Abstract
Limited response to idiotype vaccination in patients with myeloma suggests that there is a need to develop better immunotherapy strategies. It has been determined that the number of high-potency CMRF44+CD14−CD19−dendritic cells (DCs) in the blood of patients with myeloma (range, 0.03%-0.8% of mononuclear cells [MNCs]; n = 26) was not significantly different from that in controls (range, 0.05%-0.8% of MNCs; n = 13). Expression of the costimulatory molecules CD80 and CD86 on DCs from these patients (mean, 29%±17% of MNCs and 85%±10% of MNCs, respectively) was also normal (mean, 29%±17% and 86%±16% of MNCs, respectively). Up-regulation of CD80 expression in response to stimulation by human (hu)CD40LT + interleukin (IL)-2 was significantly reduced on the DCs of patients with myeloma during stable disease (n = 9) and was absent during progressive stages (n = 7) of disease. Similar effects were seen on B cells but not on monocytes of the same group of patients. CD86 expression on DCs was high before (86%) and after (89%) stimulation. Inhibition of CD80 up-regulation was neutralized by either anti–transforming growth factor (TGF)–β1 or anti–IL-10. Up-regulation of CD80 on DCs of controls was inhibited by rTGF-β1 in a dose-dependent manner. Serum TGF-β1 and IL-10 levels were normal in most patients studied. Cytoplasmic TGF-β1 was increased in plasma cells during progressive disease. Thus patients with myeloma have normal numbers of DCs, but CD80 expression may fail to be up-regulated in the presence of huCD40LT because of tumor-derived TGF-β1 or IL-10. Autologous high-potency DCs may have to be tested for CD80 up-regulation and biologically modified ex vivo before idiotype priming for immunotherapy.
Introduction
Dendritic cells (DCs) are immune surveillance cells that have a specialized ability to process antigen and to present relevant peptides to T lymphocytes in association with major histocompatibility complex class I and II molecules. Engagement of the T-cell receptor defines specificity, but a productive immune response also requires the costimulatory molecules CD80 (B7-1) and CD86 (B7-2) on antigen-presenting cells to bind to their counterreceptors CD28 and CD152 (CTLA-4) on T lymphocytes. Expression of these costimulatory molecules plays a major role in determining whether T-cell survival, apoptosis, anergy, or productive immunity occurs.1-3 There is now considerable interest in the ability of DCs to generate CD4 helper, CD8 cytotoxic, and humoral responses and to act as cellular vectors in immunotherapy protocols. Identification and enumeration of DCs from peripheral blood and optimal conditions for their collection for immunotherapy trials have been difficult to standardize because of the lack of specific markers, the heterogeneous nature of the DC population, and the immature phenotype of DCs in peripheral blood. Early quantitation methods using complex purification techniques estimated the number of DCs to be up to 4% of the circulating mononuclear cell population.4 More recently, the expression of CMRF44 on CD19−CD14− propidium iodide–negative (PI−) mononuclear cells after a brief period of culture has provided a standard method for the analysis of a population of high-potency DC precursors that comprise less than 1% of the blood mononuclear cell population.5 6 The ability to count and mobilize blood DCs makes them a valuable source for future immunotherapy trials.
It is paradoxical that idiotype vaccination is considered a treatment option for patients with myeloma because they respond poorly to immunization by viral and bacterial antigens, and it has been recognized for many years that they have a primary immune defect.7 Murine studies have suggested that this defect is associated with antigen presenting cells (APCs).8 There have been reports that the DCs of patients with myeloma are functionally normal9,10; however, the criteria used to define DCs in these latter studies included monocytes–macrophages and even B cells. Preliminary reports of immunotherapy trials in patients with myeloma have demonstrated only minor responses.11-14If immunotherapy using autologous DCs is to be a serious treatment option for patients with myeloma, it is important that we have a greater understanding of the number and function of high-potency blood DCs and the optimal conditions for their collection and administration. One important factor to consider is that without adequate expression of costimulating molecules on the APCs, immunotherapy involving idiotype presentation may induce further T-cell tolerance.
Expression of the B7 family of costimulatory molecules can be modulated by a range of cytokines. This includes down-regulation by transforming growth factor (TGF)–β1 or interleukin (IL)-10 and up-regulation by CD40L or interferon (IFN)-γ.2,3 15-19Whether immunotherapy strategies can be improved by stimulating high-potency DCs ex vivo to express high levels of costimulatory molecules is likely to be an important question not only for patients with myeloma but also for patients in other immunotherapy trials. We have determined the DC number and the expression of CD80 and CD86 on DCs in the peripheral blood of patients with myeloma. In addition, we have demonstrated that there is a functional defect in the DCs of patients with myeloma that is characterized by a reduced ability to up-regulate CD80 expression after stimulation with trimeric human CD40 ligand (huCD40LT). Finally, we have identified the cytokines responsible for this defect and have produced evidence to support a proposed mechanism that may, at least in part, explain the impaired immune response in these patients.
Materials and methods
Patient selection
Blood and bone marrow samples were collected after informed consent was obtained from patients with myeloma attending our clinic. Disease status was classified as either stable or progressive after clinical assessment and laboratory investigations that included determination of active lytic lesions, full blood count, serum calcium level, serum creatinine level and creatinine clearance, paraprotein level, Bence-Jones proteinuria, serum β2M level, and serum thymidine kinase level.20 Patients were enrolled in the Australian Leukaemia Study Group myeloma trial.21 Serum for enzyme-linked immunosorbent assay (ELISA) was separated and frozen until the day of assay.
