Abstract
K562 cells were stably transfected with cDNAs encoding the band 3 found in Southeast Asian ovalocytosis (B3SAO, deletion of residues 400-408), band 3 with a transport-inactivating E681Q point mutation (B3EQ), or normal band 3 (B3). Flow cytometric analysis and quantitative immunoblotting revealed that B3SAO expressed alone was translocated to the plasma membrane, at levels similar to B3 or B3EQ. Nine monoclonal antibodies that reacted with extracellular loops of B3 also reacted with B3SAO, although the affinity of most antibodies for the mutant protein was reduced. Both known Wrb epitopes were expressed on K562/B3SAO cells, demonstrating that B3SAO interacts with glycophorin A. The growth rates of K562 clones expressing equivalent amounts of B3 and B3EQ were the same, suggesting that the potentially toxic transport function of band 3 may be regulated in K562 cells. The band 3–mediated enhancement of Rh antigen reactivity and the depression of Rh epitopes on SAO erythrocytes were investigated by comparing the coexpression of B3, B3SAO, or B3EQ in K562 clones expressing exogenous RhcE or RhD polypeptides. The results are consistent with an interaction between band 3 and the Rh polypeptide–Rh glycoprotein (RhAG) complex, which may enhance translocation of the complex or affect its conformation in the plasma membrane. The data suggest that the interaction between band 3 and the RhD–RhAG complex is weaker than it is between band 3 and the RhCcEe–RhAG complex.
Introduction
The human red blood cell anion exchange protein (band 3, AE1) has a role in the movement of CO2 from the tissues to the lungs and in maintaining red cell (RBC) integrity.1-4 Recently, the heterologous expression of band 3 in mammalian cells has become available as a tool for studying the protein,5-8 and the ability to express mutants is particularly useful for understanding its structure and function. Southeast Asian ovalocytosis (SAO) is relatively common in areas where malaria is endemic because it provides protection against cerebral malaria in children.9 The cause of SAO is a heterozygous presence of a deletion of amino acids 400 to 408, inclusive, at the cytoplasmic boundary in the first membrane span of band 3 (B3SAO).10 The abnormal protein does not mediate chloride transport,11 and homozygosity for B3SAO appears to be lethal.12
SAO erythrocytes have been reported to show a selective depression of several RBC antigens.13,14 Quantitative experiments revealed that the binding of 3 radio-iodinated antiband 3 antibodies to SAO cells was depressed to 54% to 90% of normal levels.14 Since both normal band 3 (B3) and B3SAO are expressed in SAO erythrocytes, it was unclear whether the depressed band 3 antigen activity was caused by the loss of some epitopes in B3SAO (combined with preferential surface expression of band 3 from the normal AE1 allele) or by subtle structural differences between the normal and the mutant proteins in the plasma membrane. In addition to band 3 antigens, the Rh polypeptide (Rh30) antigens D, C, and e, as well as the Rh-associated antigens S/s (located on glycophorin B), U and LW are known to be depressed on SAO erythrocytes.13 These observations are of particular interest since the expression of band 3 in K562 erythroleukemia cells has revealed that band 3 enhances the cell surface reactivity of Rh antigens.5 K562 cells transduced with Rh polypeptide cDNAs and subsequently cotransfected with band 3 cDNA bound significantly more antibody directed against Rh polypeptides and Rh glycoprotein (RhAG) than cells transduced only with Rh polypeptide cDNAs. The antigen Wrb, which requires both band 3 and glycophorin A (GPA) for its expression,15 is also depressed on SAO erythrocytes. In addition to interacting during their translocation,16,17 band 3 and GPA are thought to associate in the plasma membrane, causing expression of the Wrb epitopes. Since binding of anti-Wrbantibody to SAO cells is reduced to 75% of normal levels,14 the SAO mutation may have a modifying effect on this interaction.
In this study, we used retroviral vector–mediated transfection to express B3SAO in the human K562 erythroleukemia cell line,18,19 which we previously used for functional cell surface expression of normal band 3 (B3).5 K562 cells express GPA but no endogenous band 3, and the exogenous expression of B3SAO simulated homozygosity for the SAO mutation in AE1,thus allowing studies that cannot be conducted in erythrocytes. We have investigated the cell surface movement and epitope expression of B3SAO. Since it was unclear whether the deficient transport activity of SAO erythrocytes indirectly contributes to their unusual antigenic properties, the chloride transport inactive band 3 E681Q mutant (B3EQ)20 21 was expressed as a control. K562 cells express endogenous RhAG but only low levels of Rh polypeptides. To gain information about the possible interactions between band 3 and the Rh complex, B3SAO and B3EQ were therefore coexpressed in K562/Rh cells previously transduced with Rh polypeptide cDNAs. We investigated whether the band 3–mediated increase in Rh antigen expression is dependent on band 3 transport activity and how the depression of Rh-related antigens on SAO erythrocytes could be explained. Finally, the expression of B3EQ in K562 cells was used to examine whether the potentially toxic transport activity of newly synthesized band 3 is likely to be regulated in K562 cells.
Materials and methods
Monoclonal antibodies and RBCs
Murine monoclonal antibodies to band 3 BRIC 6 (IgG-3), BRIC 71 (IgG-1), BRIC 170 (IgG-1), and BRIC 200 (IgG-1)14; BRIC 155 (IgG-2b) and BRIC 170 (IgG)22,23; rat monoclonal antibodies to band 3 BRAC 15 (IgG-2a), BRACs 17, 18, 21, and 23 (IgG-2b), and BRAC 25 (IgG-2a)14; murine monoclonal anti-GPA R18 (IgG-2b)24; murine monoclonal antibodies to WrbBRIC 14 (IgG-2a)25 and BRIC 201 (IgG-1)26; murine monoclonal anti-Rh polypeptide antibody BRIC 69 (IgG-1); and purified human anti-D, Brad 5, IgG-1 (50 μg/mL−1 diluted in phosphate-buffered saline–1% bovine serum albumin–0.1% NaN3)27 were available in house. Human anti-c, MS37 (IgG-3), was provided by Dr K. Thompson (IGRL, Oslo, Norway), murine monoclonal anti-RhAG, LA18.18 (IgG-1), was provided by Prof A.E.G Kr. von dem Borne (C.L.B., Amsterdam, The Netherlands), and murine monoclonal anti-E, H-4 1-G4 (IgG) was provided by Dr M. Uchikawa (Japanese Red Cross, Shibuya-ku, Japan). Fluorescein isothiocyanate–labeled F(ab′)2 fragments of goat antimouse IgG, antihuman IgG, and antirat IgG (Dako, Glostrup, Denmark) were used for flow cytometric analysis. Horseradish peroxidase–conjugated goat antimouse IgG (Biorad, Richmond, CA) was used for immunoblotting. Intact RBCs and ghosts of known phenotype were available in house.
