Abstract
Mutations of Wiskott-Aldrich syndrome protein (WASP) underlie the severe thrombocytopenia and immunodeficiency of the Wiskott-Aldrich syndrome. WASP, a specific blood cell protein, and its close homologue, the broadly distributed N-WASP, function in dynamic actin polymerization processes. Here it is demonstrated that N-WASP is expressed along with WASP, albeit at low levels, in human blood cells. The presence of approximately 160 nmol/L rapidly acting N-WASP molecules may explain the normal capacity of WASP-negative patient platelets for early agonist-induced aggregation and filopodia formation. Ex vivo experiments revealed a significant difference between WASP and N-WASP in sensitivity to calpain, the Ca++-dependent protease activated in agonist-stimulated platelets. Through the use of a series of calpain-containing broken cell systems, it is shown that WASP is cleaved in a Ca++-dependent reaction inhibitable by calpeptin and E64d and that N-WASP is not cleaved, suggesting that the cleavage of WASP by calpain functions in normal platelets as part of a Ca++-dependent switch mechanism that terminates the surface projection phase of blood cell activation processes.
Introduction
Wiskott-Aldrich syndrome protein (WASP) and its close homologue, the broadly distributed N-WASP, members of the WASP/SCAR protein family (reviewed in1), are cytoplasmic proteins thought to mediate dynamic cytoskeletal rearrangements by nucleating actin filament formation in response to specific stimuli.2 N-WASP, which was discovered in brain, has broad tissue distribution.3 WASP, in contrast, is exclusive to blood cells.4,5 Mutations of WASP lead to the Wiskott-Aldrich syndrome (WAS), a severe blood cell disease that includes immunodeficiency of variable severity and profound thrombocytopenia (reviewed in6). A recent genotype–phenotype study established that WAS patients with severe immune disease (nonsense and frameshift mutations) have WASP-negative leukocytes and that patients with mild immune disease (missense mutations) have decreased WASP levels in leukocytes.7 In contrast, 18 patients with diverse mutations all had WASP-negative platelets, providing an apparent explanation for the uniform severity of platelet dysfunction in this disease.
Although substantial inroads have been made in delineating the biochemistry, regulation, and signaling capability of N-WASP and WASP, the pathologic steps leading to the dysfunction of WAS blood cells remain undefined. Neither is it known how blood cells of patients retain a substantial number of normal cytoarchitectural responses despite the absence or deficit of WASP. For example, WAS patient platelets, though hypersusceptible to late-phase activation events such as phosphatidylserine exposure and microparticle release,8are nonetheless capable of normal early responses, including actin polymerization, shape change, and others.8-10 To determine whether N-WASP might contribute to early cytoarchitectural reorganization events, we examined blood cells from healthy control subjects and from patients for the content of this protein. We also compared WASP and N-WASP for stability under conditions of platelet activation.
Patients, materials, and methods
Patients
WAS was diagnosed in patients, all previously described,7 on the basis of male sex, thrombocytopenia with small platelets, eczema, immunodeficiency of variable clinical severity, family history (in some cases), and identification of a WASP mutation.
Cells
Paired blood samples of patients and healthy consenting control subjects were collected in acid-citrate-dextrose and fractionated immediately or after overnight shipment at ambient temperature as described.7 The blood was centrifuged at 200gfor 12 minutes to separate platelet-rich plasma (PRP) and pelleted cells. Acid-citrate-dextrose (ACD) (1 part) was added to PRP (3 parts), and platelets were pelleted at 800g for 15 minutes. Platelets were resuspended in platelet buffer (10 mM TES buffer, pH 7.2, 136 mM NaCl, 2.6 mM KCl, 0.5 mM NaH2PO4, 2 mM MgCl2, 0.1% glucose, 0.1% bovine albumin). Additional ACD (20%) and prostacyclin (1 μg/mL; Calbiochem, San Diego, CA) were added, and the platelets were pelleted at 800g for 10 minutes. Packed blood cells remaining after the removal of PRP were combined with equal-volume 2% dextran in 150 mM NaCl for 30 to 40 minutes at approximately 22°C to sediment erythrocytes. The supernatant was aspirated, and the leukocytes were fractionated by centrifugation on Histopaque 1077 (Sigma, St Louis, MO). Peripheral blood mononuclear cells (PBMCs) collected from the interface layer and neutrophils collected from the pellet were washed with Ca++/Mg2+-free Hanks balanced salt solution (HBSS) by pelleting at 200g for 15 minutes. Residual erythrocytes were removed from the neutrophils by water lysis.
