Abstract
The small molecule receptor tyrosine kinase (RTK) inhibitor SU5416 targets the vascular endothelial growth factor receptor 2 and the stem cell factor receptor c-kit. Herein is described the successful treatment of a 65-year-old woman with SU5416, in second relapse of acute myeloid leukemia (AML) and refractory toward standard chemotherapy regimens. After 12 weeks of treatment with SU5416, the blast cell counts (blood and bone marrow) decreased to undetectable levels and the peripheral blood cell counts normalized with the exception of the platelet count (50-80 × 109/L [50-80 × 103/μL]). The duration of the remission is longer than 4 months during maintenance therapy with SU5416. Microvessel density in the patient's bone marrow dropped from 33.4 to 12.3 microvessels/×500-field 8 weeks after SU5416 administration and remains in the normal range. This is the first report of a stable remission achieved after administration of the RTK inhibitor SU5416 in a patient with AML relapse.
Introduction
Angiogenesis—a complex process of pericellular proteolysis, endothelial migration, and proliferation—is an absolute requirement for the viability and growth of solid tumors.1Recently, we reported increased angiogenesis in the bone marrow of patients with acute myeloid leukemia (AML) and normalization of bone marrow microvessel density when patients achieved a complete remission (CR).2 This finding suggests an involvement of angiogenesis in the pathophysiology of AML as well.
Vascular endothelial growth factor (VEGF), which exerts proliferative and migratory effects on endothelium, and its cellular receptor VEGFR-2 (KDR, human homologue; Flk-1, murine homologue) have been implicated as the key endothelial pathways required for tumor neovascularization.3 VEGFR-2 was thought to be exclusively expressed by adult endothelial cells. However, it has recently been shown that VEGF and VEGFR-2 are expressed by leukemic blast cells, suggesting an autocrine loop that supports the proliferation and survival of leukemic cells.4-7 Moreover, increased cellular VEGF has been shown to be an independent poor prognostic factor in patients with AML.8 Therefore, the receptor tyrosine kinase (RTK) VEGFR-2 might be a promising target for antiangiogenic and antileukemic treatment strategies.
SU5416 is a small-molecule RTK inhibitor of VEGFR-2 and the stem cell factor (SCF) receptor c-kit. It has been demonstrated that SU5416 inhibits the growth of colon cancer liver metastasis by inducing tumor and endothelial cell apoptosis.9 Furthermore, SU5416 causes apoptosis in a c-kit–positive human myeloid leukemia cell line (MO7E) in a dose-dependent manner.10
In the current study, we report a stable remission after SU5416 administration in a patient with a second AML relapse refractory toward standard chemotherapy regimens. Additionally, we provide evidence that SU5416 exhibits antiangiogenic and antileukemic properties in AML.
Study design
Case report
In November 1997, AML was diagnosed in a 62-year-old woman. Diagnosis and classification according to the criteria of the French-American-British (FAB) Cooperative Group11 revealed the subtype M4 and was confirmed by centralized review of bone marrow morphology, cytochemistry, and immunophenotyping within the AML Cooperative Group (AMLCG).12 Cytogenetic analyses showed a deletion of the long arm of chromosome 9 in 5 of 23 metaphases. The patient achieved CR with standard induction chemotherapy and received consolidation and maintenance chemotherapy according to the AMLCG-protocol.12
The first relapse (March 1999) was treated with sequential high-dose cytosine arabinoside (Ara-C; 2000 mg/m2 per day on days 1, 2, 8, 9) and idarubicin (10 mg/m2 per day on days 3, 4, 10, 11) followed by a second CR that lasted 8 months. For the second relapse (March 2000) the patient received mitoxantrone (10 mg/m2, days 1-5) and etoposide (100 mg/m2, days 1-5) without inducing CR. Subsequently, thalidomide was administered within a phase I/II dose-escalating clinical study without any response (final dose: 200 mg/d for 2 months). The deteriorating clinical condition of the patient (bilateral pneumonia with severe hypoxemia, pleural and pericardial effusions) did not allow further chemotherapeutic trials. Because immunophenotyping by flow cytometry and immunohistochemistry revealed strong expression of c-kit (> 80% of leukemic blasts) and VEGFR-2, we decided to administer the RTK inhibitor SU5416 (145 mg/m2 intravenously [iv], twice a week) under compassionate use after written informed consent of the patient and approval of the local institutional review board in accordance with the Helsinki protocol. In order to prevent hypersensitivity reactions related to the drug or to excipients in the formulation, such as polyoxyethylated castor oil (Cremophor), polyethylene glycol or absolute ethanol, the patient received prophylaxis with histamine antagonists (2 mg clemastin iv and 400 mg cimetidine iv) plus 10 mg dexamethasone iv prior to each infusion of SU5416. The dose of dexamethasone was reduced to 4 mg at the fourth and to 2 mg at the subsequent infusions. The safety of SU5416 was assessed through physical examinations, vital signs, toxicity assessments, electrocardiography, and laboratory tests (hematology, coagulation, and clinical chemistry). In July 2000, treatment with SU5416 was started. Subsequently, a continuous decrease of the blast infiltration in the bone marrow and an increase of the peripheral blood cell counts were observed. The patient completely recovered from pneumonia and the pleural/pericardial effusions resolved.
