Abstract
Designing effective strategies to load human dendritic cells (DCs) with tumor antigens is a challenging approach for DC-based tumor vaccines. Here, a cytoplasmic expression system based on mRNA electroporation to efficiently introduce tumor antigens into DCs is described. Preliminary experiments in K562 cells using an enhanced green fluorescent protein (EGFP) reporter gene revealed that mRNA electroporation as compared with plasmid DNA electroporation showed a markedly improved transfection efficiency (89% versus 40% EGFP+ cells, respectively) and induced a strikingly lower cell toxicity (15% death rate with mRNA versus 51% with plasmid DNA). Next, mRNA electroporation was applied for nonviral transfection of different types of human DCs, including monocyte-derived DCs (Mo-DCs), CD34+ progenitor-derived DCs (34-DCs) and Langerhans cells (34-LCs). High-level transgene expression by mRNA electroporation was obtained in more than 50% of all DC types. mRNA-electroporated DCs retained their phenotype and maturational potential. Importantly, DCs electroporated with mRNA-encoding Melan-A strongly activated a Melan-A–specific cytotoxic T lymphocyte (CTL) clone in an HLA-restricted manner and were superior to mRNA-lipofected or -pulsed DCs. Optimal stimulation of the CTL occurred when Mo-DCs underwent maturation following mRNA transfection. Strikingly, a nonspecific stimulation of CTL was observed when DCs were transfected with plasmid DNA. The data clearly demonstrate that Mo-DCs electroporated with mRNA efficiently present functional antigenic peptides to cytotoxic T cells. Therefore, electroporation of mRNA-encoding tumor antigens is a powerful technique to charge human dendritic cells with tumor antigens and could serve applications in future DC-based tumor vaccines.
Introduction
Dendritic cells (DCs) are bone-marrow–derived leukocytes that function as professional antigen-capturing and -presenting cells for the initiation of a primary immune response in vitro and in vivo.1 Given their central role in cell-mediated immunity in vivo, they represent highly attractive targets for molecular immunotherapy of acquired diseases, such as AIDS and cancer. Recent advances in the ex vivo generation of DCs and the ability to modulate DC functions provide a rationale to design DC-based tumor vaccines.2 The outcome of such tumor vaccines will highly depend on the efficacy of the applied antigen-loading method for optimal stimulation of cytotoxic T lymphocyte (CTL)–mediated antitumor immune responses.3 Although several reports have documented viral transfer of cDNA-encoding tumor-associated antigens to load DCs for induction of TAA-specific CTLs,4-6 nonviral gene delivery systems for DC-based vaccines would provide a more attractive approach with clinical perspectives because safety issues and immunogenicity of the vector are reduced to a minimum. However, the problem of inefficient gene transfer and low level of expression by nonviral transfection remains.7
Previously, we reported high-level transgene expression in proliferating CD34+ progenitor-derived DCs (34-DCs) and Langerhans cells (34-LCs) using electroporation-mediated gene delivery.8 In contrast, nondividing monocyte-derived DCs (Mo-DCs), which represent a highly accessible and widely used source of in vitro cultured DCs, were relatively refractory to cDNA transfection techniques, either by electroporation or by lipofection.
Recently, it was shown that human DCs could be transfected with RNA and were capable of inducing primary antigen-specific CTLs.9However, there are very few data on the efficiency of mRNA transfer in Mo-DCs using passive pulsing, lipofection, or electroporation. Furthermore, the feasibility of mRNA transfection in 34-DCs or 34-LCs has not yet been established, nor compared with Mo-DCs. In this study, we sought to improve mRNA transfection efficiency using an optimized mRNA-based electroporation. To our knowledge, this is the first detailed report describing high-efficiency nonviral transfection of Mo-DCs by mRNA electroporation correlated with effective loading of tumor antigens into different types of human DCs. In addition, we compared its efficiency with other transfection methods, such as lipofection and passive pulsing of mRNA as well as cDNA electroporation. Furthermore, the effect of DC maturation on loading efficiency was investigated.
Materials and methods
Cell lines
T2 cells (TAP-deficient, HLA-A2+, TxB hybrid), EBV-LG2 (HLA-A2− EBV-transformed B lymphocytes), and SK23-MEL (Melan-A+ HLA-A2+ melanoma cell line) were kindly provided by Dr Pierre Van der Bruggen (Ludwig Institute for Cancer Research, Brussels, Belgium). K562 cells were obtained from the American Type Culture Collection (ATCC no. CCL-243; Rockville, MD). Cell lines were cultured in complete medium consisting of Iscove modified Dulbecco medium (IMDM) supplemented with L-glutamine (2 mM), penicillin (100 U/mL), streptomycin (100 μg/mL), amphotericin B (1.25 μg/mL fungizone), and 10% fetal calf serum (FCS; Sera Lab, Sussex, United Kingdom). Cells were maintained in logarithmic phase growth at 37°C in a humidified atmosphere supplemented with 5% CO2. All cell culture reagents were purchased from Gibco BRL (Paisley, United Kingdom).
Melan-A–specific CTL clone
The CD8+ TIL 1235 clone recognizing the immunodominant HLA-A0201–restricted Melan-A27-35 epitope (AAGIGILTV) was a kind gift of Dr J. Wunderlich (National Institutes of Health, Bethesda, MD) and was cultured as described earlier with minor modifications.10 Briefly, the TIL clone was maintained in AIM-V medium (Gibco BRL) supplemented with 5% pooled human AB serum (Sigma, Bornem, Belgium) and 500 IU/mL interleukin 2 (IL-2) (R&D Systems, Minneapolis, MN) and used as responder population in DC coculture experiments.
Source of primary cells
Bone marrow (BM) samples were aspirated by sternal puncture from hematologically normal patients undergoing cardiac surgery, after informed consent. Peripheral blood mononuclear cells (PBMCs) were obtained from healthy volunteers or hemochromatosis patients. Mononuclear cells were isolated by Ficoll-Hypaque gradient separation (LSM, ICN Biomedicals, Costa Mesa, CA).
CD34+ cell sorting
After Ficoll-Hypaque separation, mononuclear BM cells were indirectly stained using supernatant of the 43A1 hybridoma (anti-CD34) kindly donated by Dr H-J. Bühring (University of Tübingen, Germany),11 followed by fluorescein isothiocyanate (FITC)–conjugated rabbit antimouse immunoglobulins (Dako, Glostrup, Denmark). The CD34-labeled cells were then sorted on a FACStarPLUS cell sorter (Becton Dickinson, Erembodegem, Belgium) equipped with an air-cooled argon ion laser ILT model 5500-A (Ion Laser Technology, Salt Lake City, UT). Sort windows were set to include cells with low side scatter and with positive green fluorescence (CD34+). Purities of more than 95% were routinely obtained.