Epstein-Barr virus (EBV)–transformed cell lines from patients with WAS and healthy control subjects11 were grown in RPMI 1640 with 10% fetal calf serum, penicillin, and streptomycin. HeLa epithelial carcinoma cells strain S3 were grown as adherent cells in Dulbecco minimum essential high-glucose medium with the same additives and were detached for harvest by 10-minute incubation in 25 mM EDTA in phosphate-buffered saline at 37°C.
Western blots
After a final wash of the cells in the presence of 2 mM diisopropylfluorophosphate and 25 μg/mL leupeptin (Sigma), lysates were prepared as described7 of 15 × 106/mL PBMCs, 15 × 106/mL neutrophils, 5 × 108/mL platelets, and 10 × 106/mL HeLa cells in sodium dodecyl sulfate (SDS) containing the same protease inhibitors. Lysates were electrophoresed (reducing conditions) and transferred to polyvinylidene difluoride membrane (Immobilon-P; Millipore, Bedford, MA) for antibody staining as described.7 11 Reactive bands detected by iodine 125-labeled secondary antibodies were quantified with the PhosphorImager Storm 860 and Image Quant v1.1 program (Molecular Dynamics, Sunnyvale, CA).
Antibodies and proteins
The previously described N-WASP antibodies were generated in rabbits immunized with amino acids 388-501 (antibody [Ab]-1)12 (see also 13,14) or full-length (Ab-2)15 recombinant N-WASP. On Western blots, both antibodies recognized N-WASP and cross-reacted with WASP (shown in “Results”). Ab-1 also cross-reacted with WAVE/SCAR-1, -2, and -3 (not shown). WASP antibodies (W485) were raised in rabbits immunized with amino acids 485-502 (C-terminus) of human WASP.11B27D8 monoclonal antibody is a mouse IgG1 that recognizes μ-calpain heavy chain.16 Affinity-purified goat anti-rabbit and rabbit anti-mouse immunoglobulin (Ig) was from Cappel (Durham, NC) and Pierce Chemical (Rockford, IL), respectively. Recombinant full-length rat N-WASP was generated in insect cells and purified.15Glutathione S-transferase (GST) fusion proteins of the VCA regions of rat N-WASP (amino acids 392-505) and human WASP (amino acids 414-502) were generated in Escherichia coli and purified on glutathione–Sepharose as described.3 15 Calpain (porcine erythrocyte) was from Calbiochem.
Platelet activation
Platelets (5 × 108) in 1 mL platelet buffer with 2 mM CaCl2 were preincubated with calpeptin (50 μg/mL; Calbiochem), E64d (50 μg/mL; Sigma), or diluent (1% dimethyl sulfoxide) without stirring for 30 minutes at 37°C. A23187 (1 μM; Sigma) was added, and incubation was continued with stirring for 5 to 20 minutes at 37°C. Reactions were terminated by the addition of 2 × SDS solution and heating at 100°C. In some experiments, the reaction was stopped by the addition of EGTA and leupeptin, and platelets were pelleted in a microcentrifuge at 4000 rpm for 8 minutes, resuspended in one-fourth original volume platelet buffer, and lysed by heating with 2 × SDS.