Six weeks after initiation of SU5416 treatment, the patient developed symptomatic leukemic meningitis with right facial nerve palsy and 1245/3 leukemic blast cells in the cerebrospinal fluid (CSF). This was the first time that the patient had evidence of central nervous system involvement. SU5416 was continued and additional treatment with intrathecal chemotherapy (40 mg Ara-C, 15 mg methotrexate, 4 mg dexamethasone; twice a week) was started for 2 weeks followed by cranial irradiation (whole-brain dose of 30 Gy administered in 3 weeks). Subsequently, the patient received a second course of intrathecal chemotherapy applying the same dose and schedule for 3 weeks, thus achieving a complete resolution of the neurologic deficit and absence of leukemic blasts in repeated CSF specimens. Three months after the initiation of treatment with SU5416, the leukemic blast infiltration dropped to undetectable levels as determined by bone marrow morphology and immunophenotyping by flow cytometry with almost complete normalization of the peripheral blood cell counts.
Methods
Cytochemical assays, flow cytometry studies, and cytogenetic analyses were performed on bone marrow aspirates using standard methodology. The determination of the degree of angiogenesis was performed by immunohistochemic identification of microvascular endothelial cells with antihuman thrombomodulin antibodies as described.2 The median and interquartile range for the microvessel densities of 22 control patients was 13.2 and 11.4 to 14.8/×500-field, respectively. This corresponds to the average number of microvessels counted in a 0.126 mm2 field area at ×500 magnification using light microscopy.2 Immunostaining for VEGFR-2 was done in paraffin-embedded bone marrow specimens with a specific monoclonal antibody (anti–Flk-1, Santa Cruz Biotechnology, Santa Cruz, CA, sc-6251) using the alkaline phosphatase/anti–alkaline phosphatase double-bridge technique (Dako-APAAP Kit; Dako, Glostrup, Denmark).
Results and discussion
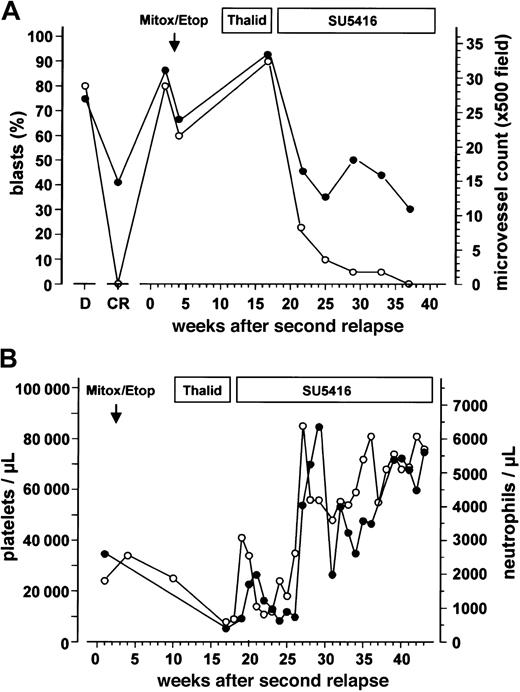
After proving to be refractory toward chemotherapy with mitoxantrone/etoposide and subsequent thalidomide, the patient received SU5416 for the second AML relapse. During SU5416 monotherapy, a steady decrease in the bone marrow blast cell infiltration was observed, accompanied by an increase in hemoglobin levels (data not shown) and the platelet and neutrophil counts (Figure 1A-B). This remission (< 5% blasts in the bone marrow by morphology and immunophenotyping with flow cytometry, neutrophils > 1.5 × 109/L [1500/μL] and hemoglobin > 110g/L [11 g/dL], untransfused) has been sustained for more than 4 months during SU5416 maintenance therapy using the same dosing schedule. The platelet counts increased to 60-80 × 109/L [60-80 × 103/μL] (Figure 1B). Thus, the patient fulfilled all but one (platelets ≥ 100 × 109/L [100 × 103/μL]) criteria for a CR according to a consensus definition13 or the criteria of CRp (CR criteria without platelets ≥ 100 × 109/L [100 × 103/μL]) as used by others.
Leukemic blast cell infiltration, bone marrow microvessel density and peripheral blood cell counts.
(A) Leukemic blast cell infiltration determined by cytologic analyses (○) and bone marrow microvessel density (●) during the course of the disease and different antileukemic treatments. Duration of the treatment with mitoxantrone/etoposide (Mitox/Etop), thalidomide (Thalid), and SU5416 is indicated at the top of the figure. D represents values obtained at initial diagnosis; CR, values obtained at the time of complete remission. (B) Platelet (○) and neutrophil counts (●) during different treatments of the patient's second AML relapse.
Leukemic blast cell infiltration, bone marrow microvessel density and peripheral blood cell counts.
