Abstract
In 1999 a working group of the World Health Organization (WHO) published a revised classification for myelodysplastic syndromes (MDS): RA, RARS, refractory cytopenia with multilineage dysplasia (RC+Dys), RAEB I and II, del (5q) syndrome, and MDS unclassifiable. Chronic myelomonocytic leukemia (CMML) and RAEB-t were excluded. Standard French-American-British (FAB) and new WHO classifications have been compared in a series of patients (n = 431) from a single center, analyzing morphologic, clinical, and cytogenetic data. According to the WHO findings, dysgranulocytopoiesis or dysmegakaryocytopoiesis only were found in 26% of patients with less than 5% medullary blasts. These patients are thus unclassified and should remain in the subgroups RA and RARS. Splitting of heterogeneous RAEB into 2 subgroups according to blast count was supported by a trend to a statistically significant difference in the single-center study population. Patients with CMML whose white blood cell counts are above 13 000/μL may be excluded from the MDS classification, as warranted by WHO, but a redistribution of patients with dysplastic CMML according to medullary blast count leads to more heterogeneity in other WHO subgroups. Although the natural courses of RAEB-T and acute myeloid leukemia (AML) with dysplasia are different, comparable median survival durations after treatment in patients with RAEB-T and AML were in favor of the proposed 20% medullary blast threshold for AML. The homogeneity of subgroups was studied by evaluating prognostic scores. A significant shift into lower IPSS risk groups was evident in the new classification. These data cannot provide evidence for the new WHO proposal, which should not be adopted for routine clinical use at present. Some of its aspects can provide a starting point for further studies involving refined cytogenetics and clinical results.
Introduction
Myelodysplastic syndromes (MDS) are clonal hematologic stem cell disorders characterized clinically and morphologically by ineffective hematopoiesis. The natural history of these diseases ranges from a chronic course that may span years to a rapid course toward leukemic progression. Unfortunately, the nomenclature and classification systems used to describe these conditions are cumbersome and contentious. These diseases were initially described as preleukemias; then, in 1976, the French-American-British (FAB) group renamed these disorders dysmyelopoietic syndromes.
In 1982, this same study group proposed a classification based on morphologic features in blood and bone marrow, namely medullary and peripheral blast cell count, ringed sideroblasts, number of monocytes in peripheral blood, and Auer rods.1 Five subgroups with significantly different prognoses were established: refractory anemia (RA), RA with ringed sideroblasts (RARS), RA with excess of blasts (RAEB), RAEB in transformation (RAEB-T), and chronic myelomonocytic leukemia (CMML). For nearly 2 decades this classification served as the standard for the evaluation of MDS, though further characteristics of MDS subtypes—for example, hypoplastic MDS,2 hypercellular MDS, and MDS with bone marrow fibrosis—were not considered by the FAB classification.3 In addition, some studies provided evidence that patients with CMML and white blood cell (WBC) counts above 13 000/μL had hematologic and clinical characteristics of myeloproliferative disorders.4-6 Other analyses showed that the entities defined by the FAB group are heterogeneous, mostly in terms of prognosis but also with regard to morphologic features.3 7-12
In 1999, the World Health Organization (WHO) published a revised classification of MDS.13 The definitions of RA and RARS became more consistent and included the presence of dysplastic features in the erythroid lineage only. At the moment RAEB remains unchanged, though some investigators suggest further discrimination into RAEB I with 5% to 10% and into RAEB II with 11% to 20% blasts in the bone marrow. Three new subgroups were incorporated: (1) refractory cytopenia with multilineage dysplasia (RC+Dys), which is equivalent to RA or RARS in the FAB classification with the presence of dysplastic features in 2 or 3 cell lineages; (2) del (5q) syndrome, characterized by dysplastic features in the erythroid lineage only, thrombocytosis, and hypolobulated micromegakaryocytic hyperplasia (an isolated interstitial deletion of the long arm of chromosome 5 is now a distinct entity); and (3) MDS unclassifiable (MDS unclass). Two other FAB subgroups have been excluded from the new MDS classification: RAEB-T, because of similarities in biologic function and treatment strategies with acute myeloid leukemia (AML), and CMML, because of its close relation to myeloproliferative diseases. CMML, together with atypical chronic myeloid leukemia (aCML) and the juvenile form of CMML, JMML, is now part of a new disease group called myelodysplastic/myeloproliferative diseases, and RAEB-T ceases to exist because of the new threshold of 20% blasts in the bone marrow set for AML.