Calpain reactions in broken cell preparations
Platelets at 109/mL in Ca++/Mg2+-free HBSS containing 2 mM EGTA and 2 mM mercaptoethanol were lysed by sonication at 4°C with a macrotip preparative probe at 50% duty cycle with 96 bursts of 1 second each delivered over 2 minutes (model W-225 Sonicator; Heat Systems, Ultrasonics, Farmingdale, NY), clarified by centrifugation, and stored in aliquots at −80°C. HeLa cells (15 × 106/mL) were lysed in 0.5% NP-40, 10 mM Tris-HCl, pH 7.4, 150 mM NaCl, 2 mM diisopropylfluorophosphate, 25 μg/mL leupeptin, and 2 mM EGTA and were clarified by centrifugation. CaCl2 (5 mM) was added to platelet sonicates or HeLa cell lysates immediately before incubation at approximately 22°C for 0 to 30 minutes. Reactions were terminated by the addition of 2 × SDS solution and incubation at 100°C.
Immunoprecipitation
Four hundred microliters platelet sonicate was incubated with 2 μg N-WASP Ab-2 or normal rabbit IgG at 4°C overnight. Washed A/G Plus agarose (100 μL; Santa Cruz Biotechnology, Santa Cruz, CA) was added, and incubation continued for 2 hours at 4°C. The resin was centrifuged for 10 seconds in a microcentrifuge and washed, and the proteins were eluted with 40 μL SDS solution at 100°C for 2 minutes.
Results
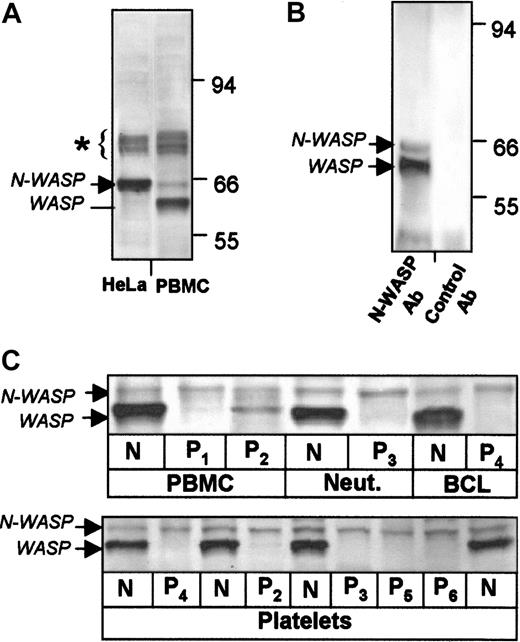
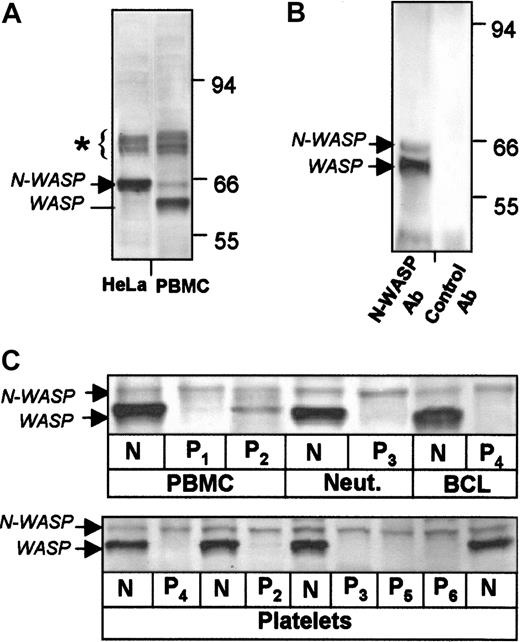
Western blots of HeLa cells with Ab-1 to the N-WASP carboxy-terminal VCA region stained N-WASP as the major band (Figure1A, lane 1) and cross-reacted with bands of 74 to 80 kd, which are apparently the 3 SCAR/WAVE proteins,17,18 known to be expressed in HeLa cells and detectable with Ab-1 (data not shown). In PBMCs, Ab-1 stained bands in the 74- to 80-kd range (which were not further investigated), and the major band WASP and N-WASP at low levels (Figure 1A, lane 2). The N-WASP band was detected in PBMCs of additional healthy control subjects (N) and WASP patients without (P1) and with (P2) low levels of WASP expression (Figure 1C, lanes 1-3). The N-WASP band was also detected in the neutrophils of control subjects and WAS patients and in EBV-transformed B-cell lines (Figure1C, upper panel) and platelets (lower panel). As previously described,7 WAS patient platelets lack WASP (lower panel). N-WASP levels were not detectably different in paired patient and healthy cells (Figure 1C), indicating that WASP-deficient patient cells do not have compensatory up-regulation of N-WASP.