In vitro culture of DCs
34-DC cultures were cultured as described previously.12 Briefly, 1-2.105CD34+ cells were cultured in 2 mL of compete medium supplemented with 100 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF; Leucomax, Novartis Pharma, Basel, Switzerland), 2.5 ng/mL tumor necrosis factor (TNF)–α (Roche Molecular Biochemicals, Mannheim, Germany), and 50 ng/mL stem cell factor (SCF; Biosource, Nivelle, Belgium) until day 5; afterward, SCF was replaced by 1000 U/mL IL-4 (R&D Systems), which was added for the next 5-9 days. After 12 days of culture, a 15- to 20-fold total cell expansion was observed and cells exhibited typical markers of mature DCs including CD1a, CD80, CD86, and HLA-DR (see further).
For 34-LCs, we used the protocol described by Herbst et al.13 Briefly, sorted CD34+ cells were first cultured for 8 days in complete medium containing 100 ng/mL IL-3, 100 ng/mL IL-6, and 50 ng/mL SCF (all from Biosource), followed by LC differentiation in GM-CSF (100 ng/mL) and IL-4 (1000 U/mL) for the next 4 weeks. After 25 days of culture, a 75- to 100-fold increase in the total number of nucleated cells was observed and cells expressed high levels of CD1a and CD40, intermediate levels of HLA-DR, and low levels of CD80 and CD86 and were able to efficiently take up FITC-dextran at 37°C (data not shown).
Immature monocyte-derived DCs (iMo-DCs) were generated from PBMCs as described by Romani et al14 Briefly, PBMCs were allowed to adhere in AIM-V medium for 2 hours at 37°C. The nonadherent fraction was removed, and adherent cells were further cultured for 5-7 days in IMDM supplemented with 2.5% autologous heat-inactivated plasma. GM-CSF (100 ng/mL) and IL-4 (1000 U/mL) were added to the cultures every 2-3 days starting from day 0. Maturation of iMo-DCs was induced by adding 2.5 ng/mL TNF-α and 100 ng/mL lipopolysaccharide (LPS; Sigma) for 24 hours starting from day 6 of the Mo-DC culture.
HLA-A typing of DCs
HLA-A2 subtyping was determined on BM-derived mononuclear cells and PBMCs by indirect staining with the supernatant of the BB7-2 hybridoma (anti–HLA-A2; ATCC), followed by FITC-conjugated rabbit antimouse immunoglobulins (Dako). HLA-A2 staining was analyzed by flow cytometry using a FACScan analytical flow cytometer (Becton Dickinson).
Synthetic peptides
The following HLA-A*0201–restricted synthetic peptides were used: influenza virus peptide (M1; amino acids [aa] 58-66, GILGFVFTL) and Melan-A peptide (MA; aa 27-35, AAGIGILTV). Peptides (> 95% pure) were purchased from Sigma-Genosys (Cambridge, United Kingdom). Both peptides were dissolved in 100% dimethyl sulfoxide (DMSO) to 10 mg/mL, further diluted to 1 mg/mL in serum-free IMDM and stored in aliquots at −70°C. Peptides were used at a final concentration of 20 mM.
Peptide-pulsing of DCs
T2 cells or HLA-A2+ iMo-DCs were washed twice with IMDM and subsequently incubated with 20 μM peptide in serum-free medium supplemented with 2.5 μg/mL β2 microglobulin (Sigma) for 2 hours at room temperature in 15-mL conical tubes. Afterward, cells were washed twice and used as peptide controls in cytokine release assays.
Plasmids
For plasmid cDNA transfection, a pEGFP-N1 plasmid (Clontech Laboratories, Palo Alto, CA) was used encoding an enhanced green fluorescent protein (EGFP) gene under the control of a cytomegalovirus (CMV) promoter, and pcDNA1.1/Melan-A containing the Melan-A/MART-1 gene driven by a CMV promoter (kindly provided by Dr Pierre Van der Bruggen). pcDNA1.1/Amp (Invitrogen, Carlsbad, CA) was used as a backbone control vector. Plasmids were propagated in E Colistrain DH5α (Gibco BRL) and purified on endotoxin-free Qiagen-tip 500 columns (Qiagen, Chatsworth, CA).
Production of in vitro–transcribed mRNA
For in vitro transcriptions, plasmids were linearized, purified using a Genieprep kit (Ambion, Austin, TX), and used as DNA templates for the in vitro transcription reaction. pcDNA1.1/Melan-A was used as such for in vitro transcription under the control of a T7 promoter. EGFP cDNA (a 0.8 kb HindIII-NotI fragment) was first subcloned into pcDNA1.1/Amp and subsequently cloned as aBamHI-XbaI fragment into pSP64 (Promega, Madison, WI) that allows in vitro transcription under the control of an SP6 promoter. Transcription was carried out in a final 20-100 μL reaction mix at 37°C for 3-4 hours using the SP6 MessageMachine kit (Ambion) to generate 5′ m7GpppG-capped in vitro–transcribed (IVT) mRNA. Purification of IVT mRNA was performed by DNase I digestion followed by LiCl precipitation and 70% ethanol wash, according to the manufacturer's instructions. For each experiment, at least 3 different batches of mRNA were used. mRNA quality was checked by agarose-formaldehyde gel electrophoresis. RNA concentration was assayed by spectrophotometrical analysis at OD260. RNA was stored at −80°C in small aliquots (1 μg/μL).
Cell transfections
Prior to electroporation, K562 cells were washed twice with serum-free IMDM and resuspended to a final concentration of 5-10.106 cells/mL in Opti-MEM (Gibco BRL). After phenotypic analysis (performed in order to confirm the presence of CD1a+HLA-DR+ DCs in the cultures), 34-DCs, 34-LCs, and Mo-DCs were routinely harvested after 12, 25, and 6 days, respectively, of culture (unless stated otherwise), washed twice with serum-free IMDM, and resuspended to a final concentration of 10-40.106 cells/mL in Opti-MEM. Subsequently, 0.5 mL of the cell suspension was mixed with 20 μg of IVT mRNA, and electroporated in a 0.4-cm cuvette using an Easyject Plus device (EquiBio, Kent, United Kingdom). In K562 cells, various voltages, capacitances, and electroporation volumes were compared in order to assess their effect on mRNA transfection efficiency (see “Results”). Plasmid DNA electroporation was performed as previously described.8After electroporation, fresh complete medium (including cytokines for DCs) was added to the cell suspension and cells were further incubated at 37°C in a humidified atmosphere supplemented with 5% CO2. Lipofection of mRNA was performed using the cationic lipid DMRIE-C (Gibco BRL) according to manufacturer's instructions with minor modifications.8 Briefly, K562 cells were washed twice with serum-free IMDM and resuspended to a final concentration of 1-2.106 cells/mL in Opti-MEM. 34-DCs, 34-LCs, and Mo-DCs were harvested after 12, 25, and 6 days, respectively, of culture, washed twice with serum-free IMDM, and resuspended to a final concentration of 1-2.106 cells/mL in Opti-MEM. 5 μg of IVT mRNA, diluted in 250 μL Opti-MEM, was mixed with DMRIE-C, also diluted in 250 μL Opti-MEM, at a lipid to RNA ratio of 4:1. After 5-15 minutes of incubation at room temperature in order to allow RNA-lipid complexation, lipoplexes were added to the cells and allowed to incubate for 2 hours at 37°C. Alternatively, 5-20 μg of IVT mRNA was pulsed to the cells in the absence of DMRIE-C for 3-4 hours at 37°C. Plasmid DNA lipofection was performed as previously described.8 After lipofection or passive pulsing, fresh complete medium (including cytokines for DCs) was added to each well.