In this study we provide a comparison of the FAB and WHO classifications by evaluating data from 431 well-documented patients with primary MDS whose disease was diagnosed at our institution between 1976 and 1999.
Patients and methods
All patients with primary MDS (n = 431) that was diagnosed between 1976 and 1999 at the Department of Hematology and Oncology of the Hanusch Hospital in Vienna were subjected to a retrospective analysis. Patients with secondary MDS were excluded from this study. Care was also taken to eliminate other borderline diseases such as aCML, paroxysmal nocturnal hemoglobinuria, and reactive bone marrow changes. Diagnosis was made by cytomorphologic and histologic methods according to the proposals made by the FAB group in 1982.1Dysplasia of the 3 cell compartments in the bone marrow was assessed according to published criteria—dyserythropoiesis, the presence of multinuclearity, nuclear fragments, cytoplasmic abnormalities, or megaloblastoid changes; dysgranulopoiesis, the hypolobulation (including pellagroid forms) and hypogranulation of granulocytes; dysmegakaryopoiesis, the presence of micromegakaryocytes or (large) mononuclear forms. Dysplasia in one cell lineage was diagnosed if at least 50% of the examined cells of this lineage had dysplastic features. All patients were reclassified according to the WHO proposals mentioned above.13
Patients were evaluated for clinical features and for blood and bone marrow parameters at the time of diagnosis and were followed up for survival and leukemic progression. In most patients, data for cytogenetic bone marrow examinations was also available. Cytogenetic analysis was carried out according to standard procedures at our institution using the tri-staining method for simultaneous production of fluorescent R and C bands.14 Whenever possible, 20 or more metaphases from each patient were analyzed to demonstrate the clonal nature of the aberrations. Analysis was performed according to the international system for human genetic nomenclature.15
We calculated prognostic scores for both classification systems using the following scoring systems: international score (IPSS) for all patients with WBC counts below 12 000/μL and available cytogenetics16 and the prognostic index (PI) score based on cytogenetics only.17
Data were given as counts, percentages, medians, ranges, and the Kendall τ rank correlation test b values. Distributional differences regarding nominal variables were tested for by the χ2 test in the case of 2 × 2 tables, including Yates correction for continuity. Correlations of ordered categorical data were tested by the Kendall τ rank correlation test b and the corresponding significance test. Survival curves were based on the Kaplan-Meier product limit estimate, and the Mantel-Cox log rank test was included.18 19 All reported P values corresponded to 2-sided hypotheses, and values less than or equal to .05 were regarded as significant. In view of the exploratory nature of the study, significant results were interpreted in a descriptive way, and no correction for multiple testing was applied.
Results
Evaluation of patients according to FAB MDS
According to the FAB group criteria, 431 patients with MDS diagnosed at our institution were classifiable as follows: 142 (33%) patients with RA, 47 (11%) patients with RARS, 92 (21%) patients with RAEB, 51 (12%) patients with RAEB-T, and 99 (23%) patients with CMML. This distribution compared well with other international MDS databases, except for a lower frequency of RARS and a considerably higher incidence of CMML in our study population. Major clinical and hematologic data of the different subgroups at time of diagnosis are shown in Table 1. The median age of 73 years (range, 31-92 years) was as expected. The sex ratio was close to 1:1, with remarkably slightly more female patients. Cytogenetic analysis was carried out for 273 (64%) patients; 128 (47%) had normal (diploid) karyotypes. Diploid karyotypes were seen in half the evaluable patients in each subgroup, except in the RAEB-T cohort, which had only 38% normal cytogenetics. Groups at lower risk (patients with RA and RARS) had median survival times of 66 and 73 months, respectively, and groups at higher risk had median survival times as follows: RAEB, 15 months; RAEB-T, 9 months; and CMML, 24 months. Median preleukemic durations showed the same trend and the same frequency of transition to overt AML—25% for RA, 16% for RARS, 38% for RAEB, 66% for RAEB-T, and 46% for CMML (Table 1).