Expression of WASP and N-WASP in human blood cells examined by Western blot.
Cell lysates were stained with N-WASP Ab-1. (A) HeLa cells and normal human PBMCs. Blots show WASP and N-WASP (low levels) in PBMCs. WASP is absent as anticipated in HeLa cells. Bands at 74 to 80 kd (∗) may represent proteins of the SCAR/WAVE family.17 18 (B) Verification of N-WASP presence in normal platelets by immune precipitation. WASP and N-WASP were precipitated from normal platelet lysate by N-WASP Ab-2 and detected with N-WASP Ab-1. Normal rabbit IgG failed to precipitate detectable bands. (C) Blood cells and platelets from patients and control subjects. N-WASP was detected at similar levels in PBMCs, neutrophils (Neut), EBV-transformed B-cell lines (BCL) (upper panel), and platelets (lower panel) of various healthy control subjects (all indicated by N) and WAS patients (P1 to P6).
Expression of WASP and N-WASP in human blood cells examined by Western blot.
Cell lysates were stained with N-WASP Ab-1. (A) HeLa cells and normal human PBMCs. Blots show WASP and N-WASP (low levels) in PBMCs. WASP is absent as anticipated in HeLa cells. Bands at 74 to 80 kd (∗) may represent proteins of the SCAR/WAVE family.17 18 (B) Verification of N-WASP presence in normal platelets by immune precipitation. WASP and N-WASP were precipitated from normal platelet lysate by N-WASP Ab-2 and detected with N-WASP Ab-1. Normal rabbit IgG failed to precipitate detectable bands. (C) Blood cells and platelets from patients and control subjects. N-WASP was detected at similar levels in PBMCs, neutrophils (Neut), EBV-transformed B-cell lines (BCL) (upper panel), and platelets (lower panel) of various healthy control subjects (all indicated by N) and WAS patients (P1 to P6).
To confirm the identity of the N-WASP band in blood cells, we performed immune precipitation of normal platelet lysate with an independent antibody generated to full-length N-WASP. N-WASP and WASP, but not the 74- to 80-kd bands, were specifically precipitated by Ab-2 and detected by Ab-1 (Figure 1B). Control rabbit IgG failed to precipitate WASP or N-WASP.
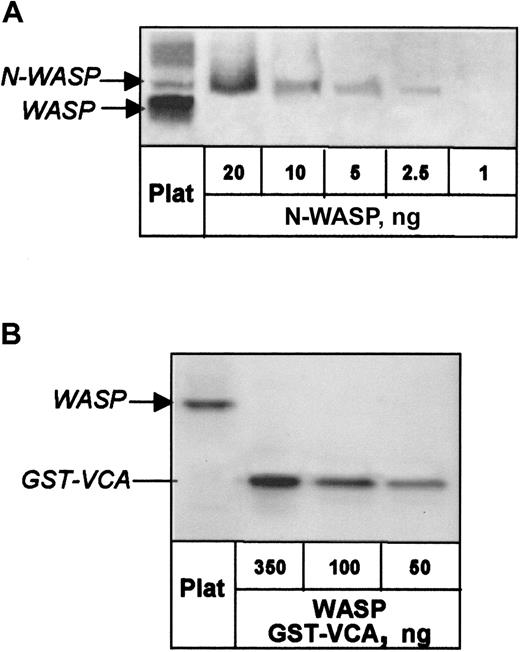
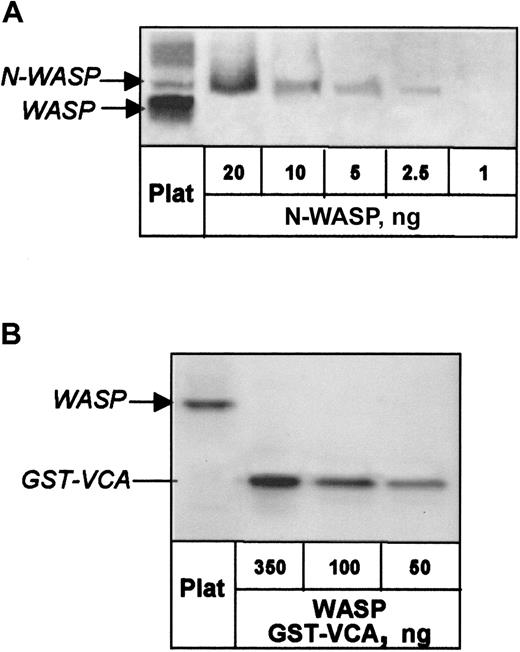
N-WASP was quantified in normal platelets by phosphor imaging of Western blots in which dilutions of recombinant N-WASP from insect cells were used as standard. N-WASP content was determined as 30 ng/mg platelet protein (Figure 2A), which, with a volume of 7 × 10−15 L/platelet,19converts to 160 nmol/L.
