Abstract
Lysophosphatidic acid (LPA) and sphingosine-1-phosphate (S1P) are agonists of the endothelial differentiation gene (Edg) family of G-protein–coupled receptors. LPA and S1P are generated by platelet activation during blood coagulation. Both lipids induce assembly of exogenous fibronectin (FN) by fibroblasts. This study examined whether LPA and S1P stimulate binding and assembly of fluoresceinated FN (FITC-FN) by adherent platelets. LPA enhanced deposition of FITC-FN into linear arrays overlying platelet surfaces and on edges of platelets adherent to FN or vitronectin (VN). Deposition was greater when platelets were adherent to FN than to VN and was elicited by platelet agonists with the following order of potency: thrombin > LPA = ADP (adenosine diphosphate) > S1P. The linear pattern of FITC-FN deposition was different from the more diffuse pattern of Alexa-fibrinogen (Alexa-FGN) binding to adherent platelets. FITC-FN was deposited by adherent platelets that had dense arrays of cytoskeletal actin when stained with rhodamine-phalloidin. The 70-kd N-terminal fragment of FN or L8 monoclonal antibody to a self-association domain of FN abolished deposition of FITC-FN but had no effect on binding of Alexa-FGN. Conversely, integrilin did not attenuate deposition of FITC-FN but abolished binding of Alexa-FGN. RGDS (Arg-Gly-Asp-Ser) or antibodies to α5β1 or αIIbβ3 integrins caused a partial decrease in LPA-induced deposition of FITC-FN. Correlative electron microscopy with anti-FITC coupled to gold beads revealed linear arrays on platelet surfaces associated with less than 20-nm–diameter filaments. These observations demonstrate that LPA, thrombin, ADP, and S1P induce adherent platelets to bind and assemble FN and suggest that platelets may contribute to early deposition of FN matrix after vascular injury.
Introduction
Fibronectin (FN) is a major cell-adhesion glycoprotein found in high concentrations in plasma and other body fluids and in an insoluble fibrillar form in the fibrin clot, connective tissues, and basement membranes.1,2 Cells assemble as well as adhere to FN matrix. FN assembly is a multistep process in which FN binds to the cell surfaces, followed by elongation and disulfide-stabilized multimerization of bound FN into insoluble fibrils.3,4 In vivo and in vitro studies have shown that insoluble tissue FN is derived from both endogenously synthesized cellular FN and circulating plasma FN.5,6 After incorporation into extracellular matrix (ECM), FN interacts with proteoglycans, collagen, and other ECM components and with cell-surface receptors.7-11 During blood coagulation, plasma FN is cross-linked by factor XIIIa into the fibrin clot, where it serves as an attachment site for cells.12-15
Assembly of FN by fibroblasts involves α5β1 integrin and molecules that interact with the 70-kd N-terminal gelatin- and heparin-binding regions of FN.16-21 Integrins are transmembrane αβ heterodimers that mediate cell adhesion to ECM components or counterreceptors on other cells.11 Ligation of integrins activates signal-transduction pathways.22-24 When α5β1 is absent, αIIbβ3, αVβ3, α3β1, or α4β1 can mediate FN matrix assembly.17,25-30 It is uncertain whether the roles of integrin in the assembly of FN involve activation of signaling pathways, tethering and clustering of to-be-assembled FN, transduction of tension to stretch-sensitive FN receptors, or some combination of these roles.4 31-33
Assembly of FN by fibroblasts and MG63 osteosarcoma cells is enhanced by lysophosphatidic acid (LPA) or sphingosine-1-phosphate (S1P).32,34-36 These lipids are produced when platelets are activated during blood coagulation and are agonists of the endothelial differentiation gene (Edg) family of G-protein–coupled receptors (GPCRs).37-41 As a result of platelet activation, the concentration of LPA in serum rises to 1 to 5 μM.37 S1P is present in plasma at a concentration of 0.3 μM and is released from platelets such that its concentration in serum is double that amount.39 Many effects of LPA and S1P have been described.41,42 Both are stimulators of platelet aggregation.43 44
An early study by Hynes et al45 demonstrated that when platelets in citrated platelet-rich plasma (PRP) attach to collagen substrate, attached platelets are surrounded by fibrils that stain for FN; that is, large, external, transformation-sensitive (LETS) protein. The conclusion drawn from the study was that the platelets by themselves do not have LETS protein, but rather recruit FN (ie, cold-insoluble globulin) from plasma coincidental with attachment to collagen. Subsequently, it was demonstrated that FN has an affinity for collagen,7 8 raising the possibility that the observed fibrillar staining represents binding to collagen.
In the present study, we examined the assembly of FN by adherent platelets in some detail. In particular, we tested whether LPA, S1P, or other platelet agonists stimulate binding and assembly of fluoresceinated FN (FITC-FN) by platelets adherent to FN or vitronectin (VN). Correlative video-enhanced differential interference contrast (VDIC) light microscopy, fluorescence microscopy, and electron microscopy were employed. Fluorescence microscopy showed that treatment with LPA, S1P, ADP, or thrombin enhances the binding and assembly of FITC-FN into discrete fibrils overlying platelet surfaces and at platelet–platelet contacts. Electron microscopy of the same platelets demonstrated linear arrangements of FN on the surface of platelets. LPA-induced enhancement of binding and assembly was abolished by concurrent treatment of platelets with the 70-kd FN fragment or L8 monoclonal antibody (mAb), which have been shown previously to block FN assembly by fibroblasts,20,46 and was attenuated by RGDS peptide or mAbs to α5β1 or αIIbβ3. In contrast, integrilin, an inhibitor of the fibrinogen (FGN) binding function of αIIbβ3,47 48 did not inhibit LPA-induced FN binding and assembly. These studies suggest that both FN assembly by activated adherent platelets and factor XIIIa–mediated crosslinking to the fibrin clot result in insolubilization of FN after wounding.
