Abstract
One hundred nine patients with hematologic malignancies, undergoing bone marrow transplants (BMT) from unrelated donors, were randomized in 2 consecutive trials to receive or not to receive antithymocyte globulin (ATG) in the conditioning regimen, as follows: (A) 54 patients (median age, 28 years; 39% with advanced disease) were randomized to no ATG (n = 25) versus 7.5 mg/kg rabbit ATG (Thymoglobulin; Sangstat, Lyon, France) (n = 29) ; (B) 55 patients (median age, 31 years, 71% with advanced disease) were randomized to no ATG (n = 28) versus 15 mg/kg rabbit ATG (n = 27). Grade III-IV graft-versus-host disease (GVHD) was diagnosed in 36% versus 41% (P = .8) in the first and in 50% versus 11% (P = .001) in the second trial. Transplant-related mortality (TRM), relapse, and actuarial 3-year survival rates were comparable in both trials. In fact, despite the reduction of GVHD in the second trial, a higher risk for lethal infections (30% vs 7%; P = .02) was seen in the arm given 15 mg/kg ATG. Extensive chronic GVHD developed overall more frequently in patients given no ATG (62% vs 39%;P = .04), as confirmed by multivariate analysis (P = .03). Time to 50 × 109/L platelets was comparable in the first trial (21 vs 24 days; P = .3) and delayed in the ATG arm in the second trial (23 vs 38 days;P = .02). These trials suggest that (1) 15 mg/kg ATG before BMT significantly reduces the risk for grade III-IV acute GVHD, (2) this does not translate to a reduction in TRM because of the increased risk for infections, and (3) though survival is unchanged, extensive chronic GVHD is significantly reduced in patients receiving ATG.
Introduction
The role of antithymocyte globulin (ATG) in preventing acute graft-versus-host disease (GVHD) has been explored in the past decades. A report by the International Matched Unrelated Study Trial was published in 1993; in this study patients, receiving transplants from unrelated donors (UD) were compared with prospectively selected patients receiving transplants from matched siblings at the same centers.1 Favorable predictors of survival were the center experience and the use of so-called serotherapy before bone marrow transplants (BMT), either as monoclonal antibodies or as antilymphocyte–antithymocyte globulin (ALG–ATG).1 During the past 10 years, some centers have reported encouraging results with the use of ATG in the conditioning regimen,2-6 whereas others have reported equally good results without the use of ATG.7 8 This has led some centers to advocate the use of ATG before all transplantations using UDs and others to state that ATG is not indicated in transplants from UDs. Strangely enough, there have been no randomized studies to test whether one or the other hypothesis is correct and whether there may be a dose effect.
We here report the results of 2 consecutive randomized studies testing the effect of ATG in the conditioning regimen, given in 2 different doses, compared with patients receiving no ATG. The primary end point of the 2 studies was acute GVHD grade III-IV.
Materials and methods
Unrelated donors
All unrelated donors were selected through the Italian Bone Marrow Donor Registry. Donors were matched by serology for HLA-A and HLA-B, including split antigens, and by high-resolution molecular biology for HLA-DRB1. Centers participating in the trial were accredited for transplantations using unrelated donors by Gruppo Italiano Trapianti di Midollo Osseo (GITMO). All patients received unmanipulated bone marrow cells.
Eligibility
Patients with a hematologic malignancy and a matched unrelated donor were eligible for this trial. Almost all (95%) patients were age 18 years or older.
Randomization
Patients were randomized centrally and were stratified by center, age (younger than 35, 35 or older), and phase of disease (first complete remission/chronic phase, beyond first complete remission/chronic phase).