Blood dendritic cell assay
Standardized blood DC assay involved separating mononuclear cells from 5 mL EDTA blood on Ficoll-Hypaque (Pharmacia, Uppsala, Sweden), followed by culture in RPMI (ICN, Costa Mesa, CA) and 10% fetal calf serum (CSL, Melbourne, Australia) for 24 hours at 37°C in 10% CO2 before assay.5 Cells were harvested and mixed with anti-CMRF44 and then with anti-mouse immunoglobulin (Ig)G fluorescein isothiocyanate (FITC; Becton Dickinson, San Jose, CA), anti-CD19 phycoerythrin (PE; Becton Dickinson), and anti-CD14 PE (Becton Dickinson). The control tube contained anti-IgG1–PE (Becton Dickinson) and an isotype-matched control, anti–CMRF75 stained with anti-mouse IgG-FITC (Becton Dickinson). At least 100 000 PI− events were analyzed on a Coulter EPICS XL flow cytometer (Beckman Coulter, Hialeah, FL).
Up-regulation of CD80 and CD86 by huCD40LT
Human trimeric CD40 ligand was kindly provided by Immunex (Seattle, WA). Ficoll-Hypaque–separated peripheral blood mononuclear cells were cultured with or without huCD40LT (5 μg/mL) and rIL-2 (0.1 μg/mL; R&D Systems, Minneapolis, MN) in 1 mL cultures of RPMI containing 10% fetal calf serum (CSL) at 37°C in 10% CO2. After 24 and 72 hours, cells were harvested and assayed for CMRF44 antigen expression on CD19−CD14−PI− cells using a Coulter Epics XL flow cytometer. Four-color staining with anti–CD80 PE (Becton Dickinson) or anti–CD86 PE (Becton Dickinson), anti–CD19 PE-Cy5 plus anti–CD14 PE-Cy5, CMRF44 (indirect FITC) on viable cells (propidium iodide) was performed to demonstrate the expression of costimulatory molecules before and after culture. Similar cultures were established from mononuclear cells of bone marrow biopsy samples from patients with myeloma and controls. Plasma cells were detected using CD38hi expression (Becton Dickinson), and plasma cell subpopulations were distinguished using anti-CD45 (Becton Dickinson) as previously described.22 These were either primitive CD38++CD45++, immature CD38++CD45+, or mature CD38++CD45−.
Cytokine neutralization and inhibition studies
Cultures of peripheral blood cells were established (as per DC assay) with and without anti–TGF-β1, anti–IL-10, or anti–IFN-γ (R&D Systems) at concentrations of 0.12 to12 μg/mL. CD80 expression on DCs was assayed after 24 hours. Normal peripheral blood cells were similarly cultured in the presence of rTGF-β1 (2-200 pg/mL), and CD80 expression on DCs was assayed after 24 hours.
TGF-β1 and IL-10 ELISA assays
Two hundred microliters monoclonal antibody to either TGF-β1 or IL-10 (R&D Systems) diluted 1:250 in 0.2 bicarbonate buffer, pH 9.2, was added to the wells of a flat-bottomed microtiter plate, incubated overnight at 4°C, and washed 3 times with phosphate-buffered saline (PBS), pH 7.4, containing 0.05% Tween 20 (wash buffer). To assay IL-10, serum was diluted 1:10 with reagent diluent (1.4% bovine serum albumin, 0.01% Tween 20 in PBS at pH 7.3). To assay TGF-β1, serum was first treated with 2.5 N acetic acid for 10 minutes, followed by neutralization with 2.7 N NaOH. Acid-treated serum was diluted 1:30 with reagent diluent. rTGF-β1 and rIL-10 (R&D Systems) were diluted to 50, 25, 12.5, 6.25, 3.12, and 1.56 ng/mL in reagent diluent. Two hundred microliters each dilution was added to triplicate wells, incubated for 3 hours, and washed 3 times with PBS. Then 200 μL biotinylated anti–TGF-β1 (PharMingen, San Diego, CA) was added to each well of the TGF-β1 plate, and 200 μL polyclonal anti–IL-10 (R&D Systems) was added to the IL-10 plate. Both plates were incubated for an hour at room temperature and were washed 3 times in wash buffer. One hundred microliters StreptAB complex (DAKO, Carpinteria, CA) was added to the TGF-β1 plate and incubated for 30 minutes. One hundred microliters biotinylated F(ab)2 fragment was added to the IL-10 plate for 1 hour and was washed before the addition of 100 μL StreptAB complex for 30 minutes. Both plates were washed 3 times, and then 100 μL o-phenylenediamine solution (20 mg in 20 mL phosphate citrate buffer containing 10 μL H2O2) was added. Color development stopped with 50 μL of 1.5 M H2SO4. Absorbance was read at 492 nm on a Multiskan RC microplate reader with Genesis software (Labsystems, Basingstoke, United Kingdom).