Materials and methods
Materials
LPA and S1P were from Avanti Polar Lipids (Birmingham, AL) and Alexis Biochemicals (San Diego, CA), respectively. ADP, RGDS, cytochalasin D, and fatty-acid–free (FA-free) bovine serum albumin were from Sigma Chemical (St Louis, MO). Human thrombin was a gift from Dr John Fenton (New York State Department of Health, Albany, NY). Thrombin receptor activating peptide (TRAP-6) was from Bachem (King of Prussia, PA). Rhodamine-phalloidin, FITC, fibrinogen conjugated to Alexa (Alexa-FGN), and rabbit antifluorescein antibodies were from Molecular Probes (Eugene, OR). Integrilin (eptifibatide) was a gift from COR Therapeutics (South San Francisco, CA). Goat anti–rabbit IgG and mAb 2253Z antibody to β1 were from Chemicon International (Temecula, CA). Anti-β1 mAb 13 was a gift from Dr Ken Yamada (National Institutes of Health, Bethesda, MD). 7E3 and 10E5 antibodies to β3 and αIIbβ3 were gifts from Dr Barry Coller (Mount Sinai Medical Center, New York, NY). Human plasma FN, the 70-kd N-terminal gelatin-binding fragment of FN, VN, and L8 anti-FN mAb have been described previously.20,35 46
Platelet preparation
Human blood from healthy adult volunteers was mixed with acid-citrate-dextrose as the anticoagulant.49 PRP was prepared by centrifugation of whole blood at 180g for 15 minutes at room temperature. Washed platelets were prepared from the PRP as described previously.50 The platelets were resuspended in HEPES Tyrode buffer (NaCl 136.5 mM, KCl 2.7 mM, MgCl2 · 6H2O 2 mM, NaH2PO4 · H2O 3.3 mM, HEPES salt 15 mM, HEPES acid 4.4 mM, and dextrose 5.5 mM) and supplemented with FA-free albumin, 1 g/L (HEPES-Tyrode/FA-free albumin). The suspensions were adjusted to 1.5 to 3 × 108/mL platelets and supplemented with 2 mM CaCl2 during the 1-hour incubation on adhesive substrates.
Preparation of FITC-pFN and colloidal-gold–antibody complexes
FITC-labeled plasma FN was prepared as described.20Spectroscopy measurements at 280 and 495 nm indicated that the labeled protein contained 2 to 3 FITC per subunit of FN. Colloidal-gold beads 20 nm in diameter (AU20) were coupled to polyclonal goat anti–rabbit IgG for localization of antifluorescein by previously published methods.51-53
Platelet adhesion and stimulation
Coverslips were coated overnight at 4°C with 10 μg/mL FN, VN, or collagen. Before the addition of platelets, the coverslips were rinsed 3 times with phosphate-buffered saline (PBS), pH 7.3. Washed platelets were incubated with coated coverslips for 15 minutes at 37°C. Adherent platelets were rinsed 3 times in HEPES-Tyrode/FA-free albumin. Samples were subsequently incubated in HEPES-Tyrode buffer, pH 7.3, with 20 mg/L (approximately 40 nmol/L) FITC-FN or 20 mg/L (approximately 60 nmol/L) ALEXA-FGN and 2 mM CaCl2 in the presence or absence of the various agonists, or agonists and inhibitors, for 10 to 60 minutes at 37°C. The samples were then rinsed with HEPES-Tyrode/FA-free albumin and fixed in 3% paraformaldehyde for 30 minutes at room temperature.
Fluorescence microscopy
For actin localization,54 55 paraformaldehyde-fixed platelets were permeabilized with 0.4% β-octylglucopyranoside in PHEM buffer (60 mM PIPES [piperazine-N,N′-bis (2-ethane sulfonic acid)], 25 mM HEPES, 10 mM EGTA, and 2 mM MgCl2), pH 6.9, for 2 minutes; rinsed 3 times in the same buffer; and incubated with 0.1 mg/L rhodamine-labeled phalloidin in PBS, pH 7.3, for 20 minutes at room temperature.
Coverslips were mounted with Vectashield mounting media (Vector Laboratories, Burlingame, CA) and sealed on the edges with nail polish. Samples were then viewed on an Olympus epifluorescence microscope. Care was taken to image a given fluorochrome at the same settings for all experimental permutations.
Correlative microscopy
Platelets adherent to polyvinyl formal–coated finder grids (Formvar, SPI, West Chester, PA) to which FN had been adsorbed were incubated in HEPES-Tyrode buffer, pH 7.3, with LPA in the presence of 20 mg/L FITC-FN and 2 mM CaCl2 for 1 hour. Samples were fixed in 3% paraformaldehyde for 20 minutes. The grids were placed on no. 1 coverslips (24 × 40 mm) in a drop of buffer and affixed with thin strips of double-sided tape overlying the edges of the grid. A single coverslip (18 × 18 mm, no. 1) was placed on top of the tape. The resultant chamber was open at 2 ends to allow introduction of buffer by capillary filling.56
Simultaneous images of both FITC-FN fluorescence via epifluorescence and platelet morphology via VDIC were obtained with a Nikon diaphot inverted microscope (Garden City, NY) connected to a cooled charged coupled device (CCD) (Photometrics PXL, Tucson, AZ) and Newvicon (DAGE-MTI, Michigan City, IN) cameras via a Nikon dual optical path tube. Analysis of fluorescence and differential interference contrast (DIC) images was performed using Metamorph Imaging Software (Universal Imaging, West Chester, PA).
