4, β-TG, and HCC-1, is compensated by a high constitutive plasma concentration (100 ng/mL). In contrast, potent and selective chemoattractants such as IL-8 or MCP-1 are strongly upregulated in multiple cell types during infection and inflammation.1,3,4 Indeed, endogenous (cytokines) and exogenous (viral and bacterial products) mediators induce these chemokines preferentially outside the vascular lumen to attract phagocytic cells to infected or inflamed tissues.1,12,20The release of PF-4 and β-TG from platelets is differently and much less strictly regulated, eg, after thrombin-induced platelet aggregation during blood coagulation. At present, nothing is known about the regulated production of HCC-1 in specific cell types, whereas the constitutive expression of HCC-1 mRNA has been demonstrated in several normal tissues.26
The exact biologic significance of the known plasma chemokines remains controversial. The weak neutrophil-degranulating activity of PF-4 was significantly increased by preincubation or coincubation of neutrophils with tumor necrosis factor-α,28 and the migration of eosinophils in response to PF-4 was markedly enhanced by IL-5.29 These findings are in parallel with the increased neutrophil and lymphocyte chemotactic responses observed with IL-8 and MCP-3 in combination with Regakine-1. The exact molecular mechanism for these synergistic actions of PF-4 and Regakine-1 with other cytokines or chemokines remains to be elucidated. Biologic functions other than chemotaxis are also ascribed to PF-4, including inhibition of tumor cell growth and endothelial cell proliferation.30,31Connective tissue–activating protein III, as well as the leukocyte-derived growth factor, both precursors of β-TG, were described to be mitogenic for connective tissue cells.32,33 However, it has not been evidenced that the biologic effects of these CXC chemokines mentioned above are mediated by G protein–coupled receptors. Indeed, it is rather speculated that PF-4 exerts its angiostatic activity by blocking fibroblast growth factor from binding to its receptor on endothelial cells.34,35 Furthermore, PF-4 induced firm adhesion of neutrophils to endothelial cells, which was dependent on specific adhesion molecules different from those involved in neutrophil-endothelium interactions in response to IL-8.36Finally, PF-4 has been reported to suppress colony formation of myeloid progenitors stimulated by granulocyte-macrophage colony-stimulating factor plus steel factor and to inhibit megakaryocytopoiesis.37 38
Post-translational modification of chemokines can enhance or reduce their chemotactic potency. For some CC chemokines, cleavage of the NH2-terminal dipeptide by the dipeptidyl peptidase IV/CD26 resulted in reduced receptor recognition and hence impaired chemotactic activity.39 In contrast, such processing of the macrophage inflammatory protein-1 isoform LD78β enhanced its CCR1 binding and monocyte chemotactic capacity.40 Similarly, most CXC chemokines, including β-TG, IL-8, ENA-78, and GRO, occur as NH2-terminally processed forms with increased in vitro and in vivo chemotactic activity.4,41 42 Further studies on post-translational processing of Regakine-1 might demonstrate the existence of more effective isoforms of this CC chemokine. Finally, current efforts to identify the human homolog of Regakine-1 might provide novel insights in its biochemical and biologic nature.
In conclusion, the identification of a novel CC chemokine with low sequence identity to any known chemokine highlights the paradox of apparent redundancy within the family of chemotactic cytokines. This phenomenon, resulting in overlapping activities, provides robustness to the chemokine network and guarantees effective immune reactions during host defense.3 An efficient and successful immune response to infection depends on endogenous and exogenous mediators that strictly regulate the production of individual chemokines by multiple cellular sources. Enhanced expression and activity of inflammatory chemokines is well controlled to prevent tissue damage. Therefore, these chemokines are only detected at high concentrations in the circulation during severe acute infections, eg, septic shock.43 In contrast to these inducible chemokines, chemokines that are constitutively produced at low levels probably fulfill homeostatic functions, eg, the regulation of leukocyte traffic under physiologic conditions.5 In this context, the constant high concentration of Regakine-1 and HCC-1 in the circulation seems to be an exception. It can at present only be speculated that Regakine-1 has a function in hematopoiesis, leukocyte homing, or angiogenesis, complex processes in which the role for chemokines has only recently been investigated.
The authors thank the members of the Laboratory of Clinical Immunology of the University of Leuven and Dr F. Shyselinck and the members of the Blood Transfusion Center of Leuven and Antwerp for providing blood samples and buffy coats. The editorial help of D. Brabants and I. Aerts, the technical assistance of R. Conings and W. Put, and the critical reading of this manuscript by Prof G. Opdenakker are greatly appreciated.
Supported by the Fund for Scientific Research of Belgium (FWO-Vlaanderen), the Concerted Research Actions of the Regional Government of Flanders, the InterUniversity Attraction Pole Initiative of the Belgian Federal Government, and the Biomed and Biotech Program of the European Community.
P.P. and S.S. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jo Van Damme, Rega Institute, Minderbroedersstraat 10, B-3000 Leuven, Belgium; e-mail:jozef.vandamme@rega.kuleuven.ac.be.