Cytoplasmic cytokine expression
Expression of the cytokines IL-10, TGF-β1, and the latency-associated peptide (LAP) in the cytoplasm of plasma cells was determined by flow cytometry. Mononuclear cells separated from bone marrow on Ficoll-Hypaque were cultured for 1 hour at 37°C in 5% CO2 in RPMI 10 and then for 5 hours with brefeldin A (10 μg/mL). Bone marrow samples were permeabilized (Harlan Sera-Lab, Loughborough, United Kingdom) and stained with anti–IL-10 PE (IQ Products, Groningen, The Netherlands), anti-TGF-β1 (R&D Systems), or anti-LAP (R&D Systems). IL-10–stained cells were washed and stained with anti-CD38–FITC (Becton Dickinson), whereas the TGF-β1 and LAP-stained cells were washed with PBS containing 0.5% saponin and 0.5% fetal calf serum and then were stained with sheep anti-mouse IgG-FITC. Normal mouse serum was used for blocking, and then these cells were stained with anti-CD38 PE (Becton Dickinson). Appropriate direct and indirect controls were used, and all tubes were read within 24 hours.
Results
Number of DCs in peripheral blood of patients with myeloma
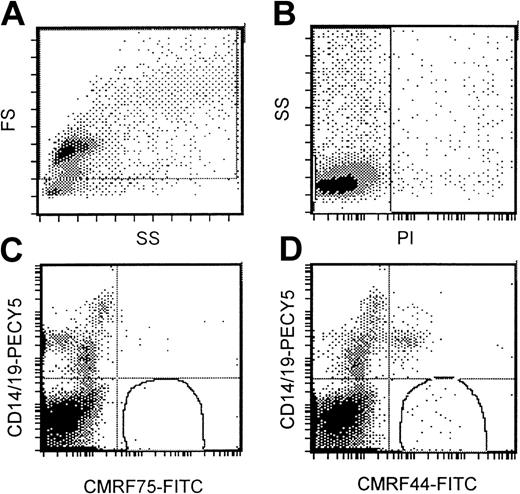
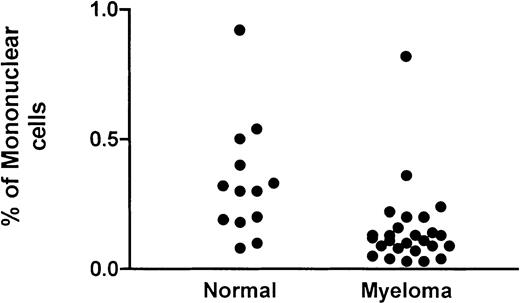
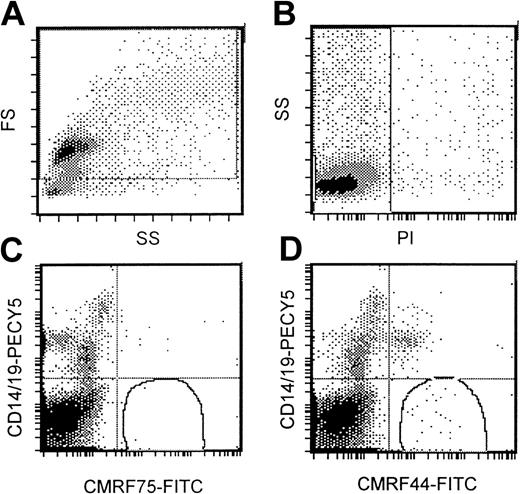
Flow cytometric DC assay involved an analysis of mononuclear cells after culture at 37°C for 24 hours. A gating strategy that incorporated all possible DCs (Figure 1A) that were PI− (Figure 1B) was used. High-potency DC populations were identified as the CMRF44+CD14−CD19−population (Figure 1D) with CMRF75-FITC–stained cells (Figure1C) used as the isotype control. Our normal range (0.05%-0.8% of MNCs; n = 13) was not significantly different from either that reported in the original publication5 or the normal range established in 2 other independent laboratories (unpublished observations, D. H., September 2000). The range of values for DC numbers in myeloma peripheral blood (0.03%-0.8% of MNCs; n = 26) was not significantly different from the normal range (Figure2). There was a trend for the mean number of DCs in the blood of patients with progressive disease (n = 12; mean, 0.12%) to be lower than in patients with stable disease (n = 14; mean, 0.18%); however, this difference was not statistically significant.
Standardized flow cytometric assay for DCs.
(A) A wide gating strategy is used to include most peripheral blood cells. (B) Only PI− cells are included. (C) CMRF75-FITC is used as an isotype control. (D) DCs stain positively for CMRF44-FITC but negatively for CD19 PE and CD14 PE.
Standardized flow cytometric assay for DCs.
(A) A wide gating strategy is used to include most peripheral blood cells. (B) Only PI− cells are included. (C) CMRF75-FITC is used as an isotype control. (D) DCs stain positively for CMRF44-FITC but negatively for CD19 PE and CD14 PE.
DCs in the blood of patients with myeloma.
The number of DCs as a percentage of the mononuclear fraction in the peripheral blood of patients with myeloma is compared with that of a control group.
DCs in the blood of patients with myeloma.
The number of DCs as a percentage of the mononuclear fraction in the peripheral blood of patients with myeloma is compared with that of a control group.
CD80 and CD86 expression on dendritic cells
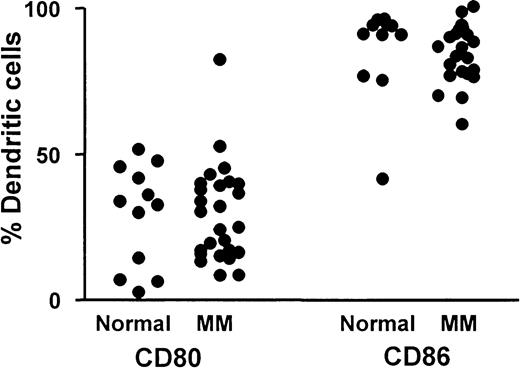
The percentage of blood DCs (CMRF44+CD19−CD14−PI−) expressing CD80 and CD86 are shown for patients with myeloma (n = 26) and for a control group (n = 12) (Figure3). Percentage CD80-expressing DCs was always lower than percentage CD86-expressing DCs, and the relatively low number of DCs expressing CD80 confirmed the immature DC phenotype on circulating DCs from patients and controls. There was no significant difference between the mean percentage CD80+DCs in the group of patients with myeloma (29%±17% MNCs) compared with a control group (29%±17% MNCs), nor was the percentage CD86 on DCs of patients with myeloma (85%±10% MNCs) significantly different from that of controls (86%±16% MNCs).