Following examination by epifluorescence and VDIC, grids were washed 3 times with HEPES-Tyrode/FA-free albumin, incubated for 45 minutes with rabbit antifluorescein antibody (1:100 dilution), rinsed with buffer, incubated for 30 minutes at room temperature with goat anti–rabbit IgG antibody conjugated to 20-nm-diameter gold beads, rinsed with PBS (pH 7.3), and postfixed for 30 minutes with 1% glutaraldehyde in 0.1 M HEPES, pH 7.3, containing 0.5% tannic acid.57 Grids were then rinsed 3 times in 0.1 M HEPES buffer and treated for 15 minutes with 0.25% osmium tetroxide, 0.1 M HEPES, pH 7.3. Samples were stained with 1.0% uranyl acetate in water for 15 minutes, dehydrated in a graded series of ethanol (30% to 100%), and dried by the critical-point procedure with a Samdri apparatus (Touisimis, Rockville, MD).58 Samples were then carbon coated and examined by transmission electron microscopy (TEM) and by scanning electron microscopy (SEM) at 5 kV. Samples were subsequently platinum coated and re-examined by SEM at 1.5 kV. SEM was performed on a Hitachi (Rolling Meadows, IL) S900 low-voltage, high-resolution instrument.
Results
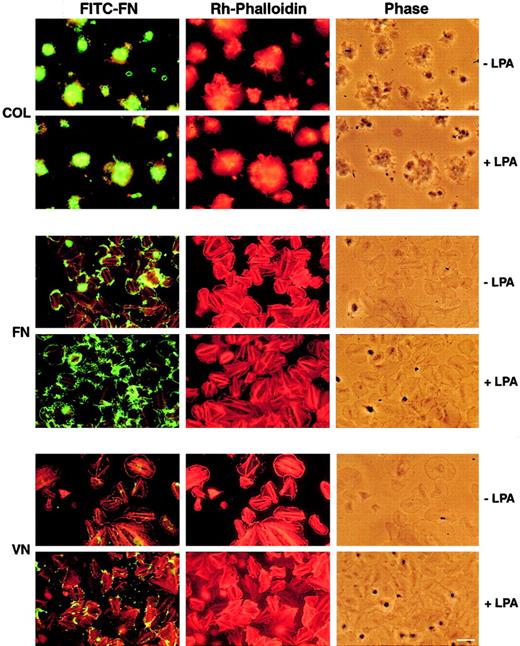
Hynes et al45 noted fibrils of FN associated with platelets after adherence of platelets to collagen from PRP. We noted deposition of FITC-labeled plasma FN by washed platelets adherent to collagen (Figure 1). However, in contrast to studies of platelets in PRP,45 washed platelets adherent to collagen surfaces were aggregated rather than spread, and the deposited FITC-FN was difficult to image by fluorescence microscopy because the aggregated platelets were in multiple focal planes. We hypothesized that the presence of plasma proteins accounted for the difference between platelets in PRP and washed platelets and therefore tested adhesive ligands for platelets that are present in plasma. These included FN, which induces adherence mediated by α5β1,59 and VN, which induces adhesion mediated by αvβ3 integrin.59 There was no aggregation of platelets and less deposition of FITC-FN on or around platelets adherent to FN or VN compared with platelets adherent to collagen (Figure 1).
Fluorescence microscopy of washed platelets adherent on collagen (COL)-, FN-, or VN-coated coverslips.
Platelets were placed on the coverslips for 15 minutes and then incubated with 20 mg/L FITC-FN, 2 mM Ca++ in HEPES-Tyrode buffer supplemented with 1 g/L FA-free albumin in the absence (−) or presence (+) of LPA, 1 μM. Platelets were washed and fixed in 3% paraformaldehyde in PBS, pH 7.3, for 30 minutes; permeabilized with 0.4% octylglucopyranoside in PHEM buffer for 2 minutes; incubated with 0.1 mg/L rhodamine-labeled phalloidin; and photographed for FITC-FN, rhodamine, and phase contrast. Comparison of the fluorescence associated with platelets on the 3 substrates shows the heaviest deposition of FITC-FN by platelet aggregates adherent on collagen without or with additional agonist. Deposition of FITC-FN by spread platelets adherent to FN or VN was enhanced by LPA. Bar = 10 μm.
Fluorescence microscopy of washed platelets adherent on collagen (COL)-, FN-, or VN-coated coverslips.
Platelets were placed on the coverslips for 15 minutes and then incubated with 20 mg/L FITC-FN, 2 mM Ca++ in HEPES-Tyrode buffer supplemented with 1 g/L FA-free albumin in the absence (−) or presence (+) of LPA, 1 μM. Platelets were washed and fixed in 3% paraformaldehyde in PBS, pH 7.3, for 30 minutes; permeabilized with 0.4% octylglucopyranoside in PHEM buffer for 2 minutes; incubated with 0.1 mg/L rhodamine-labeled phalloidin; and photographed for FITC-FN, rhodamine, and phase contrast. Comparison of the fluorescence associated with platelets on the 3 substrates shows the heaviest deposition of FITC-FN by platelet aggregates adherent on collagen without or with additional agonist. Deposition of FITC-FN by spread platelets adherent to FN or VN was enhanced by LPA. Bar = 10 μm.