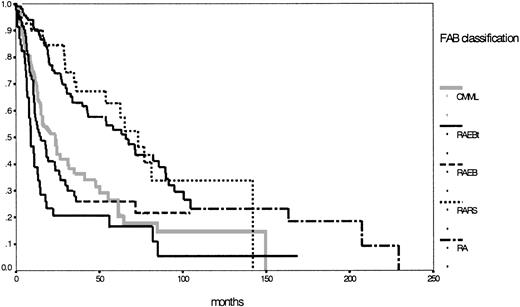
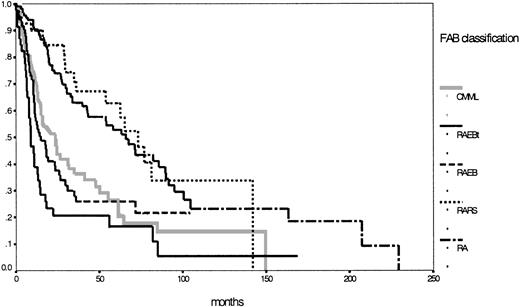
According to the IPSS definition, 222 patients were evaluable with 58% in the lower risk groups (low, 23%; intermediate 1, 35%) and 42% in the higher risk groups (intermediate 2, 23%; high, 19%). Details for the different subgroups are shown in Table 1. Figure1 shows the corresponding survival curves for the 5 MDS entities according to the FAB classification. Two different risk groups can easily be discriminated—one lower, incorporating RA and RARS, and one higher, with the 3 other entities according to the FAB proposal (P < .01).
Comparison of Kaplan-Meier estimates of overall survival in MDS classified according to the FAB proposal (n = 431) (P < .01).
Subgroups: RA (n = 142), RARS (n = 47), RAEB (n = 92), RAEB-T (n = 51), CMML (n = 99).
Comparison of Kaplan-Meier estimates of overall survival in MDS classified according to the FAB proposal (n = 431) (P < .01).
Subgroups: RA (n = 142), RARS (n = 47), RAEB (n = 92), RAEB-T (n = 51), CMML (n = 99).
Evaluation of patients according to WHO-MDS
In adopting the WHO proposals, only 281 (65%) patients remained classifiable, 150 patients with RAEB-T or CMML were excluded, and all RAEB-T cases were classified because of a blast cell count in the bone marrow between 20% and 30%. The new distribution included 43 patients with RA, 1 patient with del (5q) syndrome, 4 patients with RARS, 91 patients with RC+Dys, 50 patients with MDS unclassifiable, and 92 patients still with RAEB, of which 50 forms were RAEB I and 42 were RAEB II. Striking findings were the low frequency of del (5q) syndrome and RARS and the considerably high incidence of MDS unclassifiable. These phenomena were attributed to a significant number of patients with RA or RARS defined by the FAB classification, with presence of dysplastic features in only one cell lineage other than the erythroid and less than 5% medullary blast cells. Strictly according to the WHO definition, these patients were unclassifiable and were thus assigned to the category MDS unclassifiable. Major clinical and hematologic data of the different subgroups at the time of diagnosis are shown in Table2. The median age was 73 years (range, 31-92 years); age distribution and sex ratio were as expected and compared well with those in the FAB classification. Cytogenetic data were available for 172 (61%) patients; 81 (47%) had normal cytogenetics. Diploid karyotypes were seen in approximately half the evaluable patients in each relevant subgroup, except in the MDS unclassifiable cohort in which only 39% had normal cytogenetics.