CD80 and CD86 expression on DCs.
Comparison of percentage blood DCs expressing either CD80 or CD86 in a group of patients with myeloma (n = 26) and a control group (n = 12).
CD80 and CD86 expression on DCs.
Comparison of percentage blood DCs expressing either CD80 or CD86 in a group of patients with myeloma (n = 26) and a control group (n = 12).
CD80 and CD86 expression on peripheral blood cells after huCD40LT stimulation
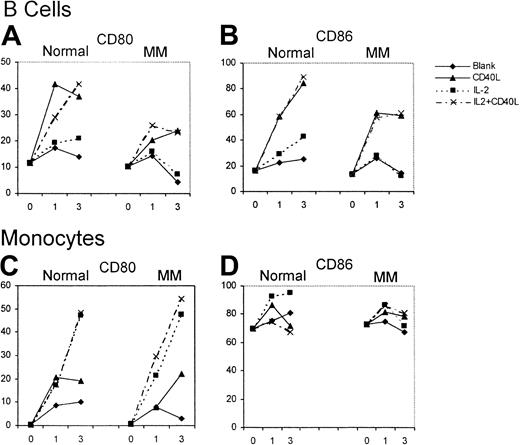
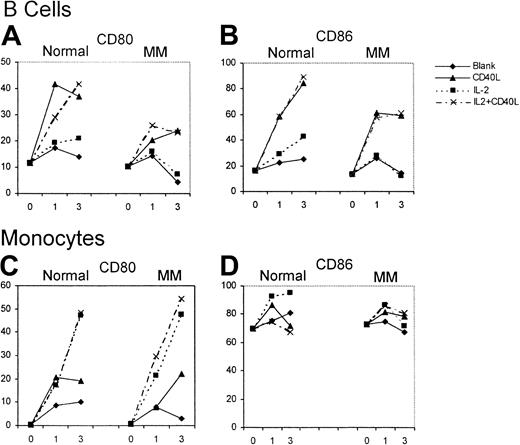
The effect of huCD40LT with or without IL-2 on the expression of CD80 and CD86 on autologous B cells (CD19+) and monocytes (CD14+) was studied over 72 hours as an internal control for the effect on DCs. B cells from patients with myeloma (n = 12) and from controls (n = 6) had low percentages of CD80+and CD86+ cells on day 0. Up-regulation of CD80 and CD86 on B cells of the control group and the patients with myeloma was attributed almost entirely to huCD40LT rather than to IL-2 (Figure4). CD80 and CD86 up-regulation on B cells, however, was greater in the controls than in the patients with myeloma. Few monocytes expressed CD80 on day 0, but most expressed CD86. In contrast to B cells, up-regulation of CD80 expression on the monocytes of patients with myeloma (n = 12) was the same as in the control group (n = 6) and was caused by IL-2, not huCD40LT. Neither huCD40LT nor IL-2 significantly altered the expression of CD86 on monocytes.
Up-regulation of CD80 and CD86 expression on B cells and monocytes.
Mean number of CD80- and CD86-expressing B cells and monocytes from patients with myeloma (n = 12) and a control group (n = 6) with and without stimulation by huCD40LT and IL-2 on days 0, 1, and 3.
Up-regulation of CD80 and CD86 expression on B cells and monocytes.
Mean number of CD80- and CD86-expressing B cells and monocytes from patients with myeloma (n = 12) and a control group (n = 6) with and without stimulation by huCD40LT and IL-2 on days 0, 1, and 3.
CD80 and CD86 expression on DCs after huCD40LT stimulation
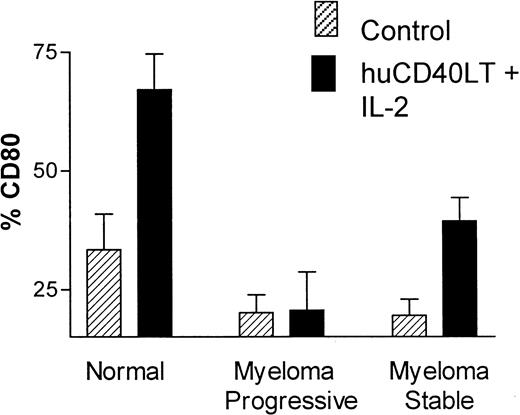
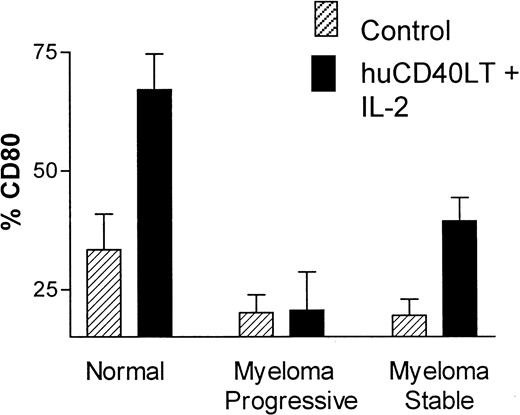
There was a significant up-regulation of the percentage of CD80+ DCs in the control group (n = 10) after stimulation with huCD40LT plus IL-2 for 24 hours. Up-regulation of CD80 on DCs from patients with myeloma occurred but was significantly less than normal in patients with stable disease (n = 9), whereas DCs from patients with progressive disease (n = 7) completely failed to up-regulate CD80 expression in response to stimulation by huCD40LT and IL-2 (Figure5). These latter patients had clinical or laboratory features of progressive disease, which included active lytic lesions, low hemoglobin level, and increasing M-protein or serum thymidine kinase levels. Percentage of CD86+ DCs in the control group was high without (mean, 86%) and with (mean, 89%) stimulation in vitro with huCD40LT and IL-2. There was no apparent defect in CD86 expression on the DCs of patients with myeloma.