Various platelet agonists were then examined for their ability to stimulate binding and assembly of FITC-FN by platelets adherent to FN- or VN-coated surfaces. Platelet agonists tested include LPA and S1P, agonists of Edg receptors41; ADP, an agonist of purinergic receptors60; and thrombin and TRAP-6, agonists of protease-activated receptors (PARs).61 62
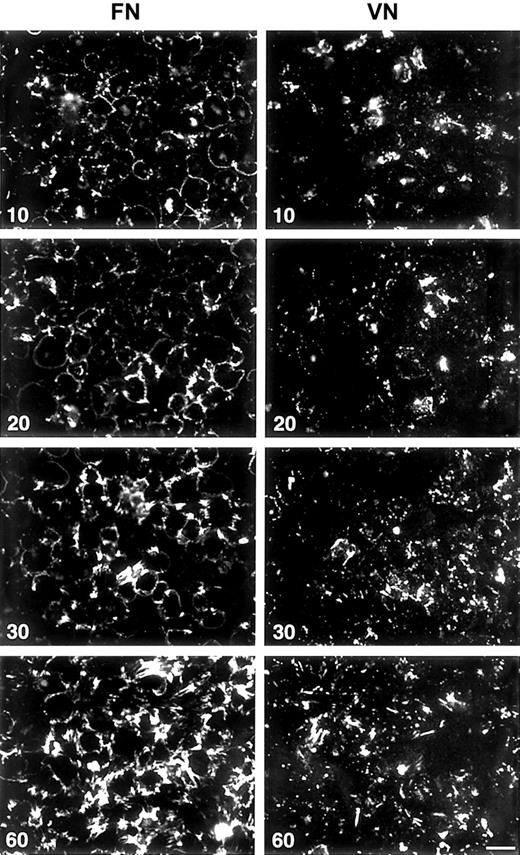
Fluorescence microscopy showed that LPA, 1.0 μM, enhanced binding and assembly of FITC-FN into discrete linear arrays, mostly at the edges of platelets and at platelet–platelet contacts (Figure 1). Dose-response studies indicated that LPA is active in the concentration range of 0.5 to 20 μM (not shown). Assembly of FITC-FN by LPA-stimulated platelets adherent on FN or VN surfaces occurred in a time-dependent manner (Figure 2). Binding of FITC-FN at all times was greater for platelets adherent to FN than for platelets adherent to VN. Initial binding to platelets adherent to FN was mostly at the periphery of the platelets. Initial binding to platelets adherent to VN was punctate. With time, FITC-FN became organized by platelets on both substrates into linear arrays.
Fluorescence microscopy of assembling FITC-FN on LPA-treated washed platelets, after a 15-minute adherence phase.
Platelets on FN-coated (upper panel) or VN-coated (lower panel) coverslips were stimulated with 1 μM LPA in the presence of FITC-FN, 20 mg/L, and fixed after an additional 10, 20, 30, and 60 minutes. Note the increase in number of fibrils and intensity of FITC-FN fluorescence with increased times. Phase contrast microscopy (not shown) revealed monolayers of platelets on both substrates at all time points. Bar = 10 μm.
Fluorescence microscopy of assembling FITC-FN on LPA-treated washed platelets, after a 15-minute adherence phase.
Platelets on FN-coated (upper panel) or VN-coated (lower panel) coverslips were stimulated with 1 μM LPA in the presence of FITC-FN, 20 mg/L, and fixed after an additional 10, 20, 30, and 60 minutes. Note the increase in number of fibrils and intensity of FITC-FN fluorescence with increased times. Phase contrast microscopy (not shown) revealed monolayers of platelets on both substrates at all time points. Bar = 10 μm.
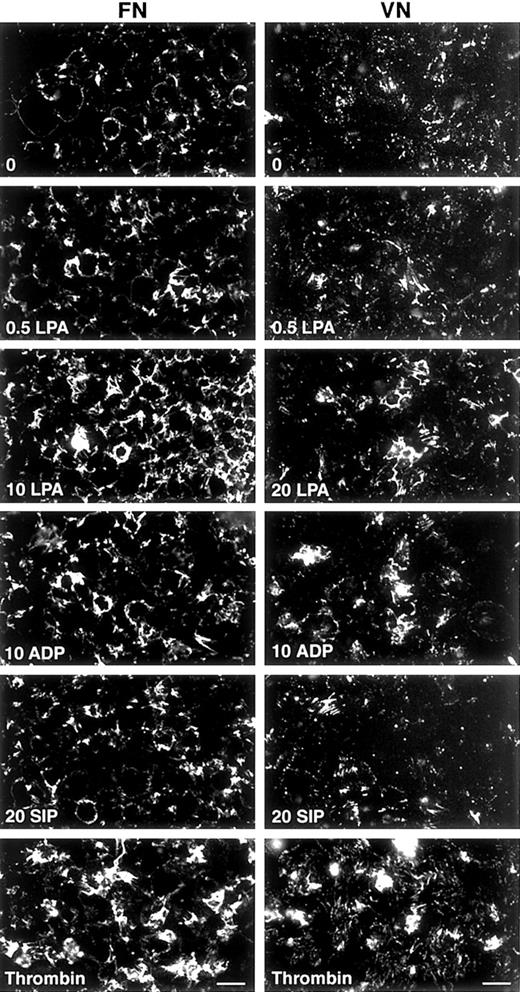
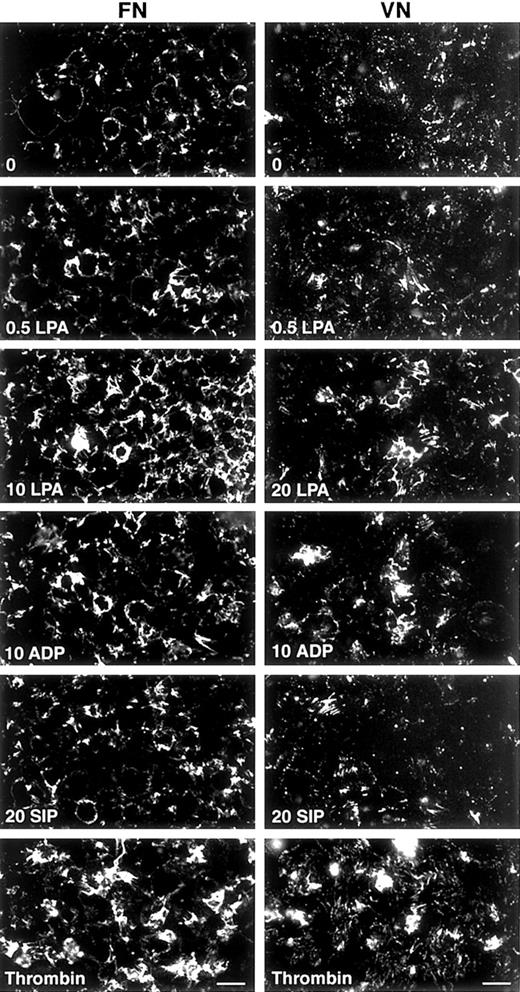
S1P (20 μM) was a weaker agonist when compared with LPA in enhancement of binding and assembly of FITC-FN by platelets (Figure3). ADP was slightly weaker or equivalent to LPA, whereas thrombin was a stronger agonist (Figure 3). TRAP-6, a peptide based on the sequence of the tethered ligand of cleaved PAR-1 thrombin receptor, also enhanced deposition of FITC-FN by adherent platelets (not shown).