Cloning of mutant band 3 cDNAs
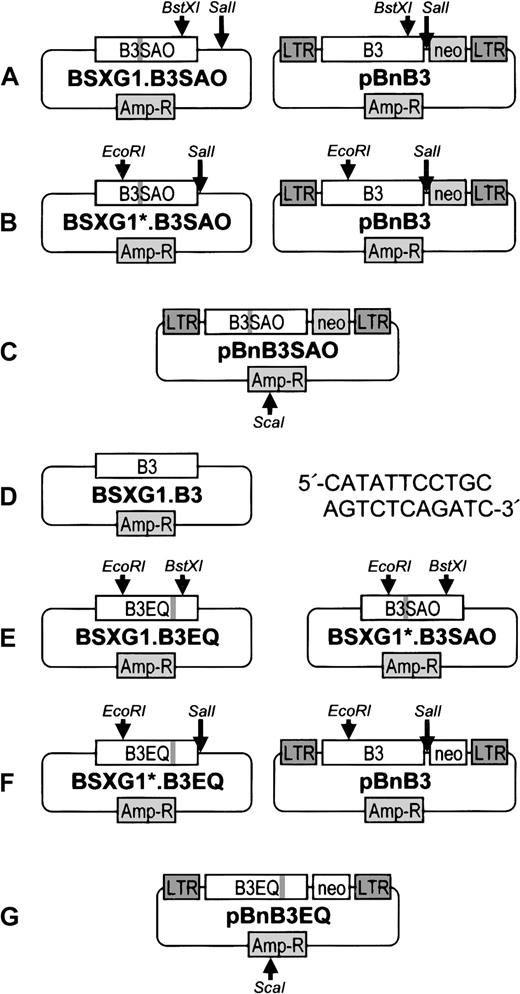
The pBabe puro (pBp) and pBabe neo (pBn) vectors28were provided by Dr H. Land (ICRF, London, United Kingdom). Band 3 Memphis I cDNA was cloned into pBp (yielding the construct pBpB3) and pBn (yielding the construct pBnB3), as previously described.5 Band 3 cDNA encoding both the Memphis I polymorphism and the SAO deletion and band 3 cDNA encoding both the Memphis I polymorphism and the E681Q point mutation were cloned into pBn, as shown in Figure 1. The resultant pBnB3SAO and pBnB3EQ constructs were analogous to the published pBnB3 and pBpB3 constructs, except that they contained the SAO deletion and the E681Q mutation, respectively.
Cloning of B3SAO and B3EQ into the pBabe neo vector.
Locations of the SAO deletion and the E681Q point mutation are indicated by a gray line in the band 3 cDNAs. For the cloning of B3SAO cDNA into pBabe neo, the 750-bp BstXI-SalI fragment of the previously described construct BSXG1.B3SAO11 was first substituted with the 412-bpBstXI-SalI fragment of pBnB3 (A), thus yielding the construct BSXG1*.B3SAO, which contained a SalI restriction site immediately downstream of the B3SAO cDNA in BSXG1. TheEcoRI-SalI fragment of BSXG1*.B3SAO that included 2066 bp of B3SAO cDNA (encoding the 9 amino acid SAO deletion) was then used to replace the EcoRI-SalI fragment of pBnB3 that included a 2093-bp fragment of B3 cDNA (B), thus generating the construct pBnB3SAO (C). Band 3 cDNA that comprised both the Memphis I polymorphism and the E681Q point mutation was cloned into pBn in 3 steps. The previously described BSXG1.B3 construct29 was used as template for site-directed mutagenesis (Sculptur kit; Amersham, Little Chalfont, United Kingdom), with the oligonucleotide 5′-CATATTCCTGCAGTCTCAGATC-3′ as primer (D). The correct mutant clone (designated BSXG1.B3EQ) was identified by PstI restriction digestion and DNA sequencing. When expressed in Xenopusoocytes, the band 3 protein (B3EQ) encoded by this clone did not mediate chloride transport (data not shown). The 1681-bpEcoRI-BstXI fragment of the B3EQ cDNA that spanned band 3 residues L217 to S773 was excised from the BSXG1.B3EQ construct and subcloned into the BSXG1*.B3SAO construct (E) to give BSXG1*.B3EQ. This construct was subjected toEcoRI-SalI restriction digestion, and the 2093-bp fragment that included the E681Q mutation was cloned into pBnB3 (F), thus generating the desired construct, pBnB3EQ (G). The band 3 coding region was verified in all constructs, using a 377 Applied Biosystems automated DNA sequencer. All vectors were linearized by using theScaI restriction site in pBabe prior to transfections.
Cloning of B3SAO and B3EQ into the pBabe neo vector.
Locations of the SAO deletion and the E681Q point mutation are indicated by a gray line in the band 3 cDNAs. For the cloning of B3SAO cDNA into pBabe neo, the 750-bp BstXI-SalI fragment of the previously described construct BSXG1.B3SAO11 was first substituted with the 412-bpBstXI-SalI fragment of pBnB3 (A), thus yielding the construct BSXG1*.B3SAO, which contained a SalI restriction site immediately downstream of the B3SAO cDNA in BSXG1. TheEcoRI-SalI fragment of BSXG1*.B3SAO that included 2066 bp of B3SAO cDNA (encoding the 9 amino acid SAO deletion) was then used to replace the EcoRI-SalI fragment of pBnB3 that included a 2093-bp fragment of B3 cDNA (B), thus generating the construct pBnB3SAO (C). Band 3 cDNA that comprised both the Memphis I polymorphism and the E681Q point mutation was cloned into pBn in 3 steps. The previously described BSXG1.B3 construct29 was used as template for site-directed mutagenesis (Sculptur kit; Amersham, Little Chalfont, United Kingdom), with the oligonucleotide 5′-CATATTCCTGCAGTCTCAGATC-3′ as primer (D). The correct mutant clone (designated BSXG1.B3EQ) was identified by PstI restriction digestion and DNA sequencing. When expressed in Xenopusoocytes, the band 3 protein (B3EQ) encoded by this clone did not mediate chloride transport (data not shown). The 1681-bpEcoRI-BstXI fragment of the B3EQ cDNA that spanned band 3 residues L217 to S773 was excised from the BSXG1.B3EQ construct and subcloned into the BSXG1*.B3SAO construct (E) to give BSXG1*.B3EQ. This construct was subjected toEcoRI-SalI restriction digestion, and the 2093-bp fragment that included the E681Q mutation was cloned into pBnB3 (F), thus generating the desired construct, pBnB3EQ (G). The band 3 coding region was verified in all constructs, using a 377 Applied Biosystems automated DNA sequencer. All vectors were linearized by using theScaI restriction site in pBabe prior to transfections.
Generation of K562 clones
Human erythroleukemic K562 cells (obtained from the European Collection of Animal Cell Cultures) were cultured and transfected by electroporation essentially as described,5 using the constructs pBpB3, pBnB3SAO, pBnB3EQ, or empty pBabe vector as control. Following the previously described selection procedure,5stably transfected clones from wells containing only a single discrete colony were expanded, and the clones expressing normal or mutant band 3 were identified by flow cytometry. Two K562/Rh clones, expressing exogenous RhD or RhcE polypeptides, were generated as previously described30 and transfected with the band 3 pBn constructs and empty pBn to yield stable K562/Rh+band 3 and K562/Rh+pBn clones.
