Abstract
Chronic granulomatous disease (CGD) is an inherited primary immunodeficiency characterized by phagocytes devoid of a functioning nicotinamide adenine dinucleotide phosphate (NADPH) oxidase. The failure of CGD phagocytes to produce reactive oxygen species (ROS) results in a marked increase in the susceptibility of affected patients to life-threatening bacterial and fungal infections. This study investigated whether loading of CGD phagocytes with glucose oxidase (GO)–containing liposomes (GOLs) could restore cellular production of bactericidal ROS (eg, H2O2 and HOCl) in vitro. Results indicate that GO encapsulated in liposomes enabled NADPH oxidase-deficient phagocytes to use H2O2 for the production of highly bactericidal HOCl. The intracellular colocalization of bacteria and liposomes (or liposome-derived ferritin) was demonstrated by confocal laser microscopy and electron microscopy. After uptake of GOLs (approximately 0.2 U/mL at 1 mM total lipid concentration, size approximately 180 nm), CGD granulocytes produced HOCl levels comparable to those of normal phagocytes. Remarkably, after treatment with GOLs, CGD phagocytes killed Staphylococcus aureus as efficiently as normal granulocytes. Moreover, treated cells retained sufficient motility toward chemotactic stimuli as measured by chemotaxis assay. Side effects were evaluated by measuring the H2O2 concentrations and the production of methemoglobin in whole blood. These studies revealed that H2O2 produced by GOLs was degraded immediately by the antioxidative capacity of whole blood. Elevated methemoglobin levels were observed only after application of extremely high amounts of GOLs (2 U/mL). In summary, the application of negatively charged GOLs might provide a novel effective approach in the treatment of patients with CGD at high risk for life-threatening infections.
Introduction
Chronic granulomatous disease (CGD) is a primary immunodeficiency with an incidence of 1:1 000 000 to 1:250 000.1 It is an inherited disorder (X chromosomal or autosomal recessive) of the phagocyte system.2 Phagocytes (granulocytes and monocytes) of patients with CGD lack a functional nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and are not able to produce reactive oxygen species (ROS), necessary for microbial killing.3 The disease is therefore dominated by high susceptibility to a variety of bacteria and fungi, especially those with catalase activity.4 Therapy and prevention of infections include the life-long administration of antibiotics and antimycotics, and in severe cases, the transfusion of allogeneic granulocytes to provide the patient with functioning phagocytes.5
In recent years the idea of encapsulating drugs such as antibiotics or cytostatics into liposomal carriers has become more feasible.6 Phagocytes tend to take up conventional liposomes very easily.7 This uptake is often a disadvantage in the use of liposomes for many medical applications and clinical trials because of rapid elimination of the encapsulated drug. We developed glucose oxidase (GO)–containing liposomes (GOLs) that were successfully phagocytosed either by normal or CGD phagocytotic cells. The positive effects of H2O2 derived from GO or GO bound to latex beads in CGD cells were reported by several investigators.8,9 The idea of restoring the H2O2 production of CGD cells with the use of GOLs was first considered by Ismail and coworkers10 20 years ago. It was not further followed up because of a poor knowledge of liposome preparation methods at that time. However, present liposome technology and research allow a wide range of possible applications. In the case of CGD, therapeutic application of GOLs for the restoration of ROS production could be useful because phagocytes of patients with CGD have a normal myeloperoxidase (MPO) activity.11 The GO-derived H2O2can be converted within CGD phagocytes into the strong oxidant HOCl during the MPO reaction:
In the present study we demonstrate the in vitro restoration of ROS production by CGD phagocytes after their treatment with different preparations of GOLs.
Materials and methods
Subjects and cell preparation
Human blood leukocytes were obtained from 10 healthy volunteers and from 4 patients with CGD (3 with an X-linked defect of NADPH oxidase [gp91phox] and one with an autosomal recessive form). Whole blood analysis and evaluation of MPO activity was performed by the routine blood analyser H3 and its successor ADVIA120 (Bayer/Technicon, Munich, Germany) based on the principles of flow cytometry: leukocytes were counted and differentiated according to their size (y-axis) and peroxidase activity (x-axis). Neutrophils with normal MPO activity range between a mean peroxidase index (MPXI) of −10 to +10. Cells were isolated from heparinized whole blood through centrifugation on a 2-step density gradient with Histopaque 1119 and 1077 (Sigma, Deisenhofen, Germany) as described elsewhere.12 Contaminating erythrocytes were lysed using ice-cold ammonium chloride buffer (0.829% w/v) for 15 minutes. Purity of the isolated granulocytes was 97% to 98%. Cells were brought to the desired concentration with phosphate-buffered saline (PBS) or Hanks balanced salt solution (HBSS, containing glucose) purchased from Biochrom (Berlin, Germany).