Conditioning regimen
All patients received cyclophosphamide and total body irradiation. The 4 participating centers used 4 different modalities of total body irradiation: 2 Gy × 2 × 3, 3.3 Gy × 3, 120 Gy × 11, and 10 Gy single dose. Each center used the same conditioning regimen throughout the study. All patients received cyclosporin A from day −7 to day −2, at the dose of 1 mg/kg as a continuous intravenous infusion. Patients were cared for according to established institutional protocols in 4 GITMO centers: Genoa, Firenze, Pavia, and Pescara. GVHD prophylaxis was cyclosporin A 2 mg/kg per day cyclosporin A from day −1 until day 20, then 6 to 10 mg/kg per day by mouth for at least 1 year. Methotrexate was also given at 15 mg/m2 on day 1 and 10 mg/m2 on days 3, 6, and 11. All patients received ciprofloxacin and fluconazole orally from day −7 and high-dose immunoglobulins (400 mg/kg) weekly from day −7 until day 100. Prophylaxis for cytomegalovirus (CMV) infections was 500 mg/m2 acyclovir every 8 hours from day −7 to day 30 and then oral acyclovir until 6 months after transplantation. One center used 30 mg/kg foscarnet twice a day for all patients from day −7 to day 30 and then 90 mg/kg foscarnet 5 days per week until day 100.
Trial 1
Patients were enrolled between December 15, 1995 and December 15, 1997 and were randomized to receive 3.75 mg/kg rabbit ATG (thymoglobulin, Sangstat, Lyon, France) as a slow intravenous infusion (lasting 12 hours) on days −4 and −3 (total dose, 7.5 mg/kg) or no ATG. Before the infusion of ATG, patients were premedicated with 1 mg/kg intravenous prednisolone.
Trial 2
Patients were enrolled between December 19, 1997 and July 12, 2000 and were randomized to receive 3.75 mg/kg ATG as a slow intravenous infusion (lasting 12 hours) on days −5, −4, −3, and −2 (total dose, 15 mg/kg) or no ATG. Before the infusion of ATG, patients were premedicated with 1 mg/kg intravenous prednisolone.
Statistical methods
Each study was designed with a planned sample size of 64 patients per arm, for a power of 80% against the hypothesis of a 20% absolute reduction from 35% of patients developing grade III-IV acute GVHD (significance level, Student 1-tailed t test,P = .05). Conditional power estimates,9 based on various assumptions concerning the underlying probability distributions, were used for interim analyses of trial 1 to determine whether the probability of obtaining a significant result at the end of the study was sufficient to warrant its continuation.
Comparisons of proportions were based on the χ2 test or the Fisher exact test, as appropriate. Survival curves in each study group were computed using the Kaplan-Meier10 method and were compared by means of the standard log-rank procedures. All tests were 2-tailed.
Results
Tolerance of ATG and organ dysfunction
The tolerance of the ATG infusion was good, given that patients were premedicated and the infusion was slow. Chills and fever, when present, were treated with antihistamine and reduced speed of the ATG infusion. All patients completed the prescribed ATG dose. Organ function, as indicated by laboratory values on day 13 after transplantation, was comparable in the non-ATG/ATG patients in both trials. Data were available for 48 patients, 20 in trial 1 and 28 in trial 2. In particular, renal function was comparable (median blood-urea-nitrogen, 32 vs 25 mg/100 mL for non-ATG vs ATG patients in trial 1 and 29 vs 27 mg/100 mL for non-ATG vs ATG in trial 2) (median creatinine clearance, 0.9 vs 0.9 mg/100 mL for non-ATG vs ATG patients in trial 1 and 0.9 vs 1.0 mg/100 mL for non-ATG vs ATG patients in trial 2) as was liver function (bilirubin, 1.5 vs 0.9 mg/100 mL for non-ATG vs ATG patients in trial 1 and 0.85 vs 0.6 mg/100 mL for non-ATG vs ATG patients in trial 2) (γ-glutamyltransferase, 90 vs 48 IU for non-ATG vs ATG patients in trial 1 and 45 vs 43 IU for non-ATG vs ATG patients in trial 2). On day 50 after transplantation, laboratory test results were available again in 48 patients and showed comparable renal and liver function; there was significant more lymphopenia in the ATG arm than the non-ATG arm in trial 2 (0.230 vs 0.054 × 109/L; P = .02).