Quantitation of N-WASP and WASP in normal human platelets.
(A) N-WASP in platelet lysate and dilutions of recombinant N-WASP were detected on Western blot by Ab-1 and quantified by phosphor imaging. (B) WASP in platelet lysate was detected with W485 antibody and quantified by comparison with dilutions of GST-VCA.
Quantitation of N-WASP and WASP in normal human platelets.
(A) N-WASP in platelet lysate and dilutions of recombinant N-WASP were detected on Western blot by Ab-1 and quantified by phosphor imaging. (B) WASP in platelet lysate was detected with W485 antibody and quantified by comparison with dilutions of GST-VCA.
For contrast, we examined normal platelets for WASP content, previously reported as 700 ng/mg total protein.20 Phosphor imaging of Western blots in which dilutions of WASP GST-VCA region were used as standard revealed the presence of approximately 900 ng WASP/mg total platelet protein (Figure 2B), corresponding to 4.7 μM.
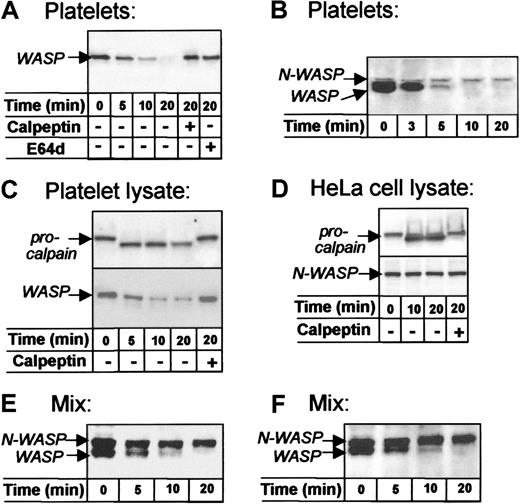
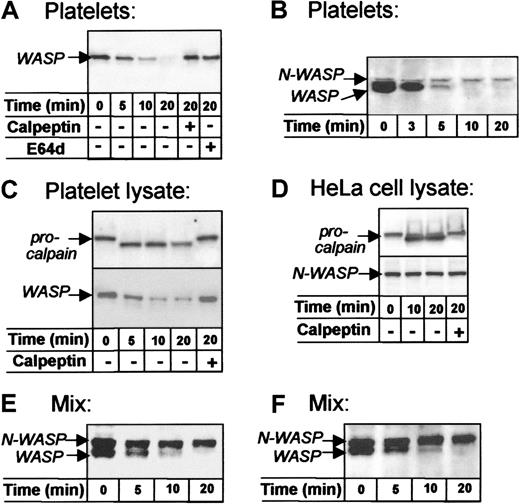
To examine the sensitivity of WASP and N-WASP to protease, platelets were activated with Ca++ ionophore A23187, a potent agonist that induces various events of platelet activation, including calpain activation.21 Western blots showed time-dependent cleavage of WASP during ionophore-induced platelet activation (Figure3A, lanes 1-4), which confirms previous results.22 Cleavage of WASP in activated platelets was abrogated by calpeptin, a specific cell permeant calpain inhibitor23 (lane 5), and by E64d (lane 6), a chemically unrelated calpain inhibitor.24 N-WASP, in contrast, remained uncleaved (Figure 3B).