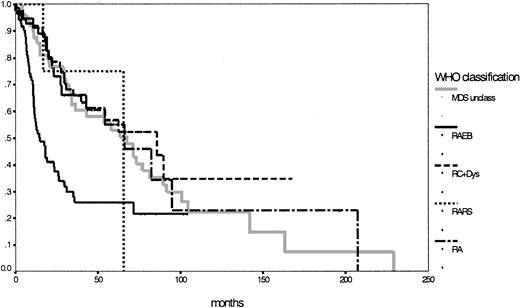
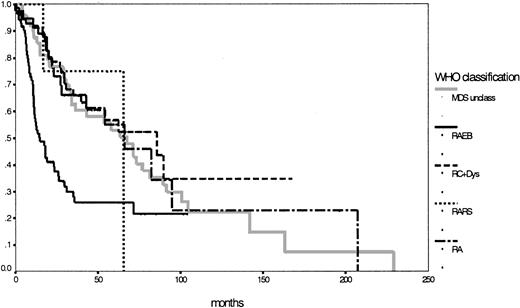
Because of the low number of patients, the subgroups with RARS and del (5q) syndrome were ineligible for statistical evaluation of survival and preleukemic duration. Remarkably, the subgroup with refractory cytopenia with multilineage dysplasia had a longer median survival duration (86 months) than the assumed low-risk group with RA (66 months; P = .68). Although MDS unclassifiable had a median survival time of 67 months, the unchanged subgroup with RAEB still had a median survival duration of 15 months. Survival time differences between RAEB I (18 months) and RAEB II (12 months) were not statistically significant, but P = .08 indicated a certain trend toward significance. Concerning the transition to AML, striking features were the low rate of 11% for the RC+Dys group (compared to 26% for the RA group; P = .02) and the high rate of 44% for the patients defined as MDS unclassifiable; of interest also was the significant difference between RAEB I and RAEB II (28% vs 52%;P = .03) (Table 2). The IPSS, according to its definition,16 could be used to evaluate 153 patients with 76% in the lower risk groups (low 31%; intermediate-1, 45%) and 24% in the higher risk groups (intermediate-2, 19%; high, 5%). Details for the different subgroups are shown in Table 2. Figure2 shows the corresponding survival curves for the 5 MDS entities according to the WHO proposal (P < .01; no survival curve for del (5q) syndrome was calculated). Based on survival curves alone, the subgroups according to the WHO classification, apart from RAEB (or RAEBI and RAEBII) were similar (P = .90).
Comparison of Kaplan-Meier estimates of overall survival in MDS classified according to the WHO proposal (n = 281) (P < .01).
Subgroups: RA (n = 43), RARS (n = 4), RC+Dys (n = 91), RAEB (n = 92), MDS unclass (n = 50).
Comparison of Kaplan-Meier estimates of overall survival in MDS classified according to the WHO proposal (n = 281) (P < .01).
Subgroups: RA (n = 43), RARS (n = 4), RC+Dys (n = 91), RAEB (n = 92), MDS unclass (n = 50).
Evaluation of patients with RAEB-T and CMML
RAEB-T (n = 51).
Patients with RAEB-T were incorporated in the AML group because the threshold for the blast cell count in the bone marrow was reduced from 30% to 20%. At our institution, 16 of 51 (31%) patients with RAEB-T were treated with AML-style induction therapy and had a median survival duration of 11 months. To evaluate this cohort retrospectively, we compared these patients with 448 unselected AML patients (de novo, or with prior MDS phase and therapy-related), 258 (58%) of whom underwent induction chemotherapy. Median overall survival duration was 6 months for all AML patients and 11 months for the treated subgroup (not significantly different from the respective RAEB-T subgroups). Among the patients with AML, 138 (31%) had a previous MDS phase. Median survival duration of these patients was 6 months untreated (compared with RAEB-T, P < .01) and 8 months in 52 (38%) patients receiving AML treatment (compared with RAEB-T, P = .13). These results are summarized in Table3.
CMML (n = 99).
In the first publication, the CMML subgroup was excluded from the WHO classification of MDS. Recently, WHO proposed a refined classification by which CMML with WBC counts lower than 13 000/μL would now be considered MDS and would be classified according to the medullary blast cell count and the number of dysplastic cell lineages in the bone marrow.20 In our CMML cohort of 99 patients, 64 had the dysplastic type of disease and 35 had the proliferative type. Fifty-two patients with dysplastic disease were reclassifiable as follows: 5 patients with RC+Dys, 46 patients with RAEB (of whom 6 had RAEB I and 40 had RAEB II), and 1 patient with MDS unclassifiable. As expected, the RA and RARS subgroups did not change. Concerning the median survival duration for each subgroup, no significant differences were observed.