Trial 1
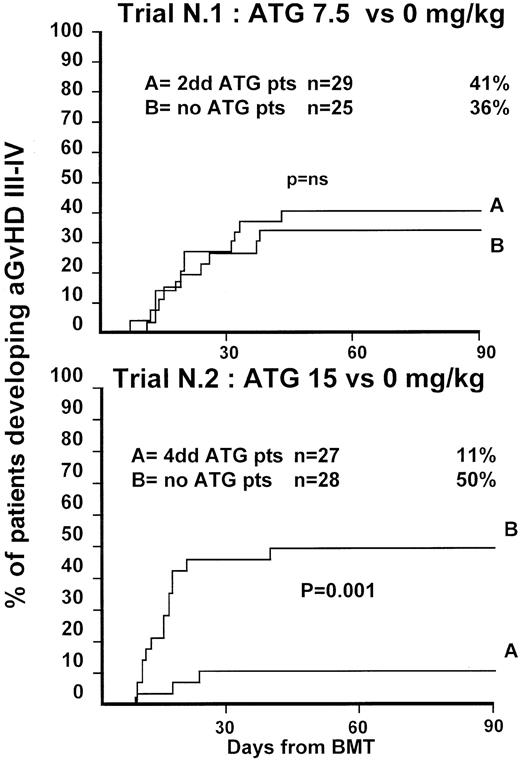
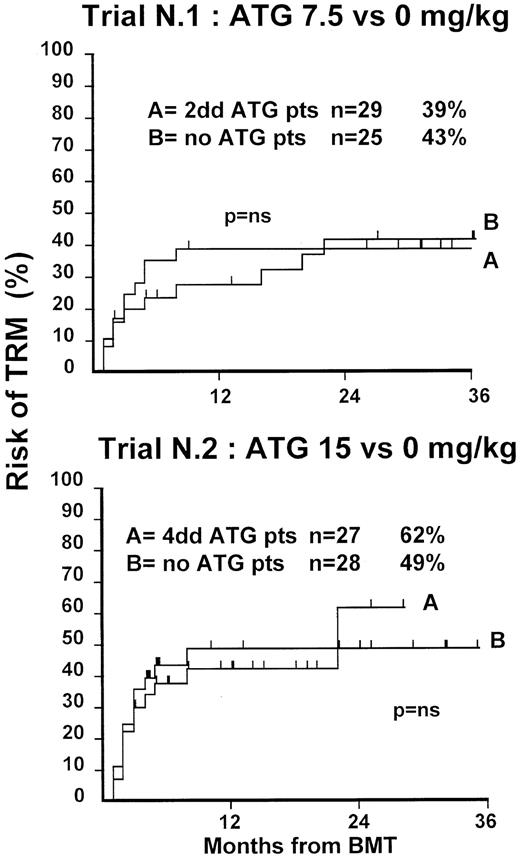
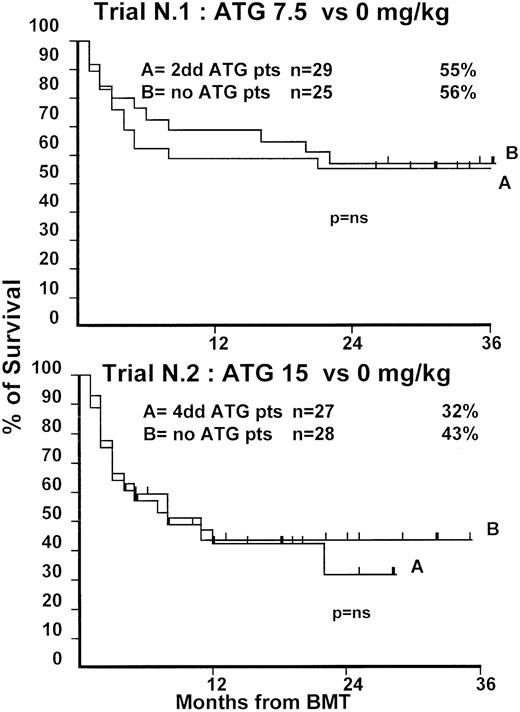
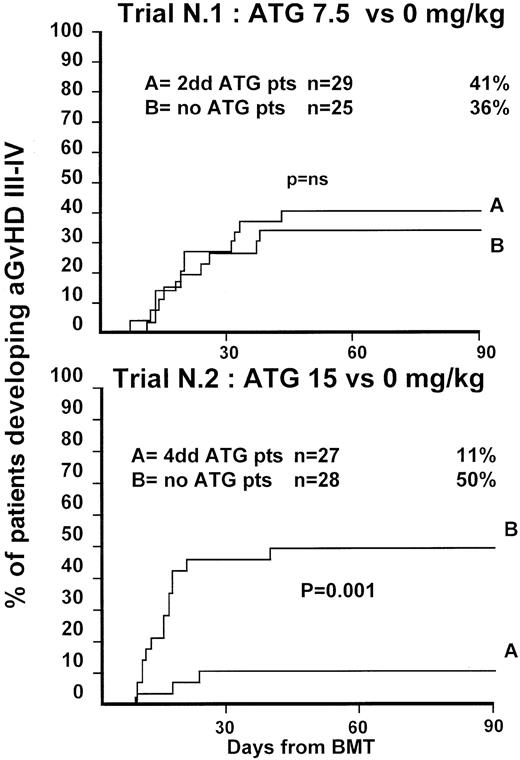
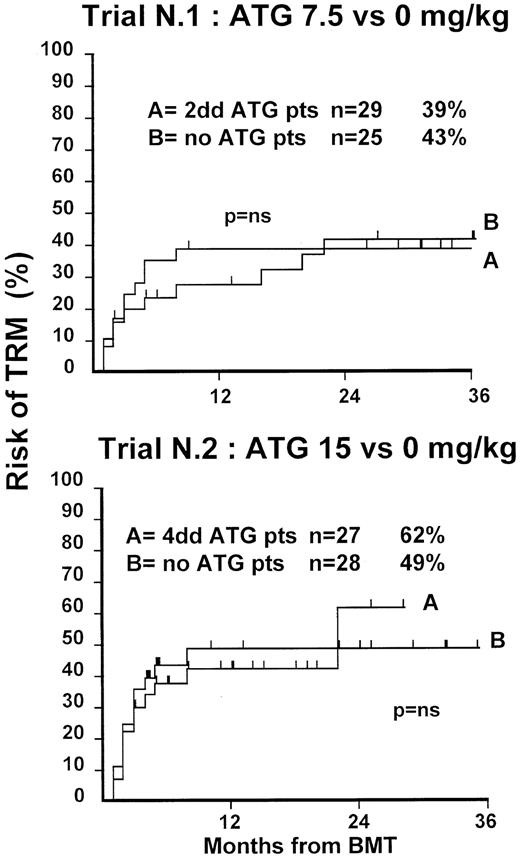
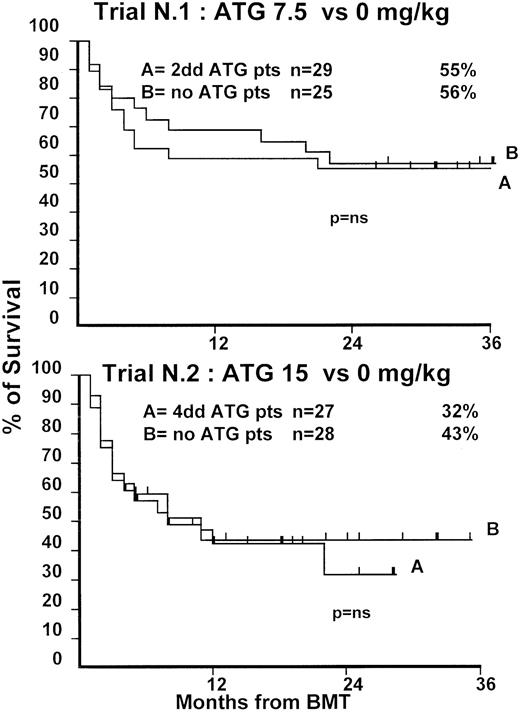
Fifty-four patients were randomized in this first trial, 25 not to receive ATG and 29 to receive 7.5 mg/kg ATG before transplantation. Clinical characteristics of these patients are outlined in Table1. Median age and phase of disease were comparable; most patients in both arms were in early phase (60% and 62%). The interval between diagnosis and treatment was longer (P = .03) in the ATG group (Table 1). Time to neutrophil engraftment was comparable in the 2 groups (P = .3), with 84% and 83% of patients achieving a neutrophil count of 0.5 × 109/L (Table2). Time to platelet engraftment was also comparable (P = .3), with 76% of patients achieving a platelet count of 50 × 109/L (Table 2). Acute GVHD grade III-IV developed in 36% and 41% of patients in the 2 arms (P = .8) (Figure 1), and acute GVHD grade II-IV developed in 72% and 69% (P = .6). CMV reactivation occurred in most patients in both arms (Table 2). At 3 years, the actuarial transplant-related mortality (TRM) rates were 39% and 43%, respectively (P = .7) (Figure 2), the actuarial survival rates were 55% and 56% (P = .8) (Figure 3), and the actuarial risk for relapse rates were 12% and 10% (P = .6).