(A) Leukemic blast cell infiltration determined by cytologic analyses (○) and bone marrow microvessel density (●) during the course of the disease and different antileukemic treatments. Duration of the treatment with mitoxantrone/etoposide (Mitox/Etop), thalidomide (Thalid), and SU5416 is indicated at the top of the figure. D represents values obtained at initial diagnosis; CR, values obtained at the time of complete remission. (B) Platelet (○) and neutrophil counts (●) during different treatments of the patient's second AML relapse.
During the SU5416 treatment period of more than 7 months, no adverse events were observed. This is in line with the rather favorable side effect profile observed in previous phase I/II clinical trials in patients with advanced malignancies (Sugen, data on file). The only side effect was temporary somnolence lasting 2 to 3 hours after each infusion and not interfering with daily activities. This somnolence was most likely attributable to the premedication with the histamine antagonist clemastin.
At the time of second relapse, microvessel density in the bone marrow of the patient was 2.5-fold higher than the median of the control group, comparable with the values obtained at first diagnosis of the patient's AML (Figure 1A). The increased microvessel density dropped from 33.4 to 12.3 microvessels/×500-field 8 weeks after the beginning of SU5416 administration and remained in the normal range during maintenance therapy with SU5416 (Figures 1A, 2A,C). The latter values are comparable to those obtained in CR after induction chemotherapy (Figure 1A). A parallel decrease of c-kit (detected by flow cytometry, data not shown) and VEGFR-2–positive leukemic blasts was observed (Figure 2B,D).
Immunohistochemic staining of bone marrow sections.
Microvascular endothelial cells were highlighted by antibodies against thrombomodulin (A,C). VEGFR-2–positive leukemic blasts were identified by anti–VEGFR-2 antibodies (B,D). Immunohistochemic localization was performed by the alkaline phosphatase/anti–alkaline phosphatase bridge technique (Dako-APAAP Kit). A and B denote bone marrow samples obtained before; C and D, 8 weeks after treatment with SU5416. Original magnification, ×500.
Immunohistochemic staining of bone marrow sections.
Microvascular endothelial cells were highlighted by antibodies against thrombomodulin (A,C). VEGFR-2–positive leukemic blasts were identified by anti–VEGFR-2 antibodies (B,D). Immunohistochemic localization was performed by the alkaline phosphatase/anti–alkaline phosphatase bridge technique (Dako-APAAP Kit). A and B denote bone marrow samples obtained before; C and D, 8 weeks after treatment with SU5416. Original magnification, ×500.
The case we present here is the first to demonstrate that the RTK inhibitor SU5416 induces a stable remission in a patient with AML relapse, refractory toward multiple standard chemotherapy regimens. SU5416 targets the VEGFR-2 as well as the SCF receptor c-kit. It has been demonstrated that SU5416 induces apoptosis in endothelial cells of a colon cancer animal model expressing VEGFR-29 and in a c-kit–positive human myeloid leukemia cell line.10 In addition to the expression of c-kit, the patient discussed here had high levels of VEGF (data not shown) and VEGFR-2 expression of the leukemic blasts as demonstrated by immunohistochemistry in the bone marrow specimens (Figure 2B). This is in line with a recent report of strong VEGFR-2 expression in human chloromas.7 Thus, VEGF produced by the patient's leukemic blasts may have supported leukemic cell growth through paracrine (by increasing the bone marrow endothelial cell mass) and autocrine (supporting leukemic cell survival) mechanisms. Therefore, the observed remission in this patient might be due to the combination of antiangiogenic (targeting VEGFR-2 on endothelial cells) and antileukemic effects (targeting both VEGFR-2 and c-kit on leukemic blast cells) thus blocking paracrine and autocrine loops induced by leukemia-derived VEGF. This hypothesis is supported by the observed decrease in bone marrow microvessel density, VEGFR-2 expression, and blast cell infiltration.
Despite the observed antiangiogenic and antileukemic effects in the bone marrow, SU5416 did not prevent the occurrence of symptomatic leukemic meningitis in the sixth week of treatment. This might suggest that SU5416 has not sufficiently crossed the blood-brain barrier. On the other hand, we cannot completely exclude that the intrathecal administration of chemotherapy, especially Ara-C, contributed to the observed remission due to systemic resorption. However, a major contribution of Ara-C is rather unlikely when considering that the decrease of the bone marrow blast infiltration from 90% to 20% and the recovery of the peripheral blood cell counts had already occurred before starting intrathecal chemotherapy (Figures 1A-B). Furthermore, the cumulative dose of Ara-C before the observed remission was low (160 mg) and the ongoing remission during SU5416 maintenance therapy for more than 3 months after the last intrathecal chemotherapy underscores the antileukemic efficacy of SU5416.
In conclusion, targeting VEGFR-2 might be a promising therapeutic option in the treatment of AML and should be further evaluated in controlled clinical trials.
P.S. and S.K. are employed by Sugen.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Note added in proof
A third AML relapse occurred 9 months after institution of SU5416 treatment.
Author notes
Rolf M. Mesters or Wolfgang E. Berdel, Department of Medicine/ Hematology and Oncology, University of Muenster, Albert-Schweitzer-Strasse 33, D-48129 Muenster, Germany; e-mail: mesters@uni-muenster.de.