EGFP analysis
EGFP-transfected cells were checked for EGFP expression 24-48 hours after transfection by flow cytometric (FCM) analysis. Briefly, cells (1-5 × 105) were washed once in phosphate-buffered saline (PBS) supplemented with 1% FCS and resuspended in 0.5 mL of PBS supplemented with 1% BSA and 0.1% sodium azide. Ethidium bromide (EB) at a final concentration of 10 μg/mL was added directly prior to FCM analysis on a FACScan analytical flow cytometer (Becton Dickinson) to assess cell viability. For EGFP analysis in DC cultures, gating was performed on cells exhibiting a large forward scatter (FSC) and side scatter (SSC) profile (ie, DCs), in order to allow exclusion of contaminating autologous lymphocytes. Gated DCs were then evaluated for EGFP expression.
Immunophenotyping of DCs
Immunophenotyping was performed as previously described.8 The following monoclonal antibodies were used: CD1a-FITC (Ortho Diagnostic Systems, Beerse, Belgium), CD1a-PE (Caltag Laboratories, San Francisco, CA), CD14-PE, HLA-DR-PE, CD4-PE, CD80-PE (Becton Dickinson), CD40-FITC (BioSource, Zoersel, Belgium), CD86-PE (Pharmingen, San Diego, CA), CD13-FITC (Dako), and CD83 (HB-15 clone; Immunotech, Marseille, France). Nonreactive isotype-matched antibodies (Becton Dickinson) were used as controls.
Interferon-γ release assay
34-DCs, 34-LCs, and iMo-DCs were used as stimulator cells 24 hours after transfection. To study the effect of maturation, 6-day–cultured iMo-DCs were allowed to mature for 24 hours in the presence of TNF-α and LPS prior to transfection and used as stimulators 24 hours after transfection. Alternatively, iMo-DCs were transfected with mRNA on day 6 of culture and, after 12-16 hours to allow protein expression, TNF-α and LPS were added to induce final DC maturation. After an additional 24 hours, mature transfected Mo-DCs were used as stimulators. In some experiments, iMo-DCs pulsed with the Melan-A or an irrelevant influenza M1 peptide were used as stimulators. Stimulators were washed twice and resuspended in AIM-V medium supplemented with 10% pooled human AB serum and 40 IU/mL IL-2. Responder CTLs were washed vigorously 3-4 times and resuspended in AIM-V medium. Then, CTLs (1 × 105 cells) were coincubated with stimulator cells (1 × 105 cells) in 96-round bottom plates for 24 hours at 37°C in a total volume of 200 μL. Triplicate supernatant samples from these cocultures were tested for specific interferon γ (IFN-γ) secretion by an IFN-γ enzyme-linked immunosorbant assay (ELISA) (Biosource). To normalize data, the background IFN-γ secretion (defined as IFN-γ released by the CTL exposed to unmodified DCs) was subtracted from each of the observed measurements. Measurements are presented as IU/mL released by 105 responder cells per 24 hours.
Results
Optimization of IVT mRNA transfection in K562 cells
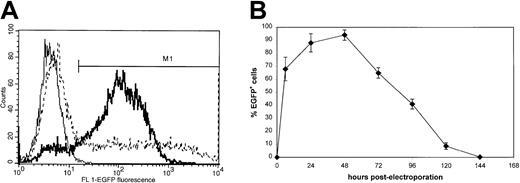
In preliminary experiments to optimize mRNA electroporation, we used leukemic K562 cells, as these cells were readily transfectable by plasmid electroporation.15 The EGFP reporter gene was used to assess mRNA transfection efficiency. Various electroporation settings were tested and transfection efficiency was determined by FCM analysis of EGFP expression (Figure 1A). Of all tested electrical settings, a voltage of 300 V combined with a capacitance of 150 μF in a total cuvette volume of 200 μL resulted in the highest EGFP expression (Table 1). The vast majority of the viable cell fraction expressed EGFP to a significant extent. The percentage of EGFP-expressing K562 cells was markedly higher following mRNA electroporation than following plasmid cDNA transfection, even when cDNA electroporation was performed at optimal DNA electroporation settings, that is, 260 V and 1050 μF (Figure 1A). Furthermore, mRNA electroporation at optimal settings showed a significantly reduced cell mortality rate as compared with cDNA electroporation at optimal settings (15% versus 51%, respectively). DMRIE-C–mediated RNA and DNA lipofection showed a somewhat similar outcome in terms of efficiency and viability although optimal lipid to nucleic acid ratio (4:1 versus 3:1) as well as incubation time (2 hours versus 6 hours) varied for RNA and DNA lipofection, respectively (Table 2).
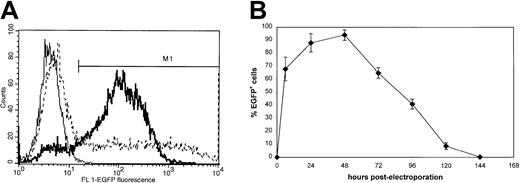
Flow cytometric analysis of transgene expression in K562 cells following EGFP mRNA electroporation.
(A) K562 cells were electroporated with EGFP mRNA at 300 V, 150 μF or with EGFP plasmid DNA at 260V, 1050 μF (dashed line) as described in “Materials and methods.” Twenty-four hours after electroporation, flow cytometric EGFP analysis was performed to estimate transfection efficiency of mRNA electroporation (bold line) and plasmid DNA electroporation (dashed line). An overlay histogram representative of 5 independent experiments is shown. Nonelectroporated cells (thin line) were used to determine background fluorescence. The M1 region indicates the EGFP-positive cell fraction. The percentage of EGFP+cells was 85% (bold line) and 50% (dashed line) following mRNA or plasmid DNA electroporation, respectively. (B) Kinetics of EGFP mRNA expression in K562 cells in function of time (n = 3). Note the rapid induction of high-level EGFP expression already 3 hours following electroporation.