Fluorescence microscopy of FITC-FN assembly in response to different agonists on FN- or VN-coated coverslips.
After a 15-minute adherence phase, coverslips were incubated for 1 hour with 20 mg/L FITC-FN in the presence of no agonist (0); 0.5, 10, or 20 μM LPA; 10 μM ADP; 20 μM S1P; or 1 U/mL thrombin. Phase contrast microscopy (not shown) revealed monolayers of platelets under all conditions. Bar = 10 μm.
Fluorescence microscopy of FITC-FN assembly in response to different agonists on FN- or VN-coated coverslips.
After a 15-minute adherence phase, coverslips were incubated for 1 hour with 20 mg/L FITC-FN in the presence of no agonist (0); 0.5, 10, or 20 μM LPA; 10 μM ADP; 20 μM S1P; or 1 U/mL thrombin. Phase contrast microscopy (not shown) revealed monolayers of platelets under all conditions. Bar = 10 μm.
The pattern of Alexa-FGN binding by platelets activated on FN- or VN-coated coverslips was examined in parallel to binding of FITC-FN. Alexa-FGN bound mainly to the central region of platelets adherent to FN (Figure 4). The pattern was similar in the absence of LPA (not shown). Alexa-FGN fluorescence was also located on platelet edges and on pseudopods (Figure 4). These results are in accord with previous studies of colloidal-gold–labeled FGN on spread platelets, which demonstrated redistribution of label from the periphery of fully spread platelets to membrane areas overlying the dense cytoskeletal network surrounding the granulomere/organelle region.63 The distributions of FITC-FN and Alexa-FGN on adherent platelets were clearly distinct in double fluorescence studies (data not shown).
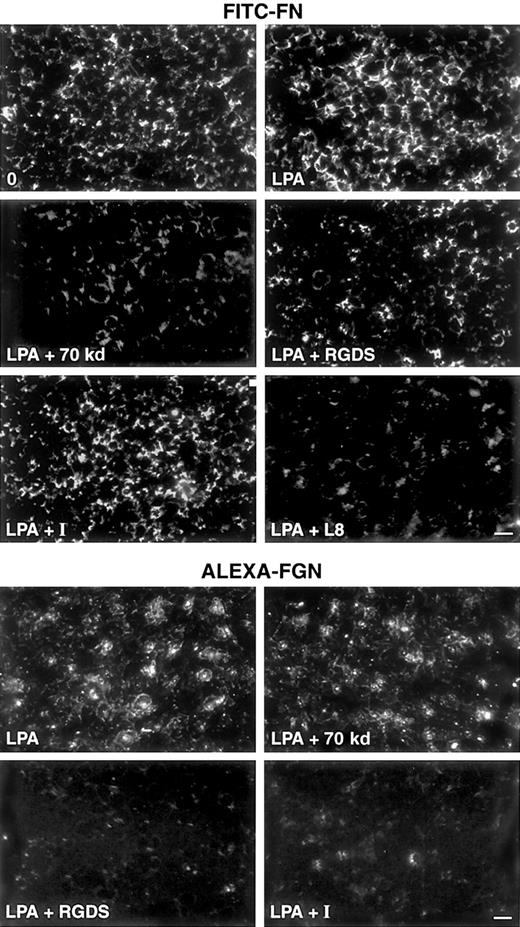
Effects of inhibitors on FITC-FN deposition and Alexa-FGN binding.
Shown are fluorescence micrographs of washed platelets adherent on FN-coated coverslips for 15 minutes and then incubated with FITC-FN or Alexa-FGN. Upper panel: platelets were incubated for 1 hour with 20 mg/L FITC-FN in the presence of no agonist and no inhibitor (0), 0.5 μM LPA, 0.5 μM LPA plus 30 mg/L 70-kd FN fragment, 0.5 μM LPA plus 0.5 mM RGDS, 0.5 μM LPA plus 0.5 μM integrilin (I), or 0.5 μM LPA plus 0.2 g/L L8 mAb. Lower panel: platelets were incubated for 1 hour with 20 mg/L Alexa-FGN and 0.5 μM LPA, 0.5 μM LPA plus 30 mg/L 70-kd FN fragment, 0.5 μM LPA plus 0.5 mM RGDS, or 0.5 μM LPA plus 0.2 μM integrilin (I). Phase contrast microscopy (not shown) revealed monolayers of platelets under all conditions. Bar = 10 μm.
Effects of inhibitors on FITC-FN deposition and Alexa-FGN binding.
Shown are fluorescence micrographs of washed platelets adherent on FN-coated coverslips for 15 minutes and then incubated with FITC-FN or Alexa-FGN. Upper panel: platelets were incubated for 1 hour with 20 mg/L FITC-FN in the presence of no agonist and no inhibitor (0), 0.5 μM LPA, 0.5 μM LPA plus 30 mg/L 70-kd FN fragment, 0.5 μM LPA plus 0.5 mM RGDS, 0.5 μM LPA plus 0.5 μM integrilin (I), or 0.5 μM LPA plus 0.2 g/L L8 mAb. Lower panel: platelets were incubated for 1 hour with 20 mg/L Alexa-FGN and 0.5 μM LPA, 0.5 μM LPA plus 30 mg/L 70-kd FN fragment, 0.5 μM LPA plus 0.5 mM RGDS, or 0.5 μM LPA plus 0.2 μM integrilin (I). Phase contrast microscopy (not shown) revealed monolayers of platelets under all conditions. Bar = 10 μm.