Analysis of a possible redistribution of unclassifiable MDS and CMML
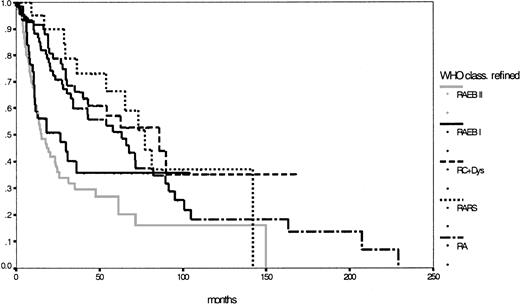
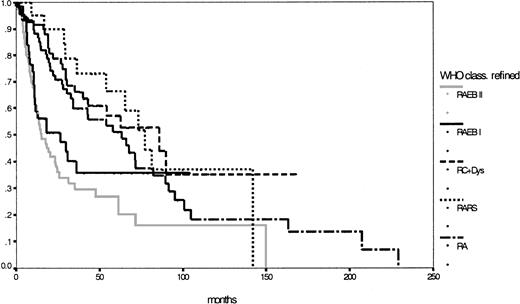
Taking into consideration patients with CMML whose WBC counts were below 13 000/μL and redistributing patients with unclassifiable MDS with less than 5% blast cells in the bone marrow and dysplastic features in only one cell lineage except the erythroid, one yielding 33 RA and 17 RARS patients, 333 patients were classifiable according to the modified WHO proposal: 76 patients with RA (median survival, 63 months), 21 patients with RARS (median survival, 77 months), 96 patients with RC+Dys (median survival, 86 months), 138 patients with RAEB (median survival, 18 months), of whom 56 with RAEB I and 82 with RAEB II (median survival, respectively, 27 and 15 months;P = .23), 1 patient with del (5q) syndrome and 1 patient with MDS unclassifiable. Finally, Table 4shows the major features of this refined WHO classification and Figure3 shows the corresponding survival curves, which, except for RAEB I and RAEB II, are still close to each other and lack statistical significance in this population (P = .42).
Comparison of Kaplan-Meier estimates of overall survival in MDS classified according to the refined WHO proposal (n = 333) (P < .01).
Subgroups: RA (n = 76), RARS (n = 21), RC+Dys (n = 96), RAEB I (n = 56), RAEB II (n = 82).
Comparison of Kaplan-Meier estimates of overall survival in MDS classified according to the refined WHO proposal (n = 333) (P < .01).
Subgroups: RA (n = 76), RARS (n = 21), RC+Dys (n = 96), RAEB I (n = 56), RAEB II (n = 82).
Discussion
For nearly 2 decades, the FAB classification of myelodysplastic syndromes was the criterion standard and was essential for clinicians, morphologists, and pathologists working in and investigating the field of MDS. Nearly all studies performed on MDS biology, morphology, treatment, and prognosis were based on the FAB classification. Nevertheless, it was obvious that this classification had some disadvantages, which were addressed by the WHO proposals for a new classification. The comparison was performed by evaluating data of a single-center MDS cohort that had characteristics similar to those of other large MDS study populations, except for a slightly higher percentage of women. However, no significant differences in outcome existed between the male and female patients, even in subgroup analysis (data not shown).
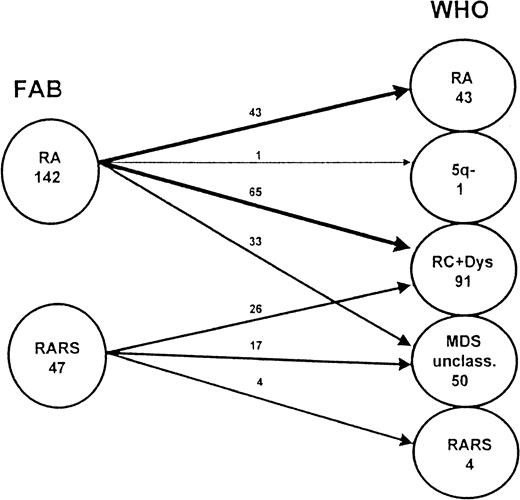
The FAB subgroups were heterogeneous. Several studies provided evidence that the division of RA (and RARS) into RA (and RARS) with dysplastic features in the erythroid cell lineage only and into refractory cytopenia with dysplasia in 2 or 3 cell lineages was useful in terms of prognosis.11,12 21 Details of the splitting, as warranted by the WHO classification, are shown in Figure4. Our data do not confirm these results. There was no significant difference in median overall survival time between RA and RC+Dys classified according to the WHO proposal. In contrast to our expectations, the transition rate into AML was considerably lower in RC+Dys than in RA (Table 2). Thus, our data cannot support the proposed splitting of RA and RARS according to FAB in the WHO classification.