Flow cytometric analysis of transgene expression in K562 cells following EGFP mRNA electroporation.
(A) K562 cells were electroporated with EGFP mRNA at 300 V, 150 μF or with EGFP plasmid DNA at 260V, 1050 μF (dashed line) as described in “Materials and methods.” Twenty-four hours after electroporation, flow cytometric EGFP analysis was performed to estimate transfection efficiency of mRNA electroporation (bold line) and plasmid DNA electroporation (dashed line). An overlay histogram representative of 5 independent experiments is shown. Nonelectroporated cells (thin line) were used to determine background fluorescence. The M1 region indicates the EGFP-positive cell fraction. The percentage of EGFP+cells was 85% (bold line) and 50% (dashed line) following mRNA or plasmid DNA electroporation, respectively. (B) Kinetics of EGFP mRNA expression in K562 cells in function of time (n = 3). Note the rapid induction of high-level EGFP expression already 3 hours following electroporation.
As RNA is extremely labile and has a short half-life time compared with DNA, we also studied kinetics of EGFP expression following mRNA electroporation (Figure 1B). Transgene expression in K562 cells peaked at 24-48 hours and rapidly declined to background levels after 6 days.
Efficiency of IVT mRNA transfection in different types of DCs
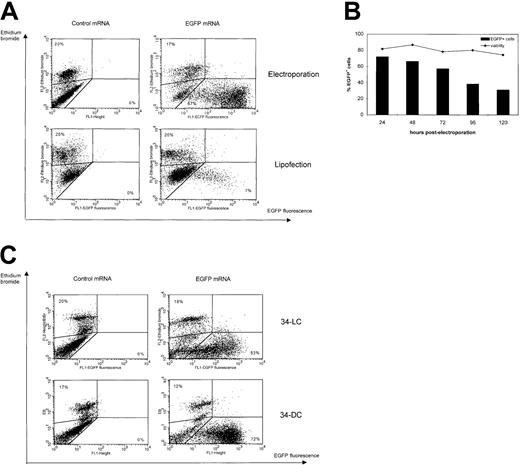
iMo-DCs were generated from adherent PBMCs in the presence of GM-CSF and IL-4. At day 5-6 of culture, Mo-DCs were electroporated with EGFP mRNA. Optimization experiments revealed optimal settings similar to those of K562 cells (300 V, 150 μF), leading to maximal transfection efficiency combined with the lowest level of cell death. FCM analysis of EGFP expression showed more than 60% EGFP-expressing iMo-DCs (Figure 2A and Table3). Mortality in Mo-DCs after mRNA electroporation ranged from 15% to 30% (mean cell mortality rate 22% ± 8%), although untransfected Mo-DC cultures already exhibited some degree of cell death (5%-10%). When gating on the viable population, 85% of viable Mo-DCs expressed EGFP to some extent. TNFα+LPS–induced maturation of Mo-DCs prior to transfection showed a significant decrease in electroporation and lipofection efficiency (Table 3). DC maturation following mRNA transfection had no effect on transgene expression (data not shown). Lipofection of EGFP mRNA in Mo-DCs resulted in a much lower efficiency (7.5% ± 0.5%) and was slightly more toxic (mean cell mortality rate 28% ± 10%) to the cells than mRNA electroporation, as shown in Figure 2A. Passive pulsing of DCs with mRNA did not result in any detectable EGFP expression. Kinetic analysis of mRNA expression in Mo-DCs showed a maximum 24 hours after electroporation, followed by a slow decline in function of time (Figure 2B). Five days after mRNA electroporation, EGFP was still detectable in a substantial proportion of Mo-DCs (31% EGFP+ cells), in contrast to transgene expression kinetics in K562 cells (9% EGFP+ cells after 5 days). Monitoring of cell viability after mRNA electroporation revealed a somewhat stable viability in function of time (Figure 2B).
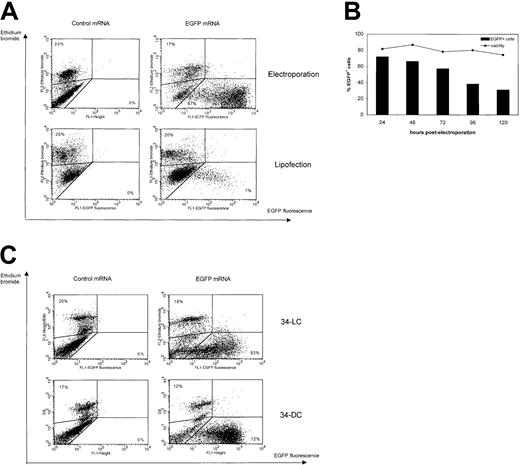
FCM analysis of transgene expression following EGFP mRNA transfection in different types of DCs.
(A) Immature Mo-DCs were cultured with GM-CSF and IL-4 and transfected at day 6 of culture with control (Melan-A) or EGFP mRNA by lipofection (bottom) or electroporation (top) and analyzed by FCM one day after transfection. The dot plots show EGFP fluorescence on the x-axis and ethidium bromide staining on the y-axis. Gates were drawn based on control mRNA–lipofected or electroporated Mo-DCs. Percentages of dead cells (upper left corner) and viable EGFP+ cells (lower right corner) are indicated. Results are representative of 8 independent experiments. (B) Monitoring of EGFP mRNA expression and cell viability in Mo-DCs following mRNA electroporation in function of time (n = 2). (C) 34-DCs (bottom) and 34-LCs (top) were cultured as described in “Materials and methods” and transfected at day 12 and day 25 of culture, respectively, with control (Melan-A) or EGFP mRNA by mRNA electroporation. FCM analysis was performed 24 hours after mRNA electroporation. The dot plots show EGFP fluorescence on the x-axis and ethidium bromide staining on the y-axis. Gates were drawn based on control mRNA-electroporated Mo-DCs (left). Percentages of dead cells (upper left corner) and viable EGFP+ cells (lower right corner) are indicated. Results are representative of 4 independent experiments.
FCM analysis of transgene expression following EGFP mRNA transfection in different types of DCs.
(A) Immature Mo-DCs were cultured with GM-CSF and IL-4 and transfected at day 6 of culture with control (Melan-A) or EGFP mRNA by lipofection (bottom) or electroporation (top) and analyzed by FCM one day after transfection. The dot plots show EGFP fluorescence on the x-axis and ethidium bromide staining on the y-axis. Gates were drawn based on control mRNA–lipofected or electroporated Mo-DCs. Percentages of dead cells (upper left corner) and viable EGFP+ cells (lower right corner) are indicated. Results are representative of 8 independent experiments. (B) Monitoring of EGFP mRNA expression and cell viability in Mo-DCs following mRNA electroporation in function of time (n = 2). (C) 34-DCs (bottom) and 34-LCs (top) were cultured as described in “Materials and methods” and transfected at day 12 and day 25 of culture, respectively, with control (Melan-A) or EGFP mRNA by mRNA electroporation. FCM analysis was performed 24 hours after mRNA electroporation. The dot plots show EGFP fluorescence on the x-axis and ethidium bromide staining on the y-axis. Gates were drawn based on control mRNA-electroporated Mo-DCs (left). Percentages of dead cells (upper left corner) and viable EGFP+ cells (lower right corner) are indicated. Results are representative of 4 independent experiments.