Analysis of cell lines
The techniques used to examine K562 clones expressing normal and mutant band 3 proteins were essentially as described.5Flow cytometric analysis of clones was performed on a Becton Dickinson FACScalibur instrument, using the mean fluorescence intensity FL1 as a measure of antibody binding. For immunoblotting experiments, some of the cells were pretreated with 2 mg/mL chymotrypsin at 4°C. A time-course study showed that the fraction of band 3 cleaved under these conditions remained constant after 6 hours, so samples were treated for 7 hours to ensure complete cleavage. Untreated and chymotrypsin-treated cells were extracted using 1% Triton X-100 buffer. After separation on 8% or 10% Laemmeli gels and immunoblotting, extracts were quantified using a Sharp (Chiba City, Japan) high-resolution color scanner and Pharmacia LKB (Uppsala, Sweden) Imagemaster software. The mean cell volume and mean cell surface area of clones were calculated as previously described.5 To measure cell growth rates, clones were seeded at approximately 5 × 104 cells/mL in 12 mL medium, and viable cells were counted over 5 days in quadruplicate, using a hemocytometer.
Results
Flow cytometric analysis of K562/B3SAO and K562/B3EQ clones
Given that Groves et al11 were able to immunoprecipitate B3 but not B3SAO protein expressed inXenopus oocytes using BRIC 6, it was unclear whether this antibody would detect B3SAO expressed at the surface of K562 cells. A panel of monoclonal anti–band 3 reagents had previously been divided into 2 groups based on ability to bind to chymotrypsin-treated erythrocytes,14 and K562 clones transfected with pBnB3SAO or pBnB3EQ constructs were analyzed using one murine monoclonal antibody from each group, BRIC 6 and BRIC 71. A clone expressing normal band 3 (K562/B3) was used as the positive control, and a clone transfected with empty pBabe (K562/pBp) was used as the negative control. Transfection of K562 cells with empty vector did not affect the binding of monoclonal antibodies directed against various erythrocyte antigens (data not shown). The flow cytometric analysis identified several K562/B3SAO clones and K562/B3EQ clones that bound both BRIC 71 and BRIC 6 much more strongly than the negative control cells. Two histograms shown in Figure 2(top) illustrate the binding of BRIC 6 to a typical K562/B3SAO clone and a typical K562/B3EQ clone. K562/B3SAO cells and K562/B3EQ cells were then analyzed using the panel of anti–band 3 antibodies described by Smythe et al,14 which comprised 9 murine and rat monoclonal antibodies directed against extracellular loops of band 3. In addition, one murine monoclonal anti-GPA and 2 murine monoclonal anti-Wrb antibodies were tested. K562/pBp cells and K562/B3 cells were used as the negative and positive controls. Results for 2 typical K562/B3SAO clones and 2 typical K562/B3EQ clones are shown in Table 1. The analysis revealed that like the K562/B3 control cells, both the K562/B3EQ clones and the K562/B3SAO clones were positive with all the antibodies. The binding of the 2 anti-Wrb reagents to K562/B3SAO cells and K562/B3EQ cells demonstrated that the mutant band 3 proteins expressed in these cells were still able to interact with the endogenously expressed GPA. The binding of one anti-Wrb, BRIC 14, is illustrated in Figure2 (bottom).
B3SAO and B3EQ bind BRIC 6 and interact with GPA.
When K562 clones were screened for B3SAO and B3EQ expression, both types of cells reacted with the murine monoclonal antibody BRIC 6. This was unexpected for K562/B3SAO cells; previous workers reported that BRIC 6 precipitates B3 but not B3SAO from Xenopusoocytes11 and that the binding of BRIC 6 to heterozygous SAO erythrocytes is reduced to 54% of normal levels.14Our flow cytometric analysis also showed that K562/B3SAO cells and K562/B3EQ cells bound all other antibodies that reacted with K562/B3 cells. The expression of both known epitopes within the Wrbantigen on K562/B3SAO cells, detected by BRIC 14 and BRIC 201 binding, revealed that the abnormal B3SAO protein was still able to interact with GPA at the cell surface.
B3SAO and B3EQ bind BRIC 6 and interact with GPA.
When K562 clones were screened for B3SAO and B3EQ expression, both types of cells reacted with the murine monoclonal antibody BRIC 6. This was unexpected for K562/B3SAO cells; previous workers reported that BRIC 6 precipitates B3 but not B3SAO from Xenopusoocytes11 and that the binding of BRIC 6 to heterozygous SAO erythrocytes is reduced to 54% of normal levels.14Our flow cytometric analysis also showed that K562/B3SAO cells and K562/B3EQ cells bound all other antibodies that reacted with K562/B3 cells. The expression of both known epitopes within the Wrbantigen on K562/B3SAO cells, detected by BRIC 14 and BRIC 201 binding, revealed that the abnormal B3SAO protein was still able to interact with GPA at the cell surface.