Preparation of liposomes
Liposomes made up of different lipid compositions were prepared by extrusion (see below). Egg phosphatidylcholine (EPC), egg phosphatidylglycerol (EPG), and soy phosphatidylserine (SPS) were kindly provided by Lipoid (Ludwigshafen, Germany). Cholesterol (Chol) was obtained from Sigma. The lipid mixture (EPC:SPS:Chol or EPC:EPG:Chol, each 4:3:3 mole ratio, or EPC:Chol, 7:3 mole ratio) was dissolved in dichloromethane or methanol or both. For fluorescence-activated cell sorter (FACS) analysis 1 mol% rhodamine-B-phosphatidylethanolamine (PE; Molecular Probes, Leiden, The Netherlands) was added as fluorescent lipid label. The organic solvent was removed completely under reduced pressure and the lipid film was dissolved in an isotonic phosphate buffer. For encapsulation of GO (Aspergillus niger, Boehringer Mannheim, Mannheim, Germany), the liposomes consisted either of EPC:SPS:Chol or EPC:EPG:Chol (each 4:3:3 mole ratio). The lipid film was dispersed in an isotonic phosphate buffer containing 6 mg/mL GO to yield a total lipid concentration of 20 mM. The dispersion was freeze-thawed 5 times before extrusion. For all liposome preparations the dispersion was extruded 21 times through a polycarbonate membrane with 0.2 μm pores (Nuclepore, Pleasanton,). It ensured sufficient sizing of liposomes and achieving an average number of lipid lamellae of 1 to 2. Unencapsulated GO was removed by gel chromatography on Sepharose 4B-CL. Liposomes were concentrated by ultracentrifugation with 150 000g for 180 minutes (LE-80 Ultracentrifuge, Rotor 50.4 Ti, Beckman, Munich, Germany). The resulting lipid concentration was 20 mM, the GO-trapping efficiency was 5%, and approximately 0.2 U/mL related to 1 mM total lipid concentration, as measured by GO liposomal H2O2 production.13 The particle size was measured by photon correlation spectroscopy using a Zetamaster S (Malvern Instruments, Malvern, United Kingdom).
Preparation of ferritin liposomes
The preparation of ferritin-containing liposomes, used for electron microscopic studies, was carried out similarly to GOLs. A solution of 50 mg/mL ferritin (type I, from horse spleen, Sigma) instead of GO was added to the lipid film. Extrusion was performed without the prior freezing-thawing step to avoid damage of the freeze-sensitive ferritin. Free ferritin was removed from liposomes by gel chromatography.
Uptake analysis
The uptake of fluorescence-labeled liposomes by leukocytes in whole blood was evaluated by FACS analysis (Becton Dickinson, Heidelberg, Germany) with a total count of 10 000 cells. Data were analyzed using the Lysis II program from Becton Dickinson. Heparinized whole blood (100 μL) was incubated with rhodamine-labeled liposomes (1 mM total lipid concentration) at 37°C for 0, 20, 40, and 60 minutes. After incubation, red blood cells were lysed using an ammonium chloride buffer; the remaining leukocytes were washed 3 times in PBS and stored on ice in the dark until measurement.
Intracellular location of GOL and S aureus
Intracellular location was evaluated by confocal laser microscopy (TCS4D, Leica Microscopy, Wetzlar, Germany) and by transmission electron microscopy (Philips EM 208S, Eindhoven, The Netherlands). For confocal analyses 500 000 granulocytes were incubated in a total volume of 500 μL PBS with 5 × 106S aureus particles (cell-to-bacteria ratio 1:10) labeled with BODIPY (Molecular Probes, Eugene, OR) at 37°C for 15 minutes. Cells were washed twice in ice-cold PBS and resuspended in PBS/glucose (0.1% w/v). EPC:EPG:Chol liposomes (1 mM lipid), rhodamine-labeled, were added and further incubated for 20 minutes. Cells were washed and fixed with 2% paraformaldehyde.
For electron microscopy, 15 million granulocytes were incubated in a total volume of 1000 μL PBS with 15 × 107S aureus particles (cell-to-bacteria ratio 1:10) and ferritin liposomes (3 mM lipid) at 37°C for 60 minutes. In parallel, granulocytes were incubated as described for confocal analyses or, as negative control, only with plain liposomes. Cells were washed and fixed with glutaraldehyde (2.5% in PBS, v/v) at 4°C for 30 minutes. Cells were further fixed with OsO4 (2% in PBS, w/v). Block staining was performed overnight in uranyl acetate (2% in aqua destillata, w/v). Samples were dehydrated in a graded series of ethanol concentrations and polymerized in Epon 812 (Fluka, Buchs, Switzerland). Ultrathin sections were cut and mounted on copper grids.