Actuarial probability of acute GVHD grade III-IV in trial 1 (upper graph) and in trial 2 (lower graph).
Trial 1 compared 7.5 mg/kg antithymocyte globulin (ATG) (given over 2 days) to patients not receiving ATG. Trial 2 compared 15 mg/kg ATG (given over 4 days) to patients not receiving ATG. There was no difference in the risk for GVHD III-IV in trial 1 but a significant difference in trial 2.
Actuarial probability of acute GVHD grade III-IV in trial 1 (upper graph) and in trial 2 (lower graph).
Trial 1 compared 7.5 mg/kg antithymocyte globulin (ATG) (given over 2 days) to patients not receiving ATG. Trial 2 compared 15 mg/kg ATG (given over 4 days) to patients not receiving ATG. There was no difference in the risk for GVHD III-IV in trial 1 but a significant difference in trial 2.
Actuarial probability of TRM in trial 1 (upper graph) and in trial 2 (lower graph).
There is no difference in TRM for patients receiving or not receiving ATG in the 2 trials.
Actuarial probability of TRM in trial 1 (upper graph) and in trial 2 (lower graph).
There is no difference in TRM for patients receiving or not receiving ATG in the 2 trials.
Actuarial probability of survival in trial 1 (upper graph) and in trial 2 (lower graph).
There is no difference in survival for patients receiving or not receiving ATG in the 2 trials.
Actuarial probability of survival in trial 1 (upper graph) and in trial 2 (lower graph).
There is no difference in survival for patients receiving or not receiving ATG in the 2 trials.
Causes of death are outlined in Table 2; there were no major differences in patients dying of GVHD, infections, multi-organ failure, or leukemia. In December 1997, conditional power analyses based on the observed proportion of GVHD in the 2 arms, 36% and 41%, respectively, indicated a low probability (less than 30% under the most extreme hypotheses) that the study would detect a significant difference if the target sample size (128 patients) were reached. Patient accrual into this study was then stopped.
Trial 2
Fifty-five patients were randomized in the second trial, 28 not to receive ATG and 27 to receive 15 mg/kg ATG before transplantation. Clinical characteristics of these patients are outlined in Table 1. Median age of patients was comparable in the 2 arms. A minority of patients in both arms was in the early phase of disease (36% and 22%;P = .8). This reflects early data on increased GVHD in patients not receiving ATG. One year before the trial was closed, a review committee recommended that randomization be continued only of patients with advanced disease. Patient outcomes are outlined in Table2. Engraftment with 0.5 × 109/L neutrophils could be documented in 89% of patients with similar time to engraftment. Time to platelet engraftment was significantly longer in patients receiving ATG (38 vs 23 days; P = .02), with 61% and 60% of patients achieving a platelet count of 50 × 109/L. Acute GVHD grade III-V developed in 50% of the non-ATG and 11% for the ATG arm (P = .001) (Figure 1), and acute GVHD grade II-IV developed in 79% of the non-ATG and 37% of the ATG arm (P = .001) (Figure1). Based on these results, an external advisory board suggested the discontinuation of patient accrual in this study. Although this was not an interim analysis planned in the study protocol, the difference in the incidence of severe acute GVHD was judged sufficient to make it unethical to continue the study.