Immunoblotting of mutant proteins
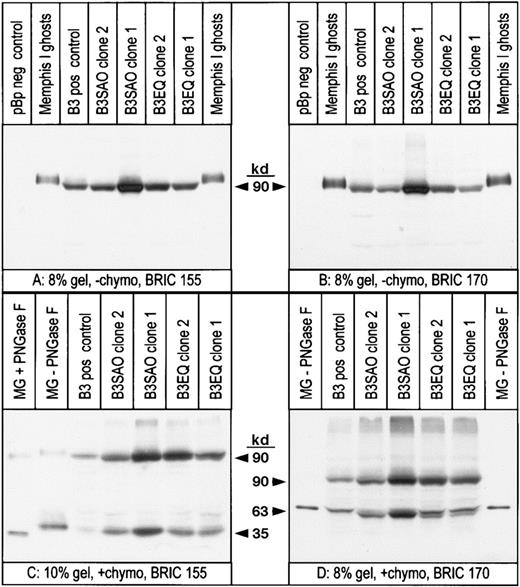
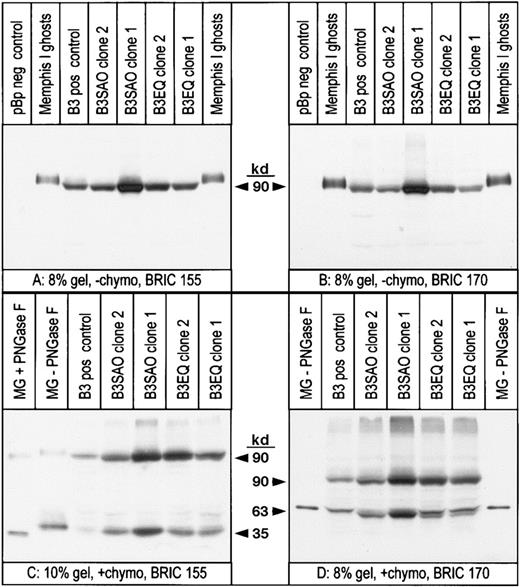
During flow cytometric analysis with anti–band 3 antibodies, it was noticed that the FL1 values obtained with the highest expressing K562/B3SAO clones were always lower than the FL1 values obtained with the highest expressing K562/B3 or K562/B3EQ clones (data not shown). This suggested that either B3SAO was not translocated to the plasma membrane as efficiently as B3, or that the number of antibody molecules bound to the cells per molecule of expressed band 3 was lower for B3SAO. Our flow cytometric results also suggested that K562/B3 cells and K562/B3SAO cells had different affinities for some of the antibodies tested. For example, Table 1 shows that cells of K562/B3SAO clone 1 and K562/B3EQ clone 1 gave almost identical FL1 values with BRIC 6 compared to the K562/B3 control clone. With BRAC 21, however, K562/B3SAO clone 2 cells and K562/B3EQ clone 2 cells gave similar FL1 values compared to K562/B3 cells, even though they did not bind the same amount of BRIC 6. To determine the actual amount of band 3 present at the cell surface of normal and mutant clones that showed similar antibody binding and to estimate the affinities of the different band 3 proteins for the various antibodies, quantitative immunoblotting was performed with antibodies previously shown to recognize linear epitopes. The monoclonal anti–band 3 antibodies BRIC 155 (recognizing an intracellular epitope near the C-terminus) and BRIC 170 (directed against an intracellular epitope toward the N-terminus) were used. The same cell populations used for flow cytometric analysis (in Table 1) were used to make Triton X-100 extracts shortly after the flow cytometric data had been collected. Some intact cells were pretreated with chymotrypsin, which cleaves band 3 Memphis I in the 4th extracellular loop to yield a 63-kd N-terminal fragment and a 35-kd C-terminal fragment.31,32 The immunoblotting results, shown in Figure 3, revealed that K562/B3, K562/B3SAO, and K562/B3EQ cells expressed a band 3 polypeptide of approximately 90 kd, whereas no corresponding band was detected for K562/pBp control cells (blots A and B). Band 3 proteins extracted from K562 cells migrated faster than erythrocyte band 3, and the 63-kd chymotrypsin fragment from some of the clones migrated as a double band (blot D). These differences in mobility were as previously observed5 and were probably caused by differences in the posttranslational modification of the expressed proteins. Cell surface fractions of the expressed band 3 were 41% for the K562/B3 control clone, 40% or 36% for the K562/B3SAO clones, and 35% or 33% for the K562/B3EQ clones (Table 2). The mean cell volume and mean cell surface area were determined for all clones before Triton extraction. These data and the scanning densitometry results were combined to calculate the density of band 3 molecules in the plasma membrane of each K562 clone, which was between 9.36-fold and 34.8-fold lower than the density of band 3 molecules in erythrocyte membranes (Table2).
Total protein expression and cell surface fractions are similar for B3, B3SAO, and B3EQ expressed in typical transfected K562 clones.
The same K562 cell samples expressing band 3 mutants that had been used for flow cytometric analysis (Table 1) were used for immunoblotting. The relative amount of band 3 expressed per packed cell volume was determined by scanning densitometry performed on 4 gels, 2 blotted with BRIC 155 (like blot A) and 2 blotted with BRIC 170 (like blot B). The surface fraction of the expressed band 3 proteins was determined by densitometric analysis of 3 gels blotted with BRIC 170 (like blot D) and the mean values of these results are shown in Table 2. Band 3 proteins expressed in K562 cells probably migrated faster than erythrocyte band 3 because of a difference in N-glycosylation. This is consistent with blot C, which shows that the 35-kd chymotrypsin fragment from K562 cells migrated faster than the glycosylated 35-kd fragment from RBCs but slower than the deglycosylated 35-kd chymotrypsin fragment from RBCs. The 63-kd chymotrypsin fragment from the 2 K562/B3EQ clones (and, to a lesser extent, the 2 K562/B3SAO clones) migrated as a double band probably because of a heterogenous posttranslational modification of the expressed protein. These unusual electrophoretic properties had previously been observed for several K562 clones expressing normal band 3.5 MG indicates Memphis I ghosts; −chymo, cells not pretreated with chymotrypsin; +chymo, cells pretreated with chymotrypsin; PNGase F, peptide-N4-(N-acetyl-β-glucosaminyl)-asparagine amidase F; pos, positive; neg, negative.
Total protein expression and cell surface fractions are similar for B3, B3SAO, and B3EQ expressed in typical transfected K562 clones.
The same K562 cell samples expressing band 3 mutants that had been used for flow cytometric analysis (Table 1) were used for immunoblotting. The relative amount of band 3 expressed per packed cell volume was determined by scanning densitometry performed on 4 gels, 2 blotted with BRIC 155 (like blot A) and 2 blotted with BRIC 170 (like blot B). The surface fraction of the expressed band 3 proteins was determined by densitometric analysis of 3 gels blotted with BRIC 170 (like blot D) and the mean values of these results are shown in Table 2. Band 3 proteins expressed in K562 cells probably migrated faster than erythrocyte band 3 because of a difference in N-glycosylation. This is consistent with blot C, which shows that the 35-kd chymotrypsin fragment from K562 cells migrated faster than the glycosylated 35-kd fragment from RBCs but slower than the deglycosylated 35-kd chymotrypsin fragment from RBCs. The 63-kd chymotrypsin fragment from the 2 K562/B3EQ clones (and, to a lesser extent, the 2 K562/B3SAO clones) migrated as a double band probably because of a heterogenous posttranslational modification of the expressed protein. These unusual electrophoretic properties had previously been observed for several K562 clones expressing normal band 3.5 MG indicates Memphis I ghosts; −chymo, cells not pretreated with chymotrypsin; +chymo, cells pretreated with chymotrypsin; PNGase F, peptide-N4-(N-acetyl-β-glucosaminyl)-asparagine amidase F; pos, positive; neg, negative.