Up-regulation of CD80 and CD86 on DCs.
Comparison of the in vitro up-regulation of CD80 expression on blood DCs after stimulation with huCD40LT and IL-2. Mean number of CD80-expressing DCs from patients with stable (n = 9) and progressive-stage (n = 7) myeloma are compared with that of a control group (n = 10).
Up-regulation of CD80 and CD86 on DCs.
Comparison of the in vitro up-regulation of CD80 expression on blood DCs after stimulation with huCD40LT and IL-2. Mean number of CD80-expressing DCs from patients with stable (n = 9) and progressive-stage (n = 7) myeloma are compared with that of a control group (n = 10).
Effect of anti-TGFβ1 and anti-IL10 on the failure to up-regulate CD80 expression on DCs and monocytes
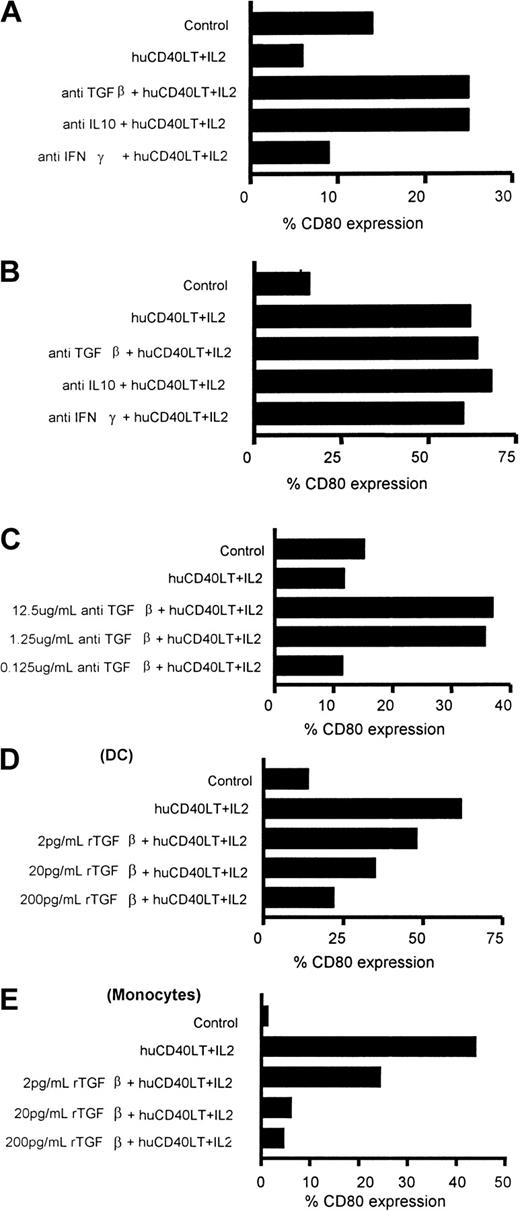
Peripheral blood mononuclear cells from patients with myeloma were stimulated with huCD40LT and IL-2 and incubated with anti-TGF-β1, anti–IL-10, or anti–IFN-γ to determine whether these cytokines were responsible for the failure of huCD40LT and IL-2 to up-regulate CD80 expression. Figure6A illustrates that either anti-TGFβ1 or anti–IL-10 (1.25 μg/mL) could independently neutralize the failure to up-regulate CD80 expression on the DCs of a patient with progressive-stage myeloma. Three other patients in progressive-stage disease demonstrated the same phenomenon. However, for 6 patients who had stable disease, the addition of either anti-TGF-β1 or anti–IL-10 did not significantly enhance the up-regulation of CD80 expression on DCs (Figure 6B). The addition of anti–IFN-γ had no significant effect on the expression of CD80 on the DCs of the patients studied (Figure 6A,B). Neutralization of any inhibitory effect on CD80 up-regulation was dose dependent and was achieved at antibody concentrations of more than 1 μg/mL (Figure6C).
Identification of factors responsible for failure to up-regulate CD80 on DCs.
Examples of up-regulation or down-regulation of CD80 expression on blood DCs of patients with myeloma (A-C), blood DCs of healthy donors (D), and blood monocytes of healthy donors (E). (A) Addition of either anti–TGF-β or anti–IL-10 (1.25 μg/mL) neutralized the inhibition of the up-regulation of percentage of CD80-expressing DCs of a patient after stimulation with huCD40LT and IL-2. (B) Addition of either anti–TGF-β or anti–IL-10 failed to alter the percentage of CD80-expressing DCs of a patient when there was normal up-regulation of CD80. (C) Neutralization of the failure to up-regulate CD80 expression on DCs of patients was dose dependent, and levels below 1 μg/mL did not completely block the inhibition. rTGF-β1 caused a dose-response inhibition of the expected up-regulation of CD80 expression on DCs (D) and monocytes (E) from a healthy donor.