We also tested mRNA transfection in bone marrow 34-DCs and 34-LCs. Up to 72% and 53%, respectively, of these DC types were readily transfected by mRNA electroporation (Figure 2C), but not by mRNA lipofection or mRNA pulsing (Table 3). Viability was always higher than 80% for both 34-DCs and 34-LCs (Figure 2C). Table 3 summarizes efficiency of mRNA-based electroporation, lipofection, and passive pulsing in the different types of DCs.
Phenotype and maturation of mRNA-electroporated DCs
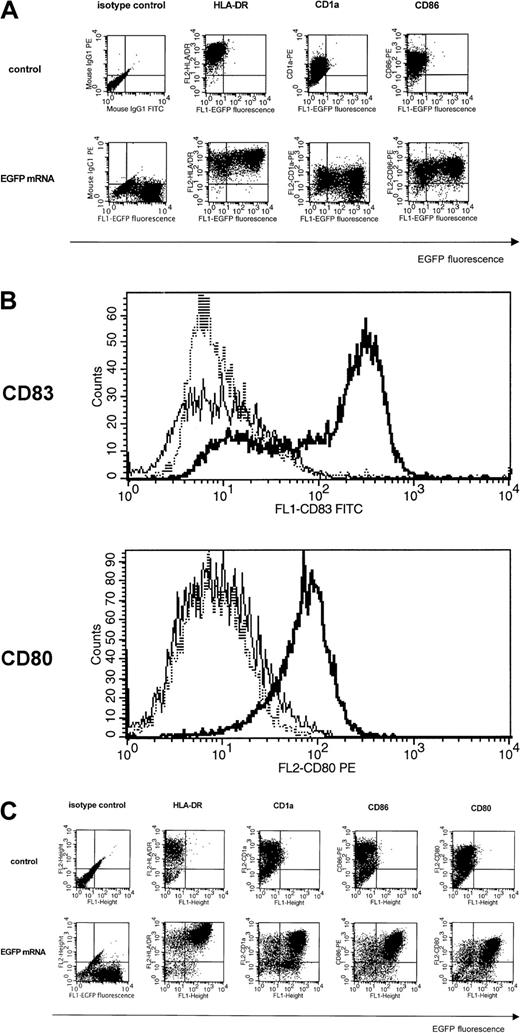
Because DCs have a delicate phenotype that can easily be disturbed by culture or transfection conditions, we assessed by FCM analysis whether electroporated DCs retained their respective phenotypes as well as their capacity to differentiate into mature DCs. Control and EGFP mRNA-transfected Mo-DCs were stained using monoclonal antibodies binding to characteristic DC markers including CD1a, HLA-DR, CD80, CD86, and CD83. Electroporation of mRNA showed no effect on the phenotype of Mo-DCs, as electroporated Mo-DCs coexpressing EGFP retained high levels of CD1a, HLA-DR, and CD86 (Figure3A). Mock electroporation (electroporation without mRNA) gave similar results (data not shown). The capacity of mRNA-electroporated Mo-DCs to differentiate to mature Mo-DCs was evaluated by expression of mature DC markers including CD80 and CD83. Figure 3B shows that mRNA electroporation itself did not induce DC maturation, but that the maturation potential after electroporation was retained since mRNA-transfected immature Mo-DCs were able to upregulate CD83 and CD80 in the presence of a maturation cocktail (TNF-α+LPS).
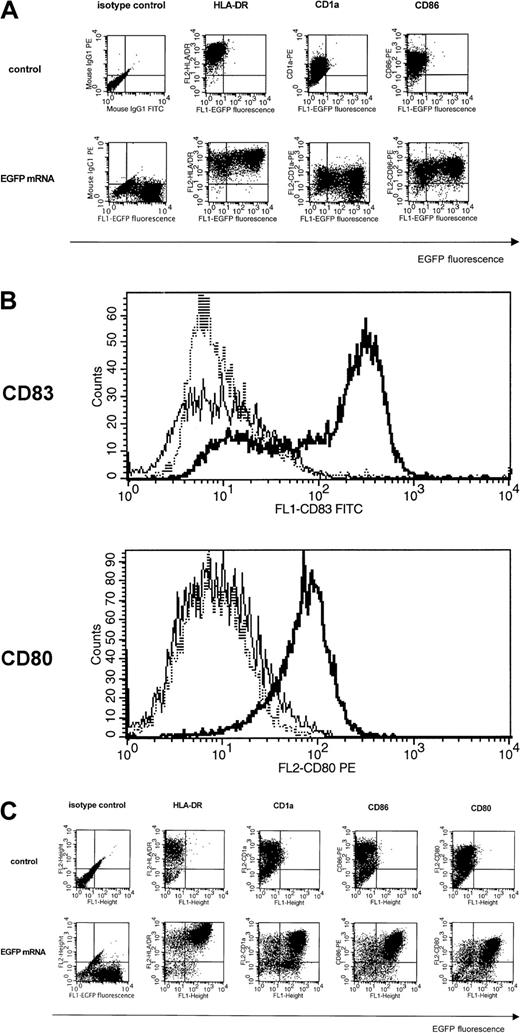
Phenotypic analysis and maturation potential of mRNA-electroporated DCs.
(A) Immature Mo-DCs (iMo-DCs) were transfected by electroporation with mRNA-encoding EGFP and stained with PE-labeled antibodies specific for CD1a, HLA-DR, and CD86 one day after electroporation (bottom). Untransfected iMo-DCs (top) served as controls and isotype-matched antibodies were used to set quadrants. Results are representative of 3 experiments. (B) iMo-DCs were transfected by electroporation with mRNA-encoding Melan-A and directly stained with a PE-labeled CD80 antibody (bottom) or indirectly stained with a CD83 antibody (top). A representative overlay histogram is shown in which the dashed line represents the control nonelectroporated iMo-DCs, the thin line the electroporated iMo-DCs, and the bold line the electroporated iMo-DCs that were allowed to mature for an additional 24 hours following mRNA electroporation in the presence of TNF-α and LPS. (C) Twelve-day-cultured 34-DCs were transfected by electroporation with mRNA-encoding EGFP and stained with PE-labeled antibodies specific for CD1a, HLA-DR, CD86, and CD80 one day after electroporation (bottom). Untransfected 34-DCs (top) served as controls and isotype-matched antibodies were used to set quadrants. Results are representative of 3 experiments.