Affinities of monoclonal antibodies
Using the flow cytometric and blotting results listed in Tables 1and 2, the binding affinities of the monoclonal antibodies reactive with exofacial loops were estimated for the different expressed band 3 proteins. Table 3 shows the relative fluorescence intensity (RFI) obtained per molecule of band 3 for the mutant clones, compared to K562/B3. This was calculated using the mean cell surface area of clones and the calculated relative density of band 3 molecules in the plasma membrane. The RFI value is arbitrary, and there is not necessarily a linear relationship (valid for a wide range of expression levels) between the number of binding sites on a cell and the fluorescence intensity obtained during flow cytometric analysis. However, all the clones used here had comparable expression levels, and the RFI value obtained per molecule of mutant band 3 gives an indication of the relative affinity of the mutated protein for each antibody. The calculated values show that B3EQ bound all the anti–band 3 reagents with similar affinity to B3, except for BRIC 6 and BRIC 71, which may have a slightly higher affinity for B3EQ. B3SAO, in contrast, had greatly reduced affinity for most antibodies, with RFI values between 41% and 56% of those obtained with B3. However, BRACs 17 and 21 gave only slightly reduced binding to B3SAO, with RFIs of 75% and 70%, respectively, of the values found for B3. These results are consistent with previous data obtained with erythrocytes, which showed that the binding of BRAC 17 to heterozygous SAO cells relative to normal RBCs was slightly reduced to 90%, whereas the binding of other antibodies was much more reduced.14The 2-fold increase in the binding of Wrb antibodies to K562/B3EQ cells compared to normal cells was probably a result of the 6-fold higher GPA expression per molecule of band 3 in the K562/B3EQ clones. The heterogeneity of K562 clones with regard to GPA expression has been described previously33,34 and was also apparent from the large differences obtained for anti-GPA binding to different K562/pBp and K562/band 3 clones (data not shown). We have shown that high Wrb expression on K562 cells is dependent on both high band 3 expression and high GPA expression.17 The 2 K562/B3SAO clones showed similar levels of GPA expression to the K562/B3EQ clones but did not bind anti-Wrb antibody more strongly than K562/B3 cells. This suggests that the affinity of the 2 anti-Wrb antibodies for GPA–B3SAO complexes may be lower than their affinity for GPA–B3 complexes or, alternatively, that GPA–B3SAO complexes are less readily formed or less stable than GPA–B3 complexes in the plasma membrane.
Coexpression of mutants in K562/Rh cells
Since untransfected K562 cells express only low levels of the Rh polypeptides,35,36 2 K562/Rh clones were used to investigate the depression of Rh-related epitopes on SAO erythrocytes and the band 3–mediated enhancement of Rh antigen reactivity on K562 cells. The K562/Rh clones were generated using retroviral transduction of K562 cells with pBabe puro Rh constructs, as previously described.30 One clone had been transduced withRHD cDNA and expressed high levels of D polypeptide (K562/D), whereas the other had been transduced with RHcEcDNA and expressed high levels of cE polypeptide (K562/cE). Each of the 2 clones was cotransfected by electroporation with pBnB3, pBnB3SAO, and pBnB3EQ constructs and empty pBabe neo vector. Single colonies were cultured and analyzed by flow cytometry. Several K562/D+B3, K562/D+B3SAO, K562/D+B3EQ, K562/cE+B3, K562/cE+B3SAO, and K562/cE+B3EQ clones were identified that expressed band 3, Rh polypeptides, and endogenous RhAG on the cell surface. For each type of cotransfectant, clones were selected that displayed varying levels of normal or mutant band 3 expression, ranging from low-level expression to the highest level of expression obtained. These clones were studied by flow cytometry using antibodies directed against band 3 and components of the Rh protein complex (Tables 4 and5). K562/D+band 3 clones were analyzed using BRIC 71 (anti–band 3), BRIC 69 (anti–Rh polypeptides), LA18.18 (anti-RhAG), and Brad 5 (anti-RhD). For negative controls, 3 K562/D+pBn clones (which showed almost identical antibody binding to the original K562/D cells) were used. Care was taken to examine all the clones on the same day and under the same conditions to obtain comparable data for clones cotransfected with different constructs. K562/cE+band 3 clones were analyzed in the same way, except that MS37 (anti-Rhc) and H4-1 G-4 (anti-RhE) were used instead of anti-RhD. For negative controls, 3 K562/cE+pBn clones (which showed almost identical antibody binding compared to original K562/cE cells) were used.
Effects of high-level B3 or B3EQ coexpression
The flow cytometric data listed in Tables 4 and 5 demonstrate that for both K562/D and K562/cE cells, the effects of coexpressing high levels of B3 (ie, FL1 for BRIC 71 being approximately equal to 200) could not be distinguished from the effects of coexpressing high levels of B3EQ. The consequences of high level coexpression of these proteins are illustrated in Figure4, which shows the histograms obtained for K562/D+B3EQ clone 1 (left column) and K562/cE+B3EQ clone 1 (right column), using K562/D+pBn clone 2 and K562/cE+pBn clone 2 as controls. The histograms illustrate that the effect of high level band 3 coexpression was the same in K562/D cells as in K562/cE cells. Both coexpressing clones gave FL1 values with BRIC 69 (recognizing Rh polypeptides), which were up to 4 times greater than those obtained with the K562/Rh+pBn control clones. Similarly, both clones gave FL1 values with LA18.18 (anti-RhAG) 3 to 5 times greater than those obtained with the control clones. The high level coexpression of B3 and B3EQ with Rh polypeptides also increased the binding of anti-RhD to K562/D cells and anti-Rhc and anti-RhE to K562/cE cells approximately 2-fold (Tables 4 and 5).
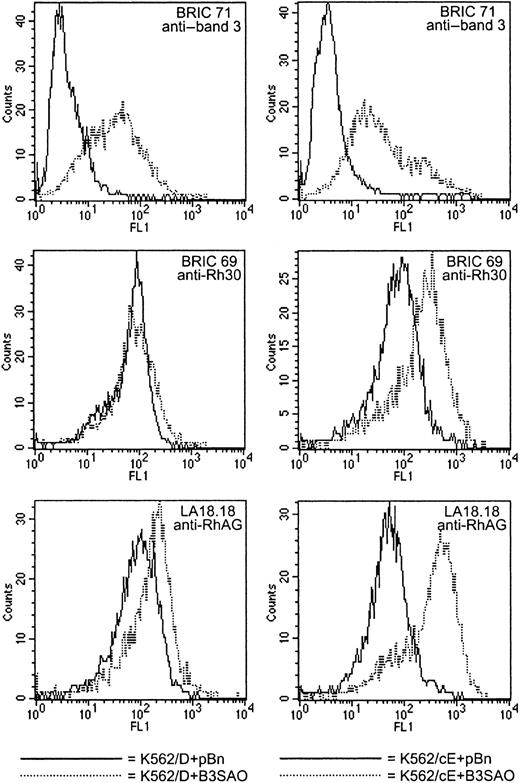
The effects of high-level band 3 coexpression are the same in K562/D and K562/cE cells.
The effects of coexpressing B3 and B3EQ in K562/Rh cells were indistinguishable, suggesting that the band 3–mediated enhancement of Rh antigen reactivity is due to the presence of the band 3 molecule itself rather than its activity. High-level coexpression was defined as the level of band 3 expression at which FL1 is approximately equal to 200 with BRIC 71. The histograms shown here illustrate the data listed in Table 4 for K562/D+B3EQ clone 1 (left column; using K562/D+pBn clone 2 as control) and in Table 5 for K562/cE+B3EQ clone 1 (right column; using K562/cE+pBn clone 2 as control). The effects of high-level band 3 coexpression were the same for K562/D and K562/cE cells. In all clones coexpressing high levels of B3 or B3EQ, the affinity of the cells was increased significantly for antibodies directed against Rh polypeptides (BRIC 69, 3- to 4-fold) and against RhAG (LA18.18, 3- to 5-fold).