Identification of factors responsible for failure to up-regulate CD80 on DCs.
Examples of up-regulation or down-regulation of CD80 expression on blood DCs of patients with myeloma (A-C), blood DCs of healthy donors (D), and blood monocytes of healthy donors (E). (A) Addition of either anti–TGF-β or anti–IL-10 (1.25 μg/mL) neutralized the inhibition of the up-regulation of percentage of CD80-expressing DCs of a patient after stimulation with huCD40LT and IL-2. (B) Addition of either anti–TGF-β or anti–IL-10 failed to alter the percentage of CD80-expressing DCs of a patient when there was normal up-regulation of CD80. (C) Neutralization of the failure to up-regulate CD80 expression on DCs of patients was dose dependent, and levels below 1 μg/mL did not completely block the inhibition. rTGF-β1 caused a dose-response inhibition of the expected up-regulation of CD80 expression on DCs (D) and monocytes (E) from a healthy donor.
In the presence of huCD40LT and IL-2, there was a normal up-regulation of CD80 expression on the monocytes of all patients with myeloma in stable and in progressive disease. Unlike DCs, CD80 up-regulation on monocytes was attributed almost exclusively to IL-2 rather than to huCD40LT (Figure 4). Also in contrast to DCs, there was no further up-regulation of CD80 on the monocytes of patients with myeloma after the addition of either anti–IL-10 or anti–TGF-β.
Effect of rTGF-β1 on CD80 expression on normal DCs and monocytes
The addition of rTGF-β1 to cultures of normal mononuclear cells (n = 5) stimulated by huCD40LT and IL-2 caused a dose-dependent inhibition of the expected CD80 up-regulation (Figure6D,E). A significant inhibition of CD80 up-regulation was demonstrated with concentrations of rTGF-β1 as low as 2 pg/mL. Inhibition of CD80 up-regulation by rTGF-β1 was more marked on monocytes than on DCs.
Serum TGF-β1 and IL-10 levels
TGF-β1 and IL-10 were measured in the serum of the same patients tested for the CD80 response to huCD40LT + IL-2 and a group of controls (n = 12). Serum IL-10 levels were within the normal range (0-30 μg/L) for all 10 patients with myeloma. One patient had a modestly elevated TGF-β1 level at 29 μg/L (normal range = 0-20 μg/L).
Cytoplasmic expression of TGF-β1, LAP, and IL-10
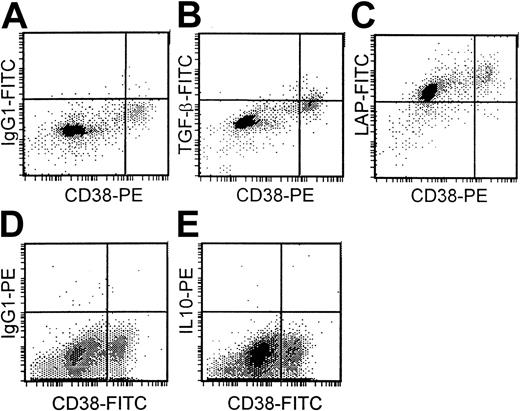
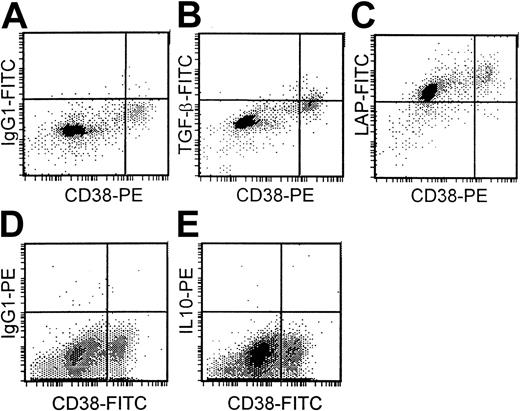
There was low or undetectable expression of TGF-β1(range, 0%-38%) and IL-10 (range, 0%-6%) in the cytoplasm of plasma cells (CD38++) of 13 different bone marrow samples from patients with myeloma. The percentage of plasma cells expressing TGF-β1 was significantly higher (P < .01) in the 7 patients with progressive disease (mean, 16.4%) than in the 6 patients with stable disease (mean, 2.9%). Cytoplasmic IL-10 expression was only detected in the plasma cells of 3 patients, all of whom also had small but detectable levels of TGF-β1expression. In contrast, LAP expression was high (more than 80% positive plasma cells) in samples from 8 patients but lower (10%-80% positive plasma cells) in samples from 5 patients. The 5 patients with the highest TGF-β1 expression had LAP expression of more than 90%. No obvious correlation existed, however, between LAP expression and either disease stage or TGF-β1 expression. Figure 7 demonstrates low TGF-β1 (Figure 7B), high LAP (Figure 7C), and absent IL-10 expression (Figure 7E) in plasma cells (CD38++) of a patient with myeloma with appropriate isotype controls (Figure 7A,D).
Cytoplasmic expression of TGF-β1, LAP, and IL-10 in plasma cells.
Isotype controls (A, D). Low TGF-β1 (B), high LAP (C), and absent IL-10 expression (E) in plasma cells (CD38++) of a patient with myeloma.