Deposition of FITC-FN and binding of Alexa-FGN were examined in the presence of potential modifiers, including 70-kd FN fragment (30 mg/L), integrilin (0.2 μM, 0.5 μM), RGDS (0.5 mM), L8 anti-FN mAb, or mAbs to α5β1 or αIIbβ3 integrin. L8 mAb and 70-kd FN fragment, which both block FN deposition by fibroblasts,20,46 attenuated LPA-induced deposition of FITC-FN but had no effect on Alexa-FGN binding to adherent platelets (Figure 4 and results not shown). Integrilin, an inhibitor of αIIbβ3,47 48 abolished Alexa-FGN binding but had no effect on FITC-FN deposition (Figure 4). Incubation of adherent platelets with RGDS in the presence of LPA and Alexa-FGN or FITC-FN resulted in abolishment of Alexa-FGN binding but only partial attenuation of LPA-induced FITC-FN deposition (Figure 4). Incubation with mAbs 13 or 2253Z to α5β1 partially attenuated the deposition of FITC-FN by LPA-stimulated platelets adherent on FN-coated coverslips (Figure 5). MAbs 7E3 to β3 or 10E5 to αIIbβ3 caused adherent platelets to detach from FN-coated substrate and also partially attenuated the deposition of FITC-FN (not shown).
Effect of anti-α5β1 mAbs on FITC-FN deposition.
Washed platelets, adherent on FN-coated coverslips for 15 minutes, were incubated with 20 mg/L FITC-FN for 1 hour in the presence of no agonists (0), 1 μM LPA, 1 μM LPA plus 10 mg/L mAb 13, or 1 μM LPA plus 10 mg/L mAb 2253Z. Bar = 10 μm.
Effect of anti-α5β1 mAbs on FITC-FN deposition.
Washed platelets, adherent on FN-coated coverslips for 15 minutes, were incubated with 20 mg/L FITC-FN for 1 hour in the presence of no agonists (0), 1 μM LPA, 1 μM LPA plus 10 mg/L mAb 13, or 1 μM LPA plus 10 mg/L mAb 2253Z. Bar = 10 μm.
High-voltage electron microscopy of Triton-extracted platelet whole mounts has demonstrated that platelet adherence and spreading are accompanied by specific reorganization of the cytoskeleton.64 Fully spread platelets have 4 distinct ultrastructural zones: a peripheral web of a densely packed meshwork of fine microfilaments, an outer filamentous zone of a loosely interwoven array of microfilaments, an inner filamentous zone of a densely packed network of anastomotic discrete filaments surrounding the granulomere region, and the granulomere region. Staining of platelets with rhodamine-phalloidin demonstrated the various zones in both unstimulated and LPA-stimulated platelets adherent to FN or VN (Figure1): an outer band of rhodamine-phalloidin–stained microfilaments (the terminal web), a loose network of microfilaments (the outer filamentous zone), and a dense polygonal microfilament network (the inner filamentous zone) that surrounds the core region containing granules (the granulomere region). An association between FITC-FN fluorescence and actin microfilaments at the terminal/peripheral web of spread platelets was apparent (Figure 1). Some platelets adherent to FN or VN had a prominent actin cytoskeleton but did not have FN deposition (Figure 1). Treatment of adherent platelets with cytochalasin D (5 μM), however, abolished both cytoskeletal organization and deposition of FITC-FN into linear arrays (result not shown).
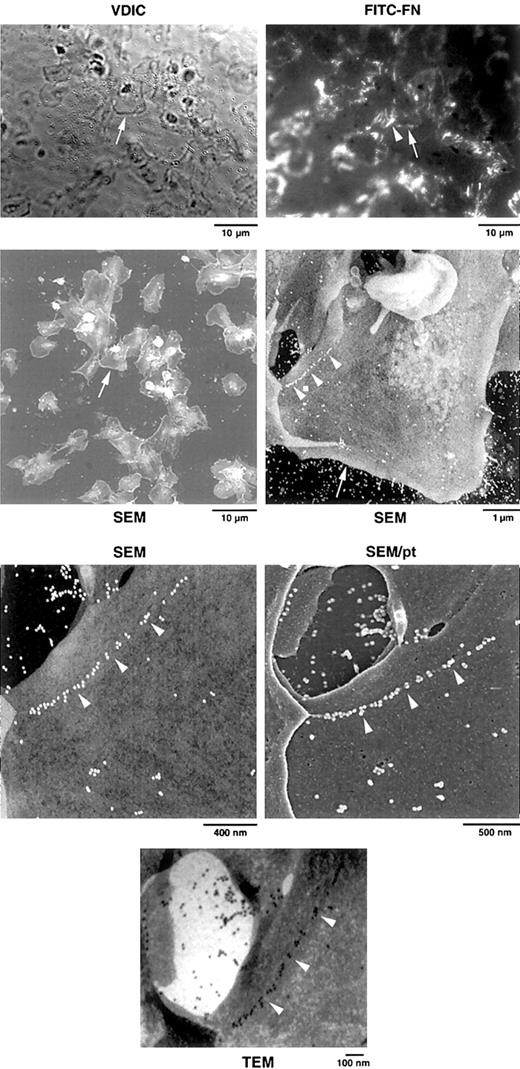
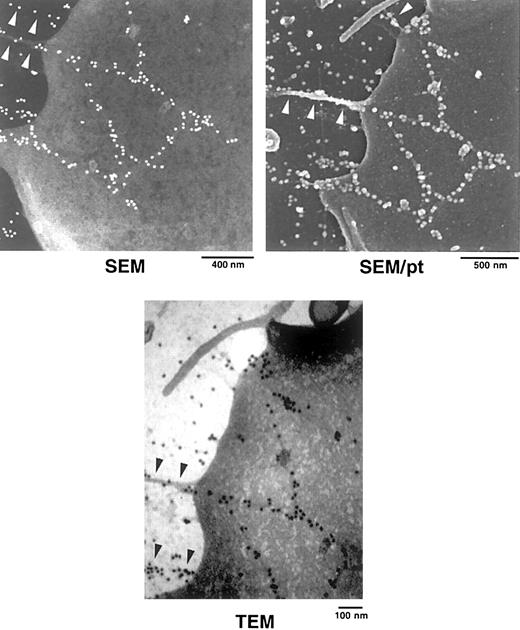
Correlative VDIC and fluorescence microscopy followed by TEM and SEM were used to confirm the presence of FITC-FN on platelet surfaces and to relate the localization to platelet ultrastructure (Figures6 and 7). Localization of FITC-FN was accomplished with anti–rabbit IgG conjugated to 20-nm gold beads. SEM and TEM of the same platelets revealed a linear string of gold labels that coincided with fluorescent signal for FITC-FN (Figure 6). Gold beads were arrayed linearly as singlets or small clusters (Figures 6 and 7). To determine whether the beads were on the surface, we analyzed carbon-coated samples at 5 kV, which penetrates into the platelets, and at 1.5 kV after platinum coating, which does not penetrate the surface. Gold beads were equally visible before and after platinum coating, indicating that FITC-FN was present on the surface of platelets. TEM and SEM demonstrated that the linearly arranged beads decorated fibrils that were less than the diameter of the bead (ie, 20 nm) (Figures 6and 7). These fibrils were in sparse networks on the platelet surface and seemed to stretch to the adjacent platelet (Figure 6) or to points where the platelet was attached to the substratum (Figure 7). As with LPA-treated fibroblasts,35 retraction processes were found in the vicinity of assembled FITC-FN (Figure 7).