The effects of high-level band 3 coexpression are the same in K562/D and K562/cE cells.
The effects of coexpressing B3 and B3EQ in K562/Rh cells were indistinguishable, suggesting that the band 3–mediated enhancement of Rh antigen reactivity is due to the presence of the band 3 molecule itself rather than its activity. High-level coexpression was defined as the level of band 3 expression at which FL1 is approximately equal to 200 with BRIC 71. The histograms shown here illustrate the data listed in Table 4 for K562/D+B3EQ clone 1 (left column; using K562/D+pBn clone 2 as control) and in Table 5 for K562/cE+B3EQ clone 1 (right column; using K562/cE+pBn clone 2 as control). The effects of high-level band 3 coexpression were the same for K562/D and K562/cE cells. In all clones coexpressing high levels of B3 or B3EQ, the affinity of the cells was increased significantly for antibodies directed against Rh polypeptides (BRIC 69, 3- to 4-fold) and against RhAG (LA18.18, 3- to 5-fold).
Effects of low-level B3 or B3EQ coexpression
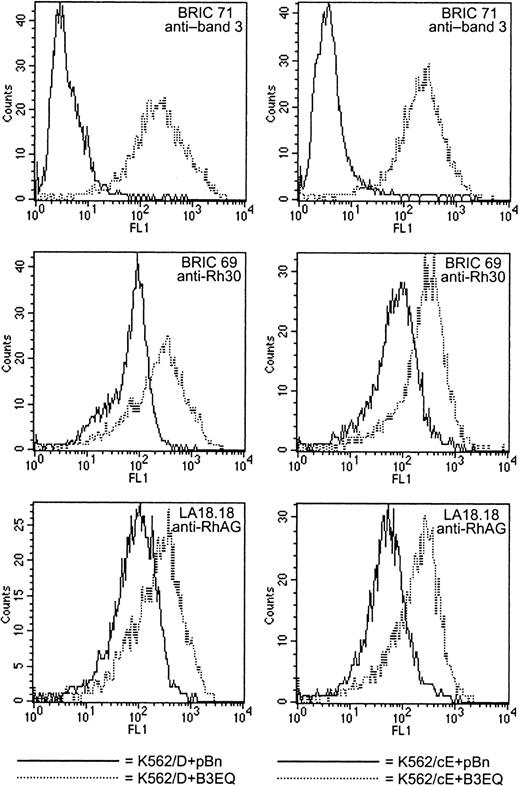
Low-level coexpression was defined as the expression level at which the FL1 value obtained with BRIC 71 was approximately 30, or 7 to 8 times higher than that recorded for the K562/Rh+pBn control clones. The data compiled in Tables 4 and 5 show that again there was no difference between the effects of B3 and B3EQ coexpression on the expression of Rh components. The consequences of coexpressing low levels of B3 or B3EQ with the Rh polypeptides are illustrated in Figure5, which shows the histograms obtained for K562/D+B3EQ clone 3 and K562/cE+B3EQ clone 4. These clones displayed similar levels of BRIC 71 binding, but the effect of the low-level presence of the band 3 protein was different in K562/D and K562/cE cells. In K562/D cells, there was only a very small increase or no increase in BRIC 69 binding and a 2-fold increase in LA18.18 binding. In K562/cE cells, however, coexpression of low levels of B3 or B3EQ was coupled to a 2- to 3-fold increase in BRIC 69 binding and a 3- to 5-fold increase in LA18.18 binding. The data listed in Table 5 for K562/cE+B3 clones 2, 3, and 4 and for K562/cE+B3EQ clones 2, 3, and 4 illustrate that there was not a strictly linear relationship between the level of band 3 expression and the increase in Rh antigen reactivity. However, even though the exact extent of the effect was unpredictable, K562/cE+band 3 cells coexpressing low levels of B3 or B3EQ always showed a marked increase in Rh antigen reactivity, whereas K562/D+band 3 cells did not. These observations were confirmed using several other clones that yielded results similar to those tabulated (data not shown).
K562/cE cells show a greater response to low-level band 3 coexpression than K562/D cells.
These histograms illustrate the data listed in Table 4 for K562/D+B3EQ clone 3 (left column) and in Table 5 for K562/cE+B3EQ clone 4 (right column). K562/D+pBn clone 2 and K562/cE+pBn clone 2 were used as negative controls. When low levels (ie, FL1 for BRIC 71 approximately equals 30, or 7 to 8 times higher than for K562/Rh+pBn clones) of B3 or B3EQ were coexpressed in K562/D cells, only a negligible increase in the binding of Rh polypeptide antibody and an approximately 1.5- to 2-fold increase in anti-RhAG binding were observed. However, when the same amount of band 3 was coexpressed in K562/cE cells, BRIC 69 binding was increased 2- to 3-fold, and LA18.18 binding was increased 3- to 5-fold.
K562/cE cells show a greater response to low-level band 3 coexpression than K562/D cells.
These histograms illustrate the data listed in Table 4 for K562/D+B3EQ clone 3 (left column) and in Table 5 for K562/cE+B3EQ clone 4 (right column). K562/D+pBn clone 2 and K562/cE+pBn clone 2 were used as negative controls. When low levels (ie, FL1 for BRIC 71 approximately equals 30, or 7 to 8 times higher than for K562/Rh+pBn clones) of B3 or B3EQ were coexpressed in K562/D cells, only a negligible increase in the binding of Rh polypeptide antibody and an approximately 1.5- to 2-fold increase in anti-RhAG binding were observed. However, when the same amount of band 3 was coexpressed in K562/cE cells, BRIC 69 binding was increased 2- to 3-fold, and LA18.18 binding was increased 3- to 5-fold.