Cytoplasmic expression of TGF-β1, LAP, and IL-10 in plasma cells.
Isotype controls (A, D). Low TGF-β1 (B), high LAP (C), and absent IL-10 expression (E) in plasma cells (CD38++) of a patient with myeloma.
Discussion
The failure of intensive chemotherapy with autologous stem cell support to provide a cure for patients with myeloma has led to considerable interest in immunotherapy protocols for patients with this disease, and several clinical trials involving immunotherapy have already begun.11-14 The most commonly used protocols involve priming autologous monocyte-derived DCs with idiotype to stimulate a T-cell response—hence, an idiotype vaccination. However, patients with myeloma respond poorly to immunization by viral and bacterial antigens,7 and murine studies have suggested that this defect is associated with APCs rather than with T cells.8 It is, therefore, not surprising that idiotype vaccination trials have had little success. Clearly, more careful consideration of the biology of host–tumor interactions, including an understanding of the induction of T-cell tolerance by tumor antigens and a characterization of the dysfunction of APCs in patients with myeloma, is required before more effective immunotherapy schedules can be developed. This study has attempted to identify and characterize one defect in the DCs of patients with myeloma that may be at least partly responsible for the poor immune response in these patients. These in vitro experiments are a model for a potential immunotherapy strategy that would involve ex vivo up-regulation of CD80 on DCs in apheresis products.
The availability of a specific marker (CMRF44) on high-potency human blood DCs has allowed the introduction of a simple and reproducible assay for DCs in blood and has provided a new tool to study DC biology.5 6 The studies in this report have demonstrated that (1) the number of DCs in the peripheral blood of patients with multiple myeloma is relatively normal throughout the course of the disease; (2) peripheral blood DCs have an immature phenotype; (3) huCD40LT + IL-2 up-regulates the expression of the costimulatory molecule CD80 on normal DCs during 24-hour culture; (4) up-regulation of CD80 after huCD40LT and IL-2 stimulation on blood DCs (but not monocytes) from patients with myeloma is reduced in stable disease and absent in progressive disease; (5) this apparent functional defect in the DCs of patients with myeloma is caused by TGF-β1, IL-10, or both, which down-regulate the expression of CD80 even in the presence of huCD40LT + IL-2; (6) neither IL-10 nor the active form of the TGF-β1 molecule was significantly increased in the serum of these patients; (7) cytoplasmic TGF-β1expression was significantly higher in the plasma cells of patients with progressive disease than with stable disease; and (8) cytoplasmic IL-10 was only found when TGF-β1 expression was also detected. We conclude that because high CD80 expression on DCs is essential to induce a T-cell response, it may be necessary to select patients suitable for immunotherapy according to their ability to biologically modify the expression of the costimulatory molecule CD80 with an agent such as huCD40LT ex vivo.
If DCs from patients with myeloma are to be used as cellular vectors in immunotherapy protocols, it is important to determine whether these cells have a functional defect. There have been some controversial findings concerning DC biology in myeloma. It was originally reported that these cells are infected with the Kaposi sarcoma herpes virus (KSHV or HHV-8)23; however, many others have failed to reproduce these observations.24-26 In contrast to the current study, 2 studies on the phenotype and function of DCs from patients with myeloma did not report any abnormality.9,10The reason for these differences may relate to the difficulties in defining DC populations and comparing analyses of DCs between laboratories. The other studies do not specifically analyze the high-potency DCs marked by the antigen CMRF44. Our studies have used the new monoclonal antibody (CMRF44) to isolate high-potency DCs and, by ensuring that these cells did not express either CD14 or CD19, have focused on a standardized DC population in blood. Previous studies included cytokine-generated DC populations involving prolonged periods of in vitro culture, which may have corrected any in vivo microenvironment defects. Our methodology detected a defect in the early up-regulation of an essential costimulatory molecule (CD80) on the high-potency blood DCs of patients with myeloma. This may be a possible cause of the primary immune defect seen in these patients. Although the expression of CD86 did not appear to be impaired, good evidence suggests that CD80 expression is essential for the induction of anti-tumor immunity3 27 and cannot be replaced by CD86.
TGF-β1 has previously been shown to suppress T-cell proliferation and to inhibit T-cell signaling. Both TGF-β1 and IL-10 have been reported to be expressed at high levels in patients with B-cell lymphoma and are considered to be responsible for tumor-induced immunosuppression.28-31Whether these cytokines down-modulate the T-cell function of the entire T-cell repertoire or only induce tolerance of antigen-specific T cells has not yet been clearly determined. TGF-β derived from malignant plasma cells has been shown to inhibit activation and IL-2 responses in T lymphocytes of patients with myeloma, and the removal of TGF-β1 renders myeloma cells highly immunogenic.31 It is, therefore, likely that TGF-β1 and IL-10 cause T-cell unresponsiveness in myeloma and in lymphoma by a similar mechanism. We have previously demonstrated that in patients with myeloma, T cells express increased levels of CTLA-4 and decreased levels of CD28, suggesting that antigen presentation without adequate costimulation might have occurred.32
Other studies have demonstrated that cross-linking of CTLA-4 induces the production of TGF-β by murine CD4+ T cells and that CTLA-4, through TGF-β1, may serve as a counterbalance for CD28 costimulation of IL-2 and CD4+ T-cell activation.33 The fact that both anti–TGF-β1 and anti–IL-10 both neutralized the inhibition of CD80 up-regulation after huCD40LT stimulation in our studies suggests that there may be a linked mechanism for the action of these 2 cytokines. It has been shown that tumor-derived TGF-β can induce an overproduction of IL-10, suggesting that the impact of IL-10 is downstream of TGF-β1.34 In our studies, cytoplasmic IL-10 was only found in plasma cells with active TGF-β1 expression. It is important to note that TGF-β1 is normally found as a latent, high-molecular–weight complex composed of TGF-β1, LAP, and a latent TGF-β–binding protein. Anti–TGF-β1 used in ELISA and flow cytometry assays usually fails to detect TGF-β1 unless released from this complex. Thus, when performing serum ELISA, serum is acidified before assay. Interpretation of the apparent low intracellular expression of TGF-β1using flow cytometry must be treated with some caution because most intracellular TGF-β1 is bound in the latent complex.