At 1 year, the actuarial TRM rates were 49% and 47%, respectively (P = .9) (Figure 2), the actuarial survival rate was 43% in each arm (P = .8) (Figure 3), and the actuarial risk for relapse rates were 18% and 36%, respectively (P = .8).
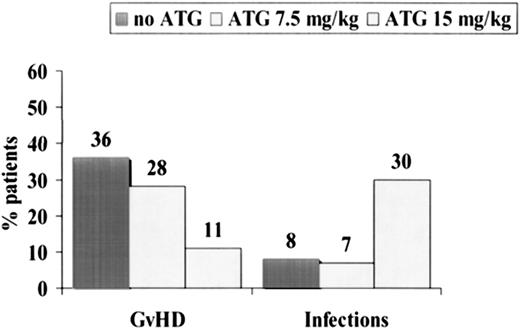
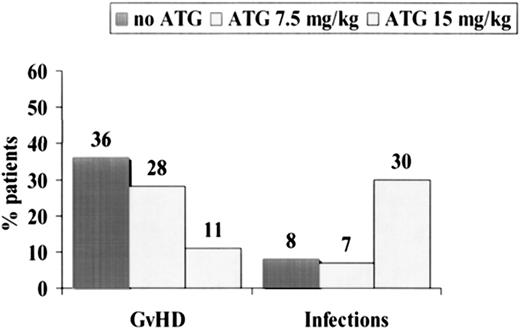
Causes of failure were as follows: in the non-ATG group, 39% of the patients died of acute GVHD and 7% of infections (Table 2). In the ATG group, the opposite was true: 30% of patients died of infections, and 11% died of acute GVHD (Table 2). The etiologies of the 8 lethal infections in the ATG group were bacterial infections/sepsis (n = 6) and fungal infection (n = 2). CMV reactivation rates were similar in the ATG and non-ATG groups (67% and 78%) (Table 2). When we pooled all patients together, we could show that acute GVHD was the cause of death in 19 (36%) of 53 patients randomized not to receive ATG (Figure 4), in 8 (28%) of 29 patients randomized to receive 7.5 mg/kg ATG, and in 3 (11%) of 27 patients randomized to received 15 mg/kg ATG (P = .06). Infections were the cause of death in 8%, 7%, and 30% (P= .01), respectively, of the same 3 groups of patients (Figure 4).
Causes of death in 109 patients randomized to receive no ATG (n = 53), 7.5 mg/kg ATG (n = 29), or 15 mg/kg ATG (n = 27).
Acute GVHD was the cause of death in 36% of patients not receiving ATG, 28% of patients receiving 7.5 mg/kg ATG, and 11% of patients receiving 15 mg/kg ATG. Infections were, on the contrary, more frequent in patients receiving high-dose ATG.
Causes of death in 109 patients randomized to receive no ATG (n = 53), 7.5 mg/kg ATG (n = 29), or 15 mg/kg ATG (n = 27).
Acute GVHD was the cause of death in 36% of patients not receiving ATG, 28% of patients receiving 7.5 mg/kg ATG, and 11% of patients receiving 15 mg/kg ATG. Infections were, on the contrary, more frequent in patients receiving high-dose ATG.
Chronic GVHD
Seventy-five (69%) of 109 patients were alive on day 100 and were at risk for chronic GVHD. In trial 1, extensive chronic GVHD was diagnosed in 65% of patients in the non-ATG group and in 38% of patients in the ATG group (P = .08). In trial 2, extensive chronic GVHD was diagnosed in 59% of patients in the non-ATG group and in 41% of patients in the ATG group (P = .3); of course, the follow up of patients in the second trial was shorter than in the first. Overall, 62% of patients not receiving ATG acquired extensive chronic GVHD compared with 39% of patients receiving ATG (P = .04).