Effects of B3SAO coexpression
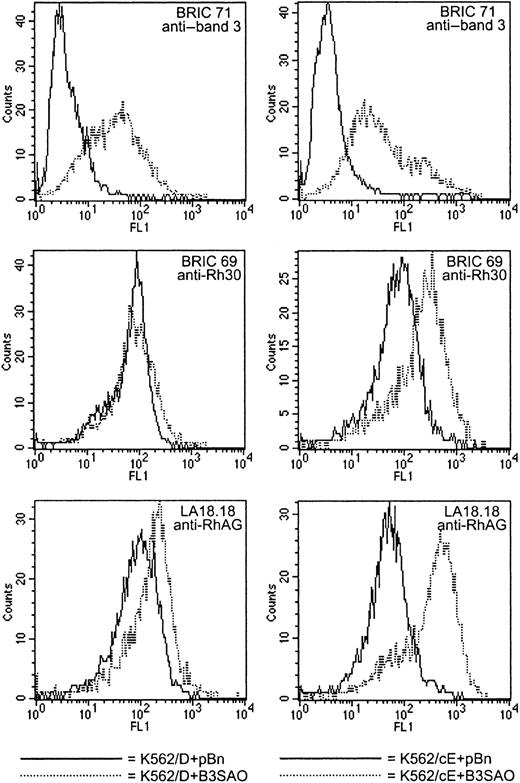
In considering the data shown in Tables 4 and 5, the fact that the fluorescence intensity with BRIC 71 for a K562/B3SAO clone is approximately 40% of the fluorescence intensity for a K562 clone expressing the same amount of normal band 3 at the cell surface (Table3) should be kept in mind. Therefore, the cell surface density of band 3 expressed in K562/D+B3SAO clone 1 (Table 4) is between the densities found in K562/D+B3 clones 1 and 2, and the cell surface density of band 3 in K562/cE+B3SAO clone 1 (Table 5) is similar to the band 3 densities in K562/cE+B3 clone 1 and K562/cE+B3EQ clone 1. The effects of coexpressing B3SAO in K562/Rh clones are illustrated in Figure6. In K562/cE cells, the effects of B3SAO coexpression were similar to the effects of low-level B3 or B3EQ coexpression (shown in Figure 5). There was always a marked (but quantitatively variable) increase in the binding of antibodies directed against Rh polypeptides (approximately 3-fold) and RhAG (approximately 4- to 5-fold). In K562/D cells, however, the coexpression of B3SAO did not produce a measurable enhancement of anti-Rh polypeptide binding, and the enhancement of anti-RhAG binding was even less (approximately 1.5-fold) than that resulting from low-level coexpression of B3. It also appeared that more B3SAO protein could be expressed in K562/cE cells than in K562/D cells (Tables 4 and 5).
Unlike K562/cE cells, K562/D cells show no response to B3SAO coexpression.
These histograms illustrate the data listed in Table 4 for K562/D+B3SAO clone 2 (left column) and in Table 5 for K562/cE+B3SAO clone 3 (right column), which showed similar amounts of B3SAO expression. It should be kept in mind that the actual amount of B3SAO expressed at the cell surface is significantly underestimated by the BRIC 71 binding data (Table 3, and “Results”). In the histograms shown here, K562/D+pBn clone 2 and K562/cE+pBn clone 2 were used as negative controls. Coexpression of B3SAO in K562/D cells did not increase their affinity for BRIC 69 and only slightly increased their affinity for LA18.18. In contrast, coexpression in K562/cE cells increased their affinity for BRIC 69 approximately 3-fold and their affinity for LA18.18 approximately 4- to 5-fold. The small increase in LA18.18 binding displayed by K562/D+B3SAO clones may have been due to interactions of endogenously expressed CcEe–RhAG complexes with B3SAO (see “Discussion”).
Unlike K562/cE cells, K562/D cells show no response to B3SAO coexpression.
These histograms illustrate the data listed in Table 4 for K562/D+B3SAO clone 2 (left column) and in Table 5 for K562/cE+B3SAO clone 3 (right column), which showed similar amounts of B3SAO expression. It should be kept in mind that the actual amount of B3SAO expressed at the cell surface is significantly underestimated by the BRIC 71 binding data (Table 3, and “Results”). In the histograms shown here, K562/D+pBn clone 2 and K562/cE+pBn clone 2 were used as negative controls. Coexpression of B3SAO in K562/D cells did not increase their affinity for BRIC 69 and only slightly increased their affinity for LA18.18. In contrast, coexpression in K562/cE cells increased their affinity for BRIC 69 approximately 3-fold and their affinity for LA18.18 approximately 4- to 5-fold. The small increase in LA18.18 binding displayed by K562/D+B3SAO clones may have been due to interactions of endogenously expressed CcEe–RhAG complexes with B3SAO (see “Discussion”).
Growth rates of K562/B3 and K562/B3EQ clones
Within the cell, unregulated anion transport activity of newly synthesized B3 could modify the pH and ion contents of intracellular compartments, thus impairing their function and slowing the growth of K562/B3 cells relative to K562/B3EQ or K562/B3SAO cells. To test whether the transport activity of newly synthesized B3 is regulated in K562 cells, we compared growth rates for K562/B3EQ clones and K562/B3 clones expressing comparable amounts of band 3 at the cell surface. For 5 K562/B3EQ clones (which gave FL1 values for BRIC 71 between 34.3 and 57.6) the doubling times were 28.2 to 34.5 hours, while for 8 K562/B3 clones (with FL1 values for BRIC 71 between 33.8 and 74.8), the doubling times were almost identical and ranged from 28.5 to 39.4 hours.
Discussion
The transfection of K562 cells with B3SAO cDNA has established stable cell lines that represent a model system for homozygous B3SAO expression. Only persons heterozygous for B3SAO are known, presumably because homozygosity for the mutation is lethal. We found that the B3SAO protein expressed on its own was translocated to the K562 cell plasma membrane and that the cell surface fraction was similar to that of B3 expressed in K562 cells (ie, slightly less than half the total protein). Flow cytometric analysis of K562 clones was used to clarify some questions regarding the structure and antigenic properties of B3SAO. First, our studies demonstrated that K562 clones expressing the mutant protein bound all the monoclonal antibodies to band 3 that reacted with K562 cells expressing B3. This indicated that all the extracellular epitopes detected by monoclonal antibodies are accessible to some extent in the mature B3SAO molecule, including the binding sites of some antibodies that were previously thought to be absent or inaccessible. However, quantitative immunoblotting studies revealed that the affinity of most antibodies for B3SAO was only 41% to 56% of that for normal band 3. In agreement with previous data,14the affinity for B3SAO was only slightly reduced for the rat monoclonal antibodies BRAC 17 and BRAC 21. The reduced affinity of monoclonal antibodies for B3SAO is probably not a result of the mutant protein having a stronger tendency than B3 to form high oligomers37,38 but is likely to be a reflection of subtle differences in folding between B3 and B3SAO. Our previous work with heterozygous SAO erythrocytes demonstrated a 46% reduction in BRIC 6 binding.14 This suggests that the abnormal folding of B3SAO may alter the tertiary structure of the normal band 3 in SAO erythrocyte membranes.
K562 cells expressing B3SAO also bound antibodies directed against both known Wrb epitopes, showing that the abnormal band 3 is still able to interact with GPA in the plasma membrane of K562 cells and emphasizing the important role of GPA in band 3 expression. However, K562/B3SAO cells bound less anti-Wrb antibody (approximately half) than might be expected when comparing the expression of band 3 and GPA with that in K562/B3 and K562/B3EQ cells. These findings are consistent with previous experiments14showing that the binding of anti-Wrb to heterozygous SAO erythrocytes was reduced to 75% of normal levels. GPA–B3SAO complexes may adopt a slightly different structure in the plasma membrane compared to GPA–B3 complexes, or, alternatively, complexes between GPA and B3 may be more readily formed than those between GPA and the mutant protein.