Oreffo et al35 reported that osteoclast-derived cells in culture can activate TGF-β. Given that osteoclasts create acidic microenvironments, it is possible that in patients with progressive-stage myeloma with active lytic lesions, TGF-β1 may be locally dissociated from the precursor complex because of local environmental conditions. Our studies have confirmed that significantly higher levels of cytoplasmic TGF-β1 are found in the plasma cells of patients with progressive rather than stable disease. If the release of active TGF-β1 is restricted to the bone marrow local microenvironment, increased levels of active TGF-β1 would not necessarily be detectable in the circulating peripheral blood. Whether TGF-β1 acts directly or indirectly on DCs is uncertain. Our hypothesis is that the cytoplasm of malignant plasma cells is the most likely source of active TGF-β1. The large reservoir of cytoplasmic LAP ensures that TGF-β1 is usually not in its active form. Increased osteoclast activity and perhaps reduced LAP concentration in progressive disease would enhance the dissociation of active TGF-β. Increased levels of tumor-derived TGF-β would stimulate IL-10 production.34 The failure of T cells to respond to stimulation then at least partly results from cytokine-induced inhibition of costimulatory molecule expression on DCs. Whether this T-cell unresponsiveness is global or antigen specific must be better understood. Recent studies have demonstrated that circulating T cells specific for tumor-associated antigens in patients with melanoma have all the hallmarks of CD8 effector cells but are functionally unresponsive and unable to control tumor growth in vitro or in vivo despite robust allogeneic responses within the CD8+ population.36
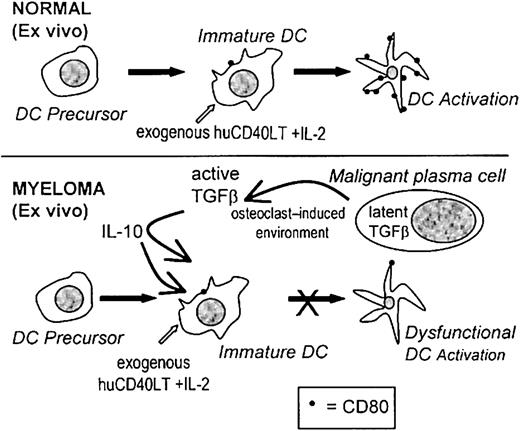
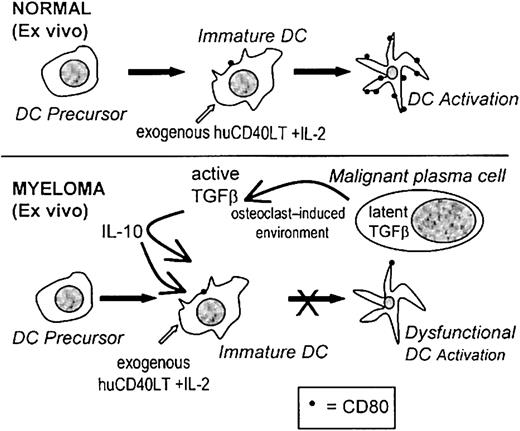
If immunotherapy is to succeed in patients with myeloma, it is important to recognize that the dysfunctional immune state that exists in these patients will result in a poor response to vaccination procedures. The real challenge for immunotherapy may well be to learn from the deficiencies in current protocols and to recognize that multiple defects in the immune response must be considered and overcome. In this study we have identified one of these defects, and we suggest that the up-regulation of CD80 expression on DC ex vivo may be an important new strategy for immunotherapy and that it may be important to determine which patients will benefit from ex vivo stimulation. A proposed mechanism for the inhibition of the activation of high-potency DCs ex vivo is represented in Figure8. Clearly, the DCs of some patients fail to respond to huCD40LT stimulation and remain dysfunctional. Reasonable evidence now suggests that failure to up-regulate the expression of costimulatory molecules on DCs will at best produce immunologic ignorance and at worst induce tolerance.2 3
Proposed mechanism causing dysfunctional DCs.
A proposed mechanism for the factors involved during the ex vivo activation of CD80 on DCs and the inhibitory effects of TGF-β1 and IL-10 in patients with myeloma.
Proposed mechanism causing dysfunctional DCs.
A proposed mechanism for the factors involved during the ex vivo activation of CD80 on DCs and the inhibitory effects of TGF-β1 and IL-10 in patients with myeloma.
Supported by the Anthony Rothe Memorial Trust and Foundation IV.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ross D. Brown, Institute of Haematology, Royal Prince Alfred Hospital, Missenden Rd, Camperdown, NSW 2120, Australia; e-mail: rbrown@haem.rpa.cs.nsw.gov.au.