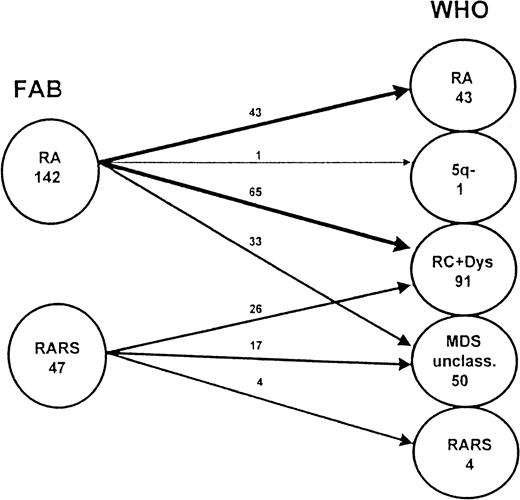
Splitting of RA and RARS (according to FAB) as warranted by the WHO classification.
This figure shows details of the redistribution of patients with RA and RARS classified according to the FAB proposal in the new proposal made by WHO. These patients are now classified among the subgroups RA, RARS, RA with multilineage dysplasia (RC+Dys), del (5q) syndrome, and MDS unclassifiable.
Splitting of RA and RARS (according to FAB) as warranted by the WHO classification.
This figure shows details of the redistribution of patients with RA and RARS classified according to the FAB proposal in the new proposal made by WHO. These patients are now classified among the subgroups RA, RARS, RA with multilineage dysplasia (RC+Dys), del (5q) syndrome, and MDS unclassifiable.
A considerable number of patients in our study population had blast cell counts in the bone marrow that were lower than 5% and dysplastic features in only one cell lineage, other than the erythroid one. Using the subtype definitions according to the first WHO proposal, these patients did not fit into the RA, RARS, or RC+Dys subgroup and remained unclassifiable, though they are still closely related to these 3 entities. To reduce the number of patients with MDS unclassifiable, it would be practicable to include patients with only dysplasia of granulocytopoiesis or megakaryocytopoiesis in the subgroups RA and RARS according to the WHO classification, as shown in Figure 4.
In the FAB classification, the RAEB entity was heterogeneous, with blast cell counts in the bone marrow ranging from 5% to 20%. Because this medullary feature is the most important single prognostic parameter and several analyses showed that a threshold of 10% is of prognostic importance, the splitting into RAEB I and RAEB II seems to be justified.16,22 23 Our results in this respect indicate a trend toward significance. Furthermore, the significant difference in transition rate into overt AML suggests that dividing RAEB into 2 subtypes according to the medullary blast cell count may be reasonable.
CMML, a heterogeneous entity, shares features with myelodysplastic syndromes and myeloproliferative disorders. However, until the WHO proposals, no agreement could be reached whether it should be assigned to the group of myeloproliferative diseases or myelodysplastic syndromes. Several studies were performed to distinguish a dysplastic and a proliferative variant of CMML, with an arbitrary leukocyte threshold of 13 000/μL. Analysis of our study group provided significant differences between both entities in terms of overall survival and other features (eg, lactate dehydrogenase).4-6,24 We therefore agree that patients with CMML whose WBC counts are above 13 000/μL should be removed from the MDS group and assigned to the group of myeloproliferative disorders. However, in our opinion, redistribution of patients with dysplastic CMML among the other subgroups, as warranted by the WHO group, would lead to a much more heterogeneous classification.20 The dysplastic variant of CMML should remain a distinct entity among the myelodysplastic syndromes.
RAEB-T was removed from the MDS classification according to WHO because of similarities with AML in biologic function and treatment strategies.25 As shown in the “Results” section, median survival times are identical in RAEB-T and AML and indicate similarity in disease prognoses. However, in subgroup analysis, patients with RAEB-T had significantly longer median survival times than AML patients with dysplasia (treated and untreated, Table 3), indicating a different stage of disease, as reflected in the original FAB classification. Comparing only treated patients, survival data are not different. The use of other prognostic instruments can be helpful for further decision making.
Prognostic scoring systems are important for risk assessment at time of diagnosis, especially if classification systems remain insufficient in this respect, and they are necessary for risk stratification in clinical trials. The most recent and powerful instrument established for MDS classified according to the FAB proposal is the IPSS, primarily based on medullary blast cell count, number of cytopenias, and cytogenetics.16 It is apparent that comparing both classifications in this respect represents a significant shift to the lower risk groups according to the IPSS. Although in the FAB classification 57% of the patients were in the lower risk (low and intermediate-1) groups and 43% were in the higher risk (intermediate-2 and high) groups, in the WHO classification 76% of the patients belonged to the lower risk groups and only 24% to the higher risk groups. Taking into consideration the refined WHO proposal, the results were still in favor of the lower risk groups, with 69% versus 31%. This phenomenon can easily be explained: in the higher risk groups, RAEB-T and (partially) CMML are removed. Consequently, this would have implications for future clinical trials, especially in high-risk MDS, because of a significantly lower number of eligible patients. Furthermore, new prognostic models, including cytogenetic analysis, for the changed AML patient population are warranted.