Phenotypic analysis and maturation potential of mRNA-electroporated DCs.
(A) Immature Mo-DCs (iMo-DCs) were transfected by electroporation with mRNA-encoding EGFP and stained with PE-labeled antibodies specific for CD1a, HLA-DR, and CD86 one day after electroporation (bottom). Untransfected iMo-DCs (top) served as controls and isotype-matched antibodies were used to set quadrants. Results are representative of 3 experiments. (B) iMo-DCs were transfected by electroporation with mRNA-encoding Melan-A and directly stained with a PE-labeled CD80 antibody (bottom) or indirectly stained with a CD83 antibody (top). A representative overlay histogram is shown in which the dashed line represents the control nonelectroporated iMo-DCs, the thin line the electroporated iMo-DCs, and the bold line the electroporated iMo-DCs that were allowed to mature for an additional 24 hours following mRNA electroporation in the presence of TNF-α and LPS. (C) Twelve-day-cultured 34-DCs were transfected by electroporation with mRNA-encoding EGFP and stained with PE-labeled antibodies specific for CD1a, HLA-DR, CD86, and CD80 one day after electroporation (bottom). Untransfected 34-DCs (top) served as controls and isotype-matched antibodies were used to set quadrants. Results are representative of 3 experiments.
Also, the phenotypes of 34-DCs were also not affected by mRNA electroporation (Figure 3C). EGFP+ 34-DCs coexpressed HLA-DR, CD1a, CD80, and CD86. Similar findings were observed in 34-LCs, with the exception that 34-LCs exhibited lower levels of CD80 and CD86, compatible with their similarity to immature Langerhans-like DCs (data not shown).
MHC class I–restricted antigen presentation by mRNA-transfected DCs
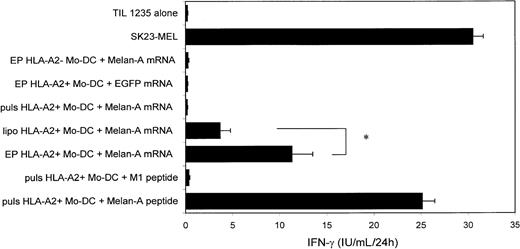
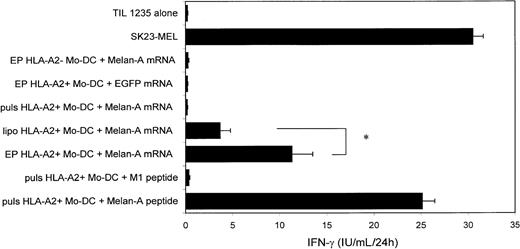
Given the high transfection efficiency in Mo-DCs, we investigated to what extent mRNA-transfected Mo-DCs could process antigen and present MHC class I–restricted antigenic epitopes to an antigen-specific CTL clone. Therefore, we introduced mRNA encoding Melan-A/MART-1 into HLA-A2+ Mo-DCs using electroporation, lipofection, or passive pulsing. Mo-DCs electroporated or lipofected with Melan-A mRNA markedly stimulated an HLA-A2+Melan-A–specific CTL clone, as judged by IFN-γ secretion (Figure4). Mo-DCs passively pulsed with Melan-A mRNA did not result in any CTL stimulation. HLA-A2+ Mo-DCs electroporated with EGFP mRNA or HLA-A2− Mo-DCs electroporated with Melan-A mRNA did not stimulate the CTL clone to produce IFN-γ. Both HLA-A2+ Melan-A+ SK23-MEL melanoma cells and HLA-A2+ Mo-DCs, pulsed with the immunodominant Melan-A27-35 peptide AAGIGILTV, were used as positive controls and induced strong IFN-γ production by the CTL clone. HLA-A2+ Mo-DCs pulsed with the M1 influenza peptide did not elicit any specific IFN-γ production. Mo-DCs electroporated with Melan-A IVT mRNA stimulated the CTL clone more than two times stronger than mRNA-lipofected Mo-DCs (Figure 4), suggesting a correlation with the difference in transfection efficiency between the 2 gene transfer methods (Table 3). The observation that transfection efficiency and CTL activation were correlated was also made when comparing efficiency of mRNA electroporation (Table 3) and the capacity to stimulate the CTL clone in the other types of DCs (Table4). Electroporation of HLA-A2+ 34-DCs and 34-LCs with Melan mRNA, but not EGFP mRNA, led to specific CTL activation. In concordance with the absence of any detectable transfection level (Table 3), lipofection of 34-DCs and 34-LCs or passive pulsing of all types of DCs with Melan-A mRNA did not result in any IFN-γ detectable above background levels (Table 4).
mRNA-based antigen loading of Mo-DCs.
Immature Mo-DCs were cultured with GM-CSF and IL-4 and transfected at day 6 of culture with Melan-A mRNA by electroporation (n = 11), lipofection (n = 8), or passive pulsing (n = 5) or with EGFP mRNA by electroporation (n = 6). The SK23-MEL melanoma cell line, HLA-A2+ Mo-DCs pulsed with a Melan-A, or an irrelevant influenza peptide and HLA-A2–negative Mo-DCs electroporated with Melan-A mRNA served as controls. Antigen-presenting cells (indicated on the left of the graph) were coincubated with a Melan-A–specific CD8+ CTL clone to determine antigen-loading efficiency, as reflected by IFN-γ production of the CTL clone. Results are shown as mean ± SD. * P < .05; EP, electroporation; lipo, lipofection; puls, passive pulsing.
mRNA-based antigen loading of Mo-DCs.
Immature Mo-DCs were cultured with GM-CSF and IL-4 and transfected at day 6 of culture with Melan-A mRNA by electroporation (n = 11), lipofection (n = 8), or passive pulsing (n = 5) or with EGFP mRNA by electroporation (n = 6). The SK23-MEL melanoma cell line, HLA-A2+ Mo-DCs pulsed with a Melan-A, or an irrelevant influenza peptide and HLA-A2–negative Mo-DCs electroporated with Melan-A mRNA served as controls. Antigen-presenting cells (indicated on the left of the graph) were coincubated with a Melan-A–specific CD8+ CTL clone to determine antigen-loading efficiency, as reflected by IFN-γ production of the CTL clone. Results are shown as mean ± SD. * P < .05; EP, electroporation; lipo, lipofection; puls, passive pulsing.