To identify the reasons for the depression of Rh antigens on SAO erythrocytes and to investigate the relationship between band 3 and Rh expression, a K562/D clone and a K562/cE clone were used in coexpression experiments. Both clones were cotransfected separately with B3, B3SAO, and B3EQ cDNAs, and the effect of band 3 expression on the expression of Rh epitopes was studied by flow cytometric analysis. The experiments revealed that the band 3–mediated increase in Rh-antigen reactivity was not dependent on band 3 function and, therefore, not due to swelling or other anion transport-induced changes in cell morphology. Instead, the effects of coexpressing B3 and coexpressing B3EQ were indistinguishable. B3EQ lacks chloride transport activity but differs from B3 by only a point mutation, indicating that it is the presence of the band 3 molecule itself rather than its activity that causes the enhancement of Rh-antigen reactivity.
Interestingly, the studies also demonstrated that the enhancement by band 3 was different for the RhD and RhcE polypeptides. When high levels of B3 or B3EQ were coexpressed, the binding of antibodies directed against the Rh complex was enhanced similarly in K562/D cells and in K562/cE cells. However, when low levels of B3 or B3EQ were coexpressed, the enhancement of antibody binding to K562/D cells was much less than the enhancement of antibody binding to K562/cE cells, in which low levels of band 3 sometimes produced as much enhancement as high levels of band 3 (Table 5, K562/cE+B3 clone 4 and K562/cE+B3EQ clone 4). Coexpression of B3SAO in K562/cE cells also gave a substantial increase in the reactivity of antibodies directed against Rh polypeptides and RhAG, but when B3SAO was coexpressed in K562/D cells, only a very small increase (RhAG) or no increase (Rh polypeptides) in the reactivity of these antibodies was observed.
We propose that an interaction occurs between band 3 and the Rh complex either during translocation of the proteins to the cell surface (and so enhancing the movement of Rh components to the surface) or in the plasma membrane (thereby changing the conformation of the complex and increasing the accessibility of antibody epitopes). Although it is unclear whether the direct interaction of band 3 occurs with the Rh polypeptides, with RhAG, or with a minor component of the Rh complex, we suggest that an interaction between band 3 and the Rh complex is the most likely mechanism for the band 3–mediated enhancement of Rh-antigen reactivity and for the depression of Rh epitopes on SAO erythrocytes. The greater response of K562/cE cells compared to K562/D cells to band 3 coexpression may be explained by an interaction that is stronger between band 3 and the Rh complex containing CcEe polypeptide than between band 3 and the Rh complex containing D polypeptide. Since the D polypeptide differs from the CcEe polypeptide by as many as 35 amino acids, the structure of the D–RhAG complex may be different from the CcEe–RhAG complex and may provide a less suitable interface for interaction with band 3. If the affinity of band 3 for D–RhAG complexes was lower, higher concentrations of band 3 would be required to enhance the expression of D–RhAG complex epitopes in K562 cells, as is observed. The lower affinity of band 3 for D–RhAG complexes, combined with structural changes present in B3SAO, may mean that the B3SAO molecule cannot interact with D–RhAG complexes. The small enhancement of anti-RhAG binding found for K562/D+B3SAO cells could be due to interactions of B3SAO with endogenous RhCcEe–RhAG complexes, which are present at low levels in K562 cells. Interestingly, Booth et al13 found that the binding of anti-D to SAO erythrocytes was reduced to 47% of normal levels, whereas the binding of anti-C was merely reduced to 80% (Table 6). This is consistent with an absence of interaction between B3SAO and D–RhAG complexes and a slightly weakened interaction existing between B3SAO and CcEe–RhAG complexes. Although the investigators found that binding of anti-e to SAO erythrocytes was on average reduced to 52% of normal levels, the c and E antigens are rare in Melanesians; hence, the C and e antigens are likely carried on the same polypeptide in most SAO cells. If the reduced reactivity with anti-C and anti-e reflects a reduction in Ce polypeptide numbers, this would be expected to be the same for both antibodies. Table 6 shows that the analysis of 9 blood samples of each type yielded a variation in anti-C binding of 12%, whereas only 6 samples of each type were analyzed with anti-e, giving a large 38% variation. Therefore, the depression of Ce polypeptide expression was most likely more accurately reflected by the anti-C binding data. For our study the monoclonal antibody BRIC 69 was available; it binds equally well to all forms of Rh polypeptides and may therefore be more suitable for estimating polypeptide numbers. Our results obtained with this antibody are consistent with the data Booth et al13 obtained using anti-C and anti-D. Furthermore, it is interesting to note that the highest expressing K562/cE+B3SAO clones isolated in our study bound more anti–band 3 antibody than the K562/D+B3SAO clones expressing the highest amounts of B3SAO. In our flow cytometric experiments, the most strongly expressing of 19 K562/cE+B3SAO clones studied gave a fluorescence intensity with BRIC 71 that was more than twice the value obtained with any of the 26 K562/D+B3SAO clones tested (data not shown). Although this result could be attributed to random differences in the copy number or site of integration of the B3SAO cDNA in the various K562 clones, it is also possible that its interaction with the cE–RhAG complex affected the translocation of B3SAO or its conformation in the plasma membrane. Whether the interactions between band 3 and the Rh complex are physiologically important remains to be determined. The function of the Rh complex is unknown, but the high frequency of persons lacking D polypeptide in some populations suggests that the CcEe polypeptide is more likely to be functionally important, and the preferential association of band 3 with the CcEe–RhAG complex may be of physiological significance.
We previously found that K562/B3 clones are stable for several weeks in culture but that they grow more slowly than untransfected K562 cells.5 It was unclear whether this was because of the strain imposed on the protein synthesis machinery of the transfected cells or because of the toxicity resulting from the transport activity of band 3 (as experiments with AEV-transformed chicken erythroblasts suggested39). In this study, we therefore compared the growth rates of K562 clones expressing B3 with those expressing B3EQ. There was no clear difference in the growth rates of K562 clones expressing comparable amounts of normal or mutant band 3, indicating that the slower growth of the transfected cells was not due to the transport function of band 3. It may be that band 3 transport activity is regulated in some way in erythroid cells, and our findings suggest that band 3 expression in K562 cells is a system in which the regulatory mechanism could be investigated.
We thank Dr G. K. Jones for preparing the BSXG1.B3EQ construct and for testing the construct in Xenopus oocytes. We also thank Dr L. J. Bruce for preparing erythrocyte ghosts, Dr H. Land for providing the pBabe vectors, and Prof A.E.G Kr. von dem Borne, Dr K. Thompson, and Dr M. Uchikawa for providing monoclonal antibodies.
Supported in part by the Wellcome Trust and by a University of Bristol Postgraduate Scholarship (R.B.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Michael J. A. Tanner, Department of Biochemistry, School of Medical Sciences, University Walk, University of Bristol, Bristol BS8 1TD, United Kingdom; e-mail:m.tanner@bristol.ac.uk.