WASP and N-WASP differ in sensitivity to calpain.
WASP was detected with rabbit W485, N-WASP with Ab-1 (D-F) or Ab-2 (B), and μ-calpain with B27D8 monoclonal antibody. (A, B) Intact platelets activated with Ca++ ionophore A23187. Note the time-dependent cleavage of WASP, which failed to occur in platelets pretreated with the calpain inhibitors calpeptin23 or E64d24 (A, lanes 5, 6). Note that N-WASP is not cleaved during platelet activation (B); similar results were obtained when blots of platelet lysates were stained with N-WASP Ab-1 (not shown). (C) Platelet sonicates (lysates) supplemented with CaCl2and incubated at approximately 22°C. Note the conversion of procalpain (80 kd) to calpain (76 kd) and the time-dependent cleavage of WASP, both inhibited by calpeptin. (D) HeLa cell lysates supplemented with CaCl2 and incubated at approximately 22°C. Note that procalpain was converted to active calpain, but N-WASP was not cleaved over 20 minutes. (E) Combined platelet and HeLa cell lysates (1:1) supplemented with CaCl2 and calpain (36 μg/mL) (Calbiochem, La Jolla, CA) incubated at approximately 22°C. Note that WASP was cleaved and N-WASP was unaffected. (F) Recombinant N-WASP (700 ng/mL) was added to platelet lysate, supplemented with CaCl2 and incubated at approximately 22°C.
WASP and N-WASP differ in sensitivity to calpain.
WASP was detected with rabbit W485, N-WASP with Ab-1 (D-F) or Ab-2 (B), and μ-calpain with B27D8 monoclonal antibody. (A, B) Intact platelets activated with Ca++ ionophore A23187. Note the time-dependent cleavage of WASP, which failed to occur in platelets pretreated with the calpain inhibitors calpeptin23 or E64d24 (A, lanes 5, 6). Note that N-WASP is not cleaved during platelet activation (B); similar results were obtained when blots of platelet lysates were stained with N-WASP Ab-1 (not shown). (C) Platelet sonicates (lysates) supplemented with CaCl2and incubated at approximately 22°C. Note the conversion of procalpain (80 kd) to calpain (76 kd) and the time-dependent cleavage of WASP, both inhibited by calpeptin. (D) HeLa cell lysates supplemented with CaCl2 and incubated at approximately 22°C. Note that procalpain was converted to active calpain, but N-WASP was not cleaved over 20 minutes. (E) Combined platelet and HeLa cell lysates (1:1) supplemented with CaCl2 and calpain (36 μg/mL) (Calbiochem, La Jolla, CA) incubated at approximately 22°C. Note that WASP was cleaved and N-WASP was unaffected. (F) Recombinant N-WASP (700 ng/mL) was added to platelet lysate, supplemented with CaCl2 and incubated at approximately 22°C.
To further compare WASP and N-WASP, we examined these molecules in broken cell preparations in which calpain activation was induced by the addition of Ca++. Although WASP underwent time- and Ca++-dependent calpeptin-inhibitable cleavage in platelet lysates (Figure 3C), N-WASP in HeLa cells lysates was not cleaved despite calpain activation (Figure 3D).
Because platelet and HeLa cell lysates are not comparable environments, WASP and N-WASP were also exposed to calpain when present at matched concentrations in the same incubation. In combined lysates of HeLa cells and platelets, the addition of Ca++ induced the activation of calpain and the cleavage of WASP but not of N-WASP (Figure 3E). In addition, recombinant N-WASP was not cleaved when incubated with endogenous WASP in Ca++-supplemented platelet lysates (Figure 3F).
Discussion
A major finding of this study is the demonstration that N-WASP is present along with WASP in normal human leukocytes and platelets. This finding contrasts with previous studies that failed to detect N-WASP in platelets.10 25 The source of the conflicting findings is unknown, though the use of detection systems with different sensitivity is a possibility because platelet N-WASP levels are low.