Correlative light and electron microscopy of platelets adherent on an FN-coated grid.
After adherence to the grid for 15 minutes, washed platelets were rinsed and incubated for 1 hour with 1 μM LPA and 20 mg/L FITC-FN. Grids were analyzed by VDIC microscopy, fluorescence microscopy for localization of FITC-FN, and SEM and TEM for immunogold localization of FITC, as described in “Materials and methods.” SEM was done without and with coating of the sample with platinum (pt). Higher magnifications center on the platelet identified by the arrow. Note the linear arrangement of beads on the platelet surface (arrowheads) that correlates with FITC fluorescence (arrowhead). One linear array extends to a neighboring platelet. TEM demonstrates that beads decorate filaments of less than 20 nm in diameter.
Correlative light and electron microscopy of platelets adherent on an FN-coated grid.
After adherence to the grid for 15 minutes, washed platelets were rinsed and incubated for 1 hour with 1 μM LPA and 20 mg/L FITC-FN. Grids were analyzed by VDIC microscopy, fluorescence microscopy for localization of FITC-FN, and SEM and TEM for immunogold localization of FITC, as described in “Materials and methods.” SEM was done without and with coating of the sample with platinum (pt). Higher magnifications center on the platelet identified by the arrow. Note the linear arrangement of beads on the platelet surface (arrowheads) that correlates with FITC fluorescence (arrowhead). One linear array extends to a neighboring platelet. TEM demonstrates that beads decorate filaments of less than 20 nm in diameter.
SEM and TEM localization of FITC-FN on a platelet adherent on an FN-coated grid.
Samples were processed as in Figure 6. SEM without platinum coating demonstrates linear arrays of beads that extend from the platelet surface to the FN-coated substratum (arrowheads). TEM of the same platelet demonstrates beads decorating filaments (arrowheads) that are less than 20 nm in diameter. SEM after platinum (pt) coating demonstrates that one of the extensions is associated with a filopodial process (arrowheads).
SEM and TEM localization of FITC-FN on a platelet adherent on an FN-coated grid.
Samples were processed as in Figure 6. SEM without platinum coating demonstrates linear arrays of beads that extend from the platelet surface to the FN-coated substratum (arrowheads). TEM of the same platelet demonstrates beads decorating filaments (arrowheads) that are less than 20 nm in diameter. SEM after platinum (pt) coating demonstrates that one of the extensions is associated with a filopodial process (arrowheads).
Discussion
Stimulation of platelets adherent and spread on FN or VN substrate with LPA, ADP, thrombin, or, to a lesser extent, S1P resulted in enhancement of the binding and assembly of FITC-FN. FN deposition by adherent platelets was lost if cytochalasin D was added, showing that an intact actin cytoskeleton is important for FN matrix assembly. This is in accord with previous characterizations of FN deposition by fibroblasts and other cells.17,35 65
LPA and S1P have been reported to cause shape change and aggregation of platelets in suspension.40,43,44,66,67 The magnitude of the platelet response in suspension is greater with LPA than with S1P.43,66 Our results support these previous observations on suspended platelets because we observed greater response with LPA than with S1P in the assembly of FN by adherent platelets. LPA and S1P signal through separate subfamilies of Edg GPCRs.41 A recent study66 demonstrated that human platelets express mRNAs for Edg-2, Edg-4, and Edg-7 LPA receptors and Edg-6 S1P receptor.
ADP is an important agonist of platelet shape change and aggregation.60,68 ADP is stored in the platelet-dense granules and, as is the case with LPA and S1P, is released upon platelet activation.69 Two distinct platelet GPCRs for ADP have been identified: the Gi-linked P2Y12receptor that mediates inhibition of adenylyl cyclase, leading to aggregation, and the Gq-coupled P2Y1 receptor that mediates elevation of intracellular Ca++, shape change, and aggregation.60,68 In addition, ADP binds to the P2X1, ligand-gated ion channel–linked receptor that mediates rapid calcium entry.60
Thrombin is the most potent activator of platelet aggregation, working on PAR GPCRs.61 The interaction of thrombin with PAR-1 cleaves and exposes a new N-terminus that functions as a tethered peptide ligand and causes receptor activation.61,62 Synthetic peptides corresponding to the tethered ligand sequence, such as TRAP-6, also induce signals via PAR-1.61,62,70 Two other thrombin receptors with homologous tethered ligand sequences, PAR-3 and PAR-4, are expressed in platelets.71 Thus, the effects of thrombin or TRAP-6 on FN deposition, as with LPA and ADP, likely result from activation of more than one type of GPCR.