Univariate analysis
We looked for variables predictive of acute GVHD and TRM in univariate analysis in all 109 patients. GVHD grade III-IV was significantly reduced in patients younger than 35 (P = .02) and in patients receiving ATG (P = .05). TRM was predicted by age (P = .003) and disease phase (P = .03). Median number of cells grafted was 3.2 × 108/kg. TRM was 47% versus 36% for patients receiving more than or less than 3.2 × 108/kg (P = .2). The advantage for the higher cell dose was more evident in patients with early-phase disease (TRM, 26% vs 50%; P = .09) than with advanced disease (TRM, 44% vs 44%; P = .9). There was an advantage for high cell dose, though not significant, in patients randomized not to receive ATG (TRM, 38% vs 47%; P = .5) or to receive ATG (TRM, 34% vs 47%).
Multivariate analysis
Phase of disease (early, advanced), patient age (continuous), TBI regimen (2 Gy × 2 × 3/others), cells dose infused × 108/kg (continuous), conditioning with ATG, CMV status of donor (CMV-positive, CMV-negative), and CMV status of recipient (CMV-positive, CMV-negative) were entered in a multivariate COX analysis with grade III-IV acute GVHD as an end point: ATG had a significant impact on acute GVHD when tested as ATG versus no ATG (P = .005) and when tested according to dose intensity (no ATG vs 7.5 mg/kg ATG vs 15 mg/kg ATG; P = .01). Similar variables were entered in a multivariate analysis on 73 patients alive and evaluable on day 100, with extensive chronic GVHD as an end point (yes vs no). ATG had a protective effect on patients with extensive chronic GVHD when entered as ATG versus non-ATG (P = .03) and when entered according to dose intensity (P = .05). ATG was not a predictor of TRM in multivariate analysis.
Event-free survival
Finally, patients were studied for event-free survival. Events included acute GVHD grade III-IV, extensive chronic GVHD, relapse, death from leukemia, and death from transplant-related causes. Actuarial event-free survival at 2 years was 20% in the non-ATG patients and 34% in the ATG patients in trial 1 (P = .6) and 15% versus 17% in the second trial (P = .8).
Discussion
We have explored the role of ATG in the conditioning regimen for patients undergoing transplantation from unrelated donors. Our results can be summarized as follows: (1) 15 mg/kg ATG in the conditioning regimen significantly reduced the risk for grade III-IV and II-IV acute GVHD; (2) this does not, however, translate to a reduction of TRM because of the increased risk for infections; and (3) survival was unchanged, but the incidence of chronic GVHD was reduced in patients receiving ATG. Reduction of severe (grade III-IV) acute GVHD was the primary end point of the 2 studies.
The first trial explored 2 days of ATG in the conditioning regimen, or a total dose of 7.5 mg/kg, compared with no ATG. We did not observe any reduction in severity of acute GVHD in the ATG arm after randomizing 54 patients, and the study was closed because it was unlikely to meet its primary end point.
The second trial compared 15 mg/kg ATG, or 4 days in the conditioning regimen, with no ATG. The difference of acute GVHD grade III-IV was already evident after 20 patients had been randomized; an external review panel suggested continuing with accrual of patients with advanced disease only, which is why most patients in the second trial are those with advanced disease. The strong and statistically significant reduction of GVHD grades III-IV and II-IV in patients receiving ATG, after randomizing 55 patients (fewer than half the planned number), suggested the trial could be closed. At that point, centers were becoming unwilling to randomize patients in the non-ATG arm. When the trial was closed, the actuarial risk rate for GVHD grade III-IV at 100 days was 50% for non-ATG patients versus 11% for ATG patients (P = .001). This indicates that ATG, at the dose of 15 mg/kg, strongly reduces the risk for severe GVHD in recipients of transplants from unrelated donors. It also suggests that there is a dose-dependent effect because 7.5 mg/kg ATG did not seem to modify significantly acute GVHD. It should be said that the 2 trials differed on the last day of ATG administration (day −3 for trial 1 and day −2 for trial 2), and we cannot exclude that this fact added to GVHD prevention in the 15 mg/kg ATG arm.