We also showed that WASP, but not N-WASP, is cleaved by calpain, which is known to be activated as a late-phase event in platelets.21 26 The difference in calpain sensitivity of WASP and N-WASP is surprising given the high degree of sequence and structural homology of these proteins and their shared in vitro functions. These findings strongly suggest that calpain sensitivity of WASP is physiologically relevant.
In WASP family proteins, the N-terminal and C-terminal regions have different functions. The acidic (A) region at the extreme C-terminus is responsible for binding the Arp2/3 complex, and the verprolin-cofilin-acidic (VCA) region (429-502 in WASP, also called the WA region) functions to dramatically enhance Arp2/3–dependent actin filament nucleation in vitro.15,27 N-terminal regions, and especially the GBD domain (230-288 in WASP), respond to cell signaling events that recruit N-WASP/WASP to the surface membrane and convert the dormant auto-inhibited folded structures to the activated open conformation.13,28,29 This unfolding and activation reaction is induced in both WASP and N-WASP by the binding of phosphatidylinositol-4,5-bisphosphate and Cdc42-GTP (reviewed in1,2). There are, however, notable differences between WASP and N-WASP in their responsiveness to these mediators.15 30
The severe platelet defect in WAS is characterized by small platelet size and low number, typically approximately 10% of normal, primarily caused by enhanced platelet destruction (reviewed in 6). Surprisingly, several laboratories found that early events of platelet activation—including shape change, formation of filopodia and lamellipodia, polymerization of actin, aggregation, and alpha granule release8-10—are normal in the WASP-negative platelets of patients. A putative explanation for these findings is that WASP is not a major contributor to these early cytoskeletal rearrangements. Cumulative data suggest that N-WASP could be the major mediator of early rapid Arp2/3-dependent cytoarchitectural responses, including filopodia formation. The first piece of evidence is the finding that the N-WASP VCA region has substantially higher actin-polymerizing activity than the WASP VCA region.14 Other evidence is provided by studies of actin filament formation induced by IcsA, a component of Shigella flexneri, which binds to, and activates, N-WASP.25 Arp2/3-mediated actin filament generation in this system became detectable with 10 nmol/L N-WASP and maximal with 100 nmol/L. These values suggest that the 160 nmol/L N-WASP in human platelets may be adequate for initial rapid Arp2/3-mediated responses.
What then is the function of platelet WASP? In contrast to the normal early events, late-phase events are abnormal in the WASP-negative patient platelets. These include altered procalpain levels,31 increased resting Ca++ levels, increased amplitude and duration of Ca++ transients, and hyperreactivity of Ca++-dependent late-phase events including phosphatidylserine exposure and microparticle release.8 These findings suggest that the role of WASP is to indirectly modulate Ca++ transients in the filopodia–lamellipodia phase and, through calpain cleavage, to contribute to the dissolution of surface projections in response to high Ca++ levels. Based on transfection studies,17 a cleavage pattern that releases an intact VCA region is expected to disrupt Arp2/3-dependent nucleation. Cleavage within the VCA region is expected to end actin nucleation and to allow decay of surface projections.
We speculate that N-WASP rather than WASP is largely responsible for the initial agonist-induced rapid generation of platelet filopodia, with the more abundant and possibly slower-acting WASP molecules sustaining actin nucleation and the filopodia– lamellipodia phase of platelet activation.
We thank Drs Hsin-yi Henry Ho, Rajat Rohatgi, and Marc W. Kirschner (Department of Cell Biology, Harvard Medical School, Boston, MA) for providing advice and reagents and Drs John Hartwig and Herve Falet (Division of Hematology, Brigham and Women's Hospital, Boston, MA) for discussing their findings before publication.
Supported by National Institutes of Health grants AI39574 and HL59561.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Eileen Remold-O'Donnell, Center for Blood Research, 800 Huntington Ave, Boston, MA 02115; e-mail:remold@cbr.med.harvard.edu.