Deposition of FN by washed platelets incubated with a collagen substrate did not require an additional agonist and was mediated by aggregated rather than spread platelets. We hypothesize that the lack of the agonist requirement by platelets adhering to collagen is due to complexity of the signaling subsequent to the interaction of platelets with collagen. GPVI and α2β1 integrin are coreceptors for collagen, and GPVI is coupled to and signals through the FcRγ chain.72 73
Organization of soluble FN into ECM by fibroblasts involves initial reversible binding of the 70-kd N-terminal region of FN to specific cell-surface binding sites and subsequent insolubilization of dimeric FN molecules into fibrils.3,4,20,74,75 FN modules I-9 and III-1 recognized by the L8 mAb are thought to play an important role in FN–FN self-association.46,76 Inhibition by 70-kd FN fragment or L8 mAb of LPA-induced FITC-FN binding and assembly by adherent platelets indicates that deposition of FN by adherent platelets is mediated by the N-terminal region of FN and occurs by a mechanism similar to that of FN matrix assembly by fibroblasts.20,35 However, the activities of agonists that enhance FN assembly by adherent platelets are different from those that enhance assembly by fibroblasts. On fibroblasts, S1P is as potent as LPA,36 whereas S1P is less potent than LPA on platelets. TRAP-6, which is active on platelets, is inactive on fibroblasts.35 ADP also does not enhance assembly of FN by fibroblasts (B. R. Tomasini-Johansson, D. F. M., unpublished data, January 2001) but is active on platelets. The variations in responses of platelets and fibroblasts probably are due to differences in the repertoires of GPCRs for LPA, S1P, thrombin, and ADP and in downstream effector pathways.
Treatment with integrilin or RGDS abolished binding of Alexa-FGN to adherent platelets but caused little or no attenuation of FN deposition. RGDS blocks events mediated by αIIbβ3, αvβ3, or α5β1 integrins,77,78 whereas integrilin is specific for αIIbβ3.48,79,80 Binding of FN to thrombin-treated platelets in suspension is inhibited completely by RGDS and antibodies to αIIbβ3.81,82 The distinctive inhibitor profiles suggest that assembly of FN by adherent platelets is different from binding of FN to suspended platelets and is not mediated solely by αIIbβ3. There are 2 other differences in the binding of FN to suspended platelets versus assembly by adherent platelets. FN binds to suspended platelets stimulated by thrombin but not to suspended platelets stimulated by ADP,83 whereas both agonists stimulate deposition of FN by adherent platelets. Specific and saturable binding of FN to thrombin-stimulated suspended platelets is half maximal at an FN concentration of approximately 300 nmol/L,83,84 8-fold higher than the concentration of 40 nmol/L used in the present experiments. α5β1 has been implicated in FN fibrillogenesis by fibroblasts.16-19,21,26 The finding that deposition of FITC-FN by adherent platelets was greater on a substrate of FN than on a substrate of VN and was partially attenuated by the presence of anti-β1 mAbs indicates that α5β1 is also functional in the assembly of FN by adherent platelets. In the absence of α5β1, αIIbβ3, αVβ3, α3β1, or α4β1 can mediate FN matrix assembly by fibroblasts.17,25-30 Antibodies to αIIbβ3 and β3 caused platelets to deposit less FN and to detach from FN-coated substratum. These results suggest that αIIbβ3 and possibly αVβ3 are accessories in the deposition process by platelets, in the same way that αVβ3 functions in deposition by fibroblasts.21 It is uncertain whether the roles of integrin in the assembly of FN by fibroblasts involve appropriate activation of signaling pathways, tethering and clustering of to-be-assembled FN, transduction of tension to stretch-sensitive FN receptors or already assembled endogenous FN, or some combination of these roles.4,31-33 Platelets contain endogenous FN in α-granules that is secreted upon activation.69 85Therefore, the ambiguities surrounding FN assembly by fibroblasts are also true for adherent platelets.
Correlative electron microscopy demonstrated linear arrangement of FN on the surfaces of platelets, at platelet–platelet contacts, and associated with the peripheral web of spread platelets. Other linear arrangements of FN extended beyond the edge of platelets to the nearby substratum. Previous studies35,86 have demonstrated linear FN patches on the surface of fibroblasts. It was postulated that the linear patches may represent “nucleation sites” for fibril formation and serve to align FN into fibrils.86 Linear arrays of FN at fibroblast-substratum “matrix contacts” have been shown to be associated with cytoplasmic tensin.18 We did not determine critically whether cytoskeletal structures underlie the FN fibrils associated with adherent platelets. Further studies of whole-mount platelets using differential extraction, higher-voltage TEM, and immunostaining of cytoskeletal proteins are needed to determine whether the mechanisms of FN deposition at “matrix contacts” of fibroblasts and on the dorsal surface of adherent platelets are the same or different.
A number of functional responses of suspended platelets to various agonists—shape change, FGN-dependent aggregation, secretion of granular contents, and contraction of fibrin clots—are well characterized and of obvious importance in platelet physiology.64,87,88 Our experiments indicate that agonists generated during blood coagulation stimulate adherent platelets to deposit a FN matrix and that such deposition is modulated by the nature of the adhesive substrate. We speculate that such an FN matrix may overlap functionally with FN incorporated into fibrin clots to provide a substrate for cellular ingrowth during the repair process. Platelets in mice lacking both von Willebrand factor and FGN have unexpectedly normal hemostatic function.89 Such platelets specifically accumulate extra FN in platelet α-granules, and it has been suggested that this FN substitutes for the adhesive and matrix-building functions of von Willebrand factor and FGN.89 Further, deposition of platelet FN has been suggested to account, at least partially, for the surprising finding that mice lacking plasma FN have normal wound healing.90
Supported by National Institutes of Health grant HL21644.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Deane F. Mosher, Department of Medicine, University of Wisconsin, 4285 MSC, 1300 University Ave, Madison, WI 53706; e-mail: dfmosher@facstaff.wisc.edu.