The second result of these 2 studies is that preventing severe acute GVHD does not modify TRM. This is a disappointing result and shows that reducing the severity of acute GVHD is insufficient to reduce transplant-related complications. The propensity of patients receiving 15 mg/kg ATG to contract severe infections is associated with significantly lower lymphocyte counts on day 50. In addition, there was a delay in platelet recovery for patients receiving high-dose ATG, which could only be explained in part by the larger number of patients with advanced disease and lower cell dose in this arm. We could have anticipated these result by looking at T-cell depletion programs, though they were not obtained within randomized trials. Patients receiving marrow depleted ex vivo of T cells experienced little GVHD, slower immune recovery, significant infectious complications, and no overall reduction in TRM.11 In vivo T-cell depletion produced similar results. Infections were the cause of death in 8% of patients not receiving ATG, in 7% of patients given 7.5 mg/kg ATG, and in 30% of patients receiving 15 mg/kg ATG. This is also seen in an independent, nonprospective study using 7.5 mg/kg or 15 mg/kg Thymoglobulin (Sangstat), suggesting that the higher dose has a negative impact on immune reconstitution.12 One recent report suggests that 10 mg/kg Thymoglobulin (Sangstat) may reduce TRM, compared to a matched cohort of patients not given ATG.13That was a retrospective study, however, and patients receiving or not receiving ATG were actually treated in 2 different institutions. In addition, the patients given ATG had a nonrelapse mortality rate of 6%, which is out of the normal range for this type of transplantation and may be attributed to factors independent of the use of ATG.13
The third result of our studies is that overall survival was unaffected by the use of ATG, both at 7.5 mg/kg and 15 mg/kg. There is, however, an important difference in the 2 treatment arms in the long term. Extensive chronic GVHD developed in 39% of the ATG-treated patients versus 62% of the non-ATG patients (P = .04), and the difference was more pronounced in multivariate analysis. We have not compared the quality of life of surviving patients, but when they are followed up for longer periods of time—if the reduction of extensive chronic GVHD is consistent—patients who received ATG in the conditioning regimen should experience better quality of life14 and may actually have fewer events long term. Indeed event-free survival (acute and chronic GVHD, relapse, and TRM) is superior, though not significantly, in the ATG arm.
Where do we go from here? Transplantations using unrelated donors without ATG in the conditioning regimen, when donors are matched by molecular biology for DRB1 and by serology for HLA-A and HLA-B, expose the patient to a high risk for severe acute and extensive chronic GVHD. In the current study, patients who did not receive ATG had an overall risk of 42% for grade III-IV acute GVHD and 62% for extensive chronic GVHD. Matching for class I (A, B, and C) by sequence-based typing will lead to better selection of unrelated donors and may improve results. In a recent analysis, patients receiving grafts from unrelated donors matched by sequence-based typing for HLA-A, HLA-B, HLA-C, and class II had a risk of 28% for grade III-IV GVHD if they were not given ATG.15 Therefore, with the best available matching (sequence-based typing) and no ATG, approximately 1 in 3 patients will experience significant acute GVHD. A high dose of ATG (15 mg/kg) clearly prevents GVHD grade III-IV but exposes the patient to a high risk for lethal infections, in keeping with a recent report.12 One possible approach would be to give all patients a small dose of ATG in the conditioning regimen (7.5 mg/kg). We have shown this does not increase the risk for infection, and it reduces the risk for extensive chronic GVHD. A third dose early after transplantation (day 7) could be given only to patients at high risk for GVHD.16 A randomized study published many years ago indicates that early use of ATG after HLA-identical BMT reduces the risk for acute GVHD.17 We have been using low-dose ATG in transplantations from high-risk alternative donors and have confirmed in a pilot study that this approach is feasible and may reduce GVHD and transplant-related death.18 We are exploring this approach in a prospective randomized trial.
We thank the nursing staffs of the transplant units for their great work.
Supported by grants from the Associazione Italiana Ricerca contro il Cancro, Milan (A.B.) and Associazione Ricerca Trapianto Midollo Osseo, Genoa, and by an educational grant from Sangstat.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
A. Bacigalupo, Divisione Ematologia 2 (PAD 5/II), Ospedale San Martino, Largo Rosanna Benzi 10, 16132 Genova, Italy; e-mail: apbacigalupo@smartino.ge.it.