Thus, the role of the IPSS as the predominant prognostic instrument seems to be uncertain in the new WHO classification. As mentioned above, it was established by evaluating data of patients classified according to the FAB proposal. Given that inclusion criteria (especially the upper limit for the medullary blast cell percentage) have been changed in the WHO classification, the IPSS maintains its validity even if its use in the WHO remains unclear.
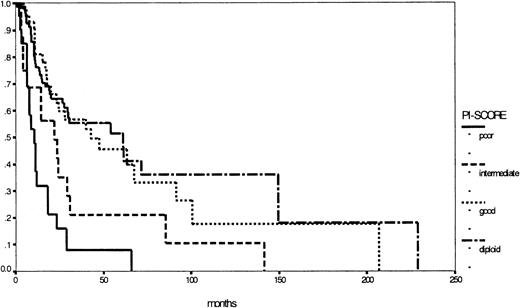
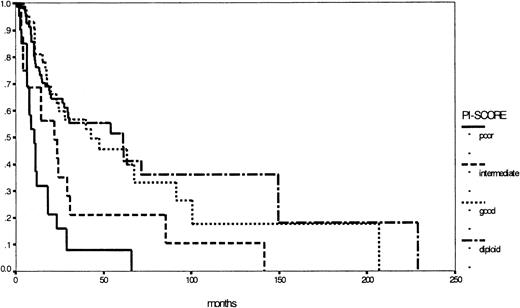
Nevertheless the major prognostic importance of cytogenetics in MDS is evident and could be confirmed by our study group by establishing the PI score.17,23,26,27 Based on cytogenetics only, it is easily applicable in both classification systems: Figure 5 illustrates the corresponding survival curves, supporting once more the importance of cytogenetics in MDS. In this respect, the WHO proposal took into account the well-known del (5q) syndrome, characterized by refractory anemia (FAB) with thrombocytosis, hypolobulated micromegakaryocytic hyperplasia, and an isolated interstitial deletion of the long arm of chromosome 5 as a distinct entity. According to this definition, the del (5q) syndrome is a rare disease with good prognosis.28 29 In contrast, a subgroup of all patients with an isolated deletion of the long arm of chromosome 5, excluding the above-mentioned criteria, would be too heterogeneous, indicating against the formation of a distinct subgroup. Therefore, prospective multicenter studies with detailed evaluation of cytogenetics, preferably metaphase and interphase analyses, are warranted to define other low- or high-risk entities based on structural or numerical aberrations, such as the 7q syndrome in childhood MDS.
Comparison of Kaplan-Meier estimates of overall survival in patients with MDS (considered by the FAB proposal) according to the PI score (n = 273) (P < .01).
Subgroups: diploid (n = 129), good risk (n = 35), intermediate risk (n = 62), poor risk (n = 47).
Comparison of Kaplan-Meier estimates of overall survival in patients with MDS (considered by the FAB proposal) according to the PI score (n = 273) (P < .01).
Subgroups: diploid (n = 129), good risk (n = 35), intermediate risk (n = 62), poor risk (n = 47).
Our data from a single-center experience cannot provide clear evidence that the new WHO proposal improves the standard FAB classification. The original goal of the WHO study group was to establish a classification, for the use of pathologists and clinicians, that included morphologic, biologic, and clinical features of MDS. The new WHO proposal has to be validated for all these aspects in unselected patient populations. Because of the heterogeneity of MDS, larger multicenter studies will be needed to finally resolve the issue of a new classification for these disorders. At present, with respect to our data, adopting the WHO proposal as a final classification seems not to be justified. Nevertheless, some aspects of this proposal could be a starting point for further investigations toward a classification for MDS.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Thomas Nösslinger, Third Medical Department for Hematology and Oncology, Hanusch Hospital, Heinrich Collinstrasse 30, A-1140 Vienna, Austria; e-mail:thomas.noesslinger@wgkk.sozvers.at.