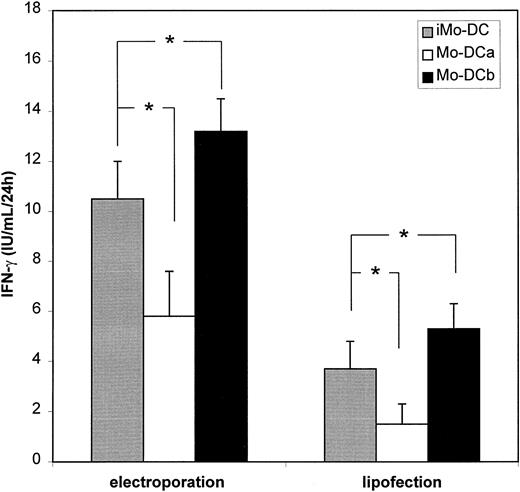
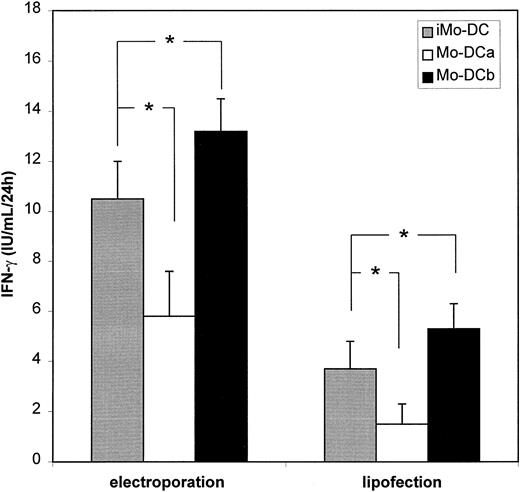
Effect of maturation on mRNA loading in Mo-DCs
Mo-DCs obtained by culturing PBMCs in the presence of GM-CSF and IL-4 for 5-7 days exhibit predominantly an immature phenotype.16 These immature Mo-DCs are specialized in capturing large amounts of antigens from the environment.17 However, for optimal presentation to CTL, Mo-DCs need to undergo a maturation process which can be induced by bacterial products (eg, LPS), inflammatory cytokines (eg, TNF-α), and/or CD40 ligation by T-helper cells.1 Therefore, in order to test whether maturation and the sequence of loading affected the antigen-presenting capacity of Mo-DCs, we evaluated the ability of Mo-DCs loaded with Melan-A by mRNA electroporation to stimulate the CTL clone prior to and after maturation with LPS+TNF-α. Figure5 clearly indicates that the most potent CTL activation was obtained when mRNA loading by electroporation or lipofection was performed prior to maturation of Mo-DCs. When maturation occurred prior to mRNA loading, there was a significant decrease in IFN-γ secretion by TIL cells (Figure 5), likely to be correlated with the lower degree of transfectability of mature Mo-DCs (Table 3).
Effect of DC maturation on tumor antigen presentation of mRNA-transfected Mo-DCs.
IFN-γ production by the CTL clone was measured after coculture with HLA-A2+ Mo-DCs electroporated with Melan-A mRNA. iMo-DCs, Mo-DCs transfected at the immature stage and used as such; Mo-DCa, Mo-DCs transfected at the mature stage after LPS+TNF-α stimulation; Mo-DCb, Mo-DCs transfected at the immature stage, matured by LPS+TNF-α and then assayed for Melan-A–specific CTL clone stimulation. Results are shown as mean ± SD (n = 4). * P < .05.
Effect of DC maturation on tumor antigen presentation of mRNA-transfected Mo-DCs.
IFN-γ production by the CTL clone was measured after coculture with HLA-A2+ Mo-DCs electroporated with Melan-A mRNA. iMo-DCs, Mo-DCs transfected at the immature stage and used as such; Mo-DCa, Mo-DCs transfected at the mature stage after LPS+TNF-α stimulation; Mo-DCb, Mo-DCs transfected at the immature stage, matured by LPS+TNF-α and then assayed for Melan-A–specific CTL clone stimulation. Results are shown as mean ± SD (n = 4). * P < .05.
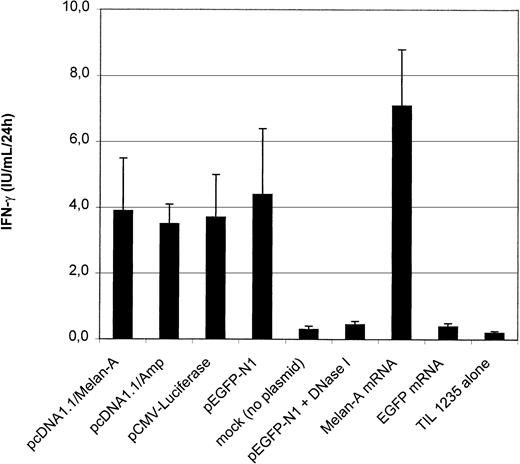
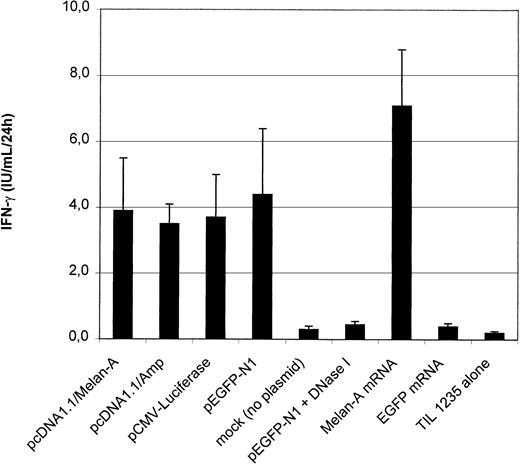
cDNA loading versus mRNA loading
In contrast to Mo-DCs, 34-LCs and 34-DCs can also be transfected by plasmid DNA electroporation.8 Therefore, we evaluated whether plasmid DNA–transfected DCs can also induce antigen-specific CTL activation. HLA-A2+ 34-LCs electoporated with plasmid DNA or IVT mRNA encoding Melan-A were incubated with the Melan-A–specific CTL to evaluate IFN-γ secretion (Figure6). Strikingly, we reproducibly obtained similar IFN-γ levels with Melan-A–cDNA as with control vector–transfected 34-LCs, indicating nonspecific CTL stimulation. Transfection with 2 other irrelevant plasmids (pEGFP-N1 and pCMV-luciferase) resulted in a similar nonspecific CTL stimulation. This phenomenon was never observed in mock-transfected (electroporation without plasmid DNA) 34-LCs or when the DNA was digested by DNase I prior to electroporation (Figure 6). Similar observations were made in 34-DCs (data not shown).
Outcome of plasmid cDNA-based antigen loading of 34-LCs.
IFN-γ production by the CTL clone was measured after coculture with HLA-A2+ 34-LCs electroporated with various plasmid DNA constructs encoding Melan-A (pcDNA1.1/Melan-A; n = 12), EGFP (pEGFP-N1; n = 12), luciferase (pCMV-Luc; n = 3), or with a backbone vector (pcDNA1.1/Amp; n = 6) lacking a eukaryotic cDNA sequence. Alternatively, 34-LCs were electroporated with in vitro–transcribed mRNA encoding EGFP or Melan-A (n = 3). Results are shown as mean ± SD. * P < .05.
Outcome of plasmid cDNA-based antigen loading of 34-LCs.
IFN-γ production by the CTL clone was measured after coculture with HLA-A2+ 34-LCs electroporated with various plasmid DNA constructs encoding Melan-A (pcDNA1.1/Melan-A; n = 12), EGFP (pEGFP-N1; n = 12), luciferase (pCMV-Luc; n = 3), or with a backbone vector (pcDNA1.1/Amp; n = 6) lacking a eukaryotic cDNA sequence. Alternatively, 34-LCs were electroporated with in vitro–transcribed mRNA encoding EGFP or Melan-A (n = 3). Results are shown as mean ± SD. * P < .05.
Discussion
Here, we developed an electroporation-based mRNA transfection protocol suitable for highly efficient antigen loading in Mo-DCs, as well as in 34-DCs and 34-LCs. This technique proved to be superior to mRNA lipofection or passive mRNA pulsing in terms of loading efficiency and subsequent activation of an antigen-specific CD8+ CTL clone. Recently, we described a electroporation-based EGFP cDNA transfection for in vitro–generated DCs showing high-efficiency gene transfer in 34-LCs and 34-DCs, but not in Mo-DCs.8However, in the present study we demonstrate that at present the plasmid DNA electroporation approach is not applicable for tumor antigen loading of DCs, because the T-cell stimulation it provokes is indistinguishable from nonspecific T-cell stimulation mediated by plasmid DNA, either directly or indirectly. This nonspecific stimulation was not observed when using mRNA electroporation, establishing the superiority of that technique for specific DC-based T-cell stimulation, at least in our hands.
In general, nonviral DNA transfection methods are inefficient, particularly in nondividing cells, as there is only very limited DNA trafficking to the nucleus where transcription occurs.18Therefore, several groups demonstrated the feasibility of mRNA transfection as a valid alternative for nonviral gene delivery, because this strategy avoids the need for entry into the nucleus as well as the complex issues of transcriptional regulation associated with DNA vectors.19-21 The RNA approach has several advantages that render it attractive in developing DC-based tumor vaccines. First, DCs can be transfected to comparable levels as compared with transduction by recombinant viruses, such as poxviruses22 or adenoviruses,4 while circumventing the drawbacks of viral vectors.23,24 Second, DCs can be charged with the full antigenic spectrum using total mRNA instead of IVT mRNA as a source of tumor antigens without prior identification of tumor-associated antigens.25 Moreover, RNA has a short cellular half-life and lacks the potential to integrate into the host genome, thereby obviating safety concerns (eg, insertional mutagenesis), in the context of clinical gene therapy trials.19 26
Using mRNA-based electroporation, transfection efficiency in Mo-DCs, 34-DCs, and 34-LCs was at least 25, 6, and 3 times, respectively, more efficient as compared with plasmid DNA electroporation.8Also, mRNA electroporation was superior to mRNA lipofection and passive pulsing. This increased transfection efficiency was translated in a superior biologic effect in vitro, as confirmed by our CTL activation experiments, and could be used as a tool to investigate as to whether it results in a higher immunopotency in vivo.27Importantly, mRNA-transfected DCs were able to efficiently process the introduced antigen and present antigenic epitopes in an MHC class I–restricted manner to a specific CD8+ TIL clone (Figure4). Furthermore, in concordance with previous reports on the effect of maturation on the antigen-presenting capacity of DCs,28antigen loading by mRNA electroporation was preferably performed prior to DC maturation in order to achieve the most optimal antigen presentation (Figure 5), indicating the importance of the sequence of loading and DC maturation for future DC-based vaccine design.29
The significant decrease in toxicity observed with mRNA electroporation could in part be explained by the less stringent electrical settings required for introduction of the RNA (Table 1). Nevertheless, mRNA electroporation performed at stringent DNA settings resulted in a lower cell toxicity as well, suggesting that cell toxicity is not solely due to the electroporation procedure itself, but can also be related to the nature of the introduced nucleic acids. Moreover, cointroduction of bacterial contaminants (eg, LPS) often found in plasmid preparations, could affect cell viability.30
In an attempt to compare DNA and mRNA loading of DCs, we unexpectedly observed nonspecific stimulation of the TIL clone with DNA- electroporated but not with mRNA-electroporated 34-DCs and 34-LCs (Figure 6), which could be abolished by DNase I–treatment of the plasmid DNA. Although this stimulatory effect of plasmid DNA confounded data interpretation, the impact of this phenomenon on DC loading with respect to antigen-presenting capacity needs further investigation. Possible involvement of immunostimulatory sequences present in plasmid DNA (ie, unmethylated CpG motifs) should be considered.31 32
Although mRNA lipofection was overall less efficient than mRNA electroporation for loading DCs, especially 34-DCs and 34-LCs, these data were derived from experiments with only one cationic lipid, DMRIE-C. Therefore, we cannot exclude the possibility that other lipids would accomplish comparable, or even higher, efficiencies of transfection and/or of MHC class I–restricted antigen loading of Mo-DCs, 34-LCs, or 34-DCs as compared with mRNA electroporation. Using passive mRNA pulsing, we were not able to detect any EGFP expression or CTL activation by any type of DC examined. Therefore, these results seem somewhat in contrast to the findings of Nair et al,9who showed in pulsing experiments that immature Mo-DCs can take up mRNA without the use of a transfection agent, and subsequently prime tumor-specific CTL in vitro.
In conclusion, we have shown that IVT mRNA-based electroporation is a highly efficient and simple nonviral method to gene-modify human Mo-DCs, 34-DCs, and 34-LCs with tumor antigens. The technique described in this study can serve applications in DC-based tumor vaccine development and in other gene transfer protocols requiring high-level, short-term transgene expression in hematopoietic cells.
V. F. I. V. T. is a research assistant of the Fund for Scientific Research, Flanders, Belgium. We thank Dr Pierre Van der Bruggen (Ludwig Institute for Cancer Research, Brussels Branch, UCL, Belgium) for his useful discussions and critical review of the manuscript.
Supported by grants no. 3.0109.96, no. G.0157.99, and no. G.0313.01 from the Fund for Scientific Research, Flanders, Belgium; by grants from the Scientific Committee of the Fortis Bank-financed Cancer Research; and from the Belgian Federation against Cancer. P.P. is holder of an Institute for Science and Technology (IWT) grant.
V.F.I.V.T and P.P. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Zwi N. Berneman, Laboratory of Experimental Hematology, Antwerp University Hospital (UZA), Wilrijkstraat 10, B-2650 Edegem, Belgium; e-mail: zwi.berneman@uza.uia.ac.be.