Flow cytometric analysis of GO activity in granulocytes
Granulocytes (500 000) were incubated with GOLs (1 mM lipid) in a total volume of 500 μL in PBS at 37°C for 15 minutes. Cells were washed 3 times in ice-cold buffer and resuspended in 500 μL PBS/glucose (0.1% w/v) and incubated at 37°C for different time periods. At the end of the incubation time cells were placed on ice, and dihydrorhodamine 123 (DHR; 0.01 mg/mL; Molecular Probes) was added and the cells were further incubated for 15 minutes. Tubes were placed on ice, washed once with PBS, and measured immediately by FACS analysis (Becton Dickinson) using live-gate for at least 10 000 cells.
Production of HOCl
Production of HOCl was measured by chlorination of taurine followed by the oxidation of 5-thio-2-nitrobenzoic acid (TNB) to 5,5′-dithio-bis(2-nitrobenzoic) acid (DTNB, Sigma). Briefly, 5 × 106 granulocytes/mL were incubated in HBSS plus taurine (15 mM) with GOLs (1 mM total lipid concentration) at 37°C for 30 minutes. The reaction was stopped by adding catalase (290 U/mL) and samples were centrifuged at 12 000g for 10 minutes. Supernatant was mixed with TNB (2 mM) and immediately measured at 412 nm. The amount of HOCl was calculated using the molar linear absorption coefficient of ε = 1410 m2/mol.
Killing assay
The killing assay was performed according to Metcalf and colleagues14 with slight modifications. In brief, S aureus (American Type Culture Collection 25923) was opsonized with 10% pooled human serum and then added to normal and CGD granulocytes in HBSS/0.1% (w/v) gelatin at a bacterium-to-granulocyte ratio of 1:1 (5 × 105 bacteria and granulocytes, respectively). Granulocytes were either pretreated with GOLs (1 mM lipid, approximately 0.2 U/mL GO) at 37°C for 15 minutes in PBS without glucose, then washed 3 times in PBS and mixed with bacteria, or incubated directly with liposomes and bacteria. Tubes were rotated at 37°C and the killing was stopped after 0, 1, and 2 hours by adding ice-cold distilled water. Tubes were mixed and diluted 1:50 and 1:250 with HBSS. Fifty microliters of the dilution was plated on trypticase-soy agar (Bio-Mérieux, Berlin, Germany). Total viable bacteria were counted as colonies after 12 hours of incubation at 37°C.
Degranulation of granulocytes by liposomes
About 3 million granulocytes were incubated with plain liposomes (1 mM lipid) or as positive control, with cytochalasin B and N-formyl-Meth-Leu-Phe (NfMLP; 0.5 μg/mL and 10−6 M, respectively) in dimethylsulfoxide (DMSO) at 37°C for 0 and 30 minutes. Degranulation was followed by measuring the samples by flow cytometry using the Bayer ADVIA120.
Chemotaxis assay
Chemotaxis assay was performed by the gelatin-agarose migration assay according to Cline15 with slight modifications. One million granulocytes in HBSS were incubated in slots of prepared gelatin-agarose slides and migrated against 1 nmol/mL NfMLP (Sigma) and 1 nmol/mL leukotriene B4 (LTB4, Sigma) at 37°C in a humidified atmosphere for 2 hours. Granulocytes were incubated with GOLs (1 mM total lipid) in PBS without glucose (to prevent premature H2O2 production) at 37°C for 15 minutes and then washed 3 times in PBS. Migration was stopped by fixation of the slides in 2.5% (v/v) glutaraldehyde (Merck, Darmstadt, Germany) for 30 minutes. Agarose was washed away and slides were stained by Pappenheim. Spontaneous migration was subtracted from migration against chemotactic agent by microscopic analysis.
Methemoglobin production and glucose consumption
Methemoglobin and glucose were determined using the blood gas analyser ABL 700 (Radiometer, Willich, Germany). Liposomal GO was incubated in different concentrations (liposomal GO: 2, 0.2, and 0.002 U/mL; respectively 10, 1, and 0.01 mM total lipid concentration) together with 0.1 mL heparinized whole blood at 37°C for 0 to 120 minutes. Samples were measured immediately after stopping the reaction on ice.
H2O2 concentration
The concentration of H2O2 in whole blood or PBS/0.1% (w/v) glucose was measured according to Thurman and coworkers.13 Liposomal GO was incubated in different concentrations (liposomal GO: 2, 0.2, and 0.002 U/mL; 10, 1, and 0.01 mM total lipid concentration) at 37°C for 0 to 120 minutes.
Results
Uptake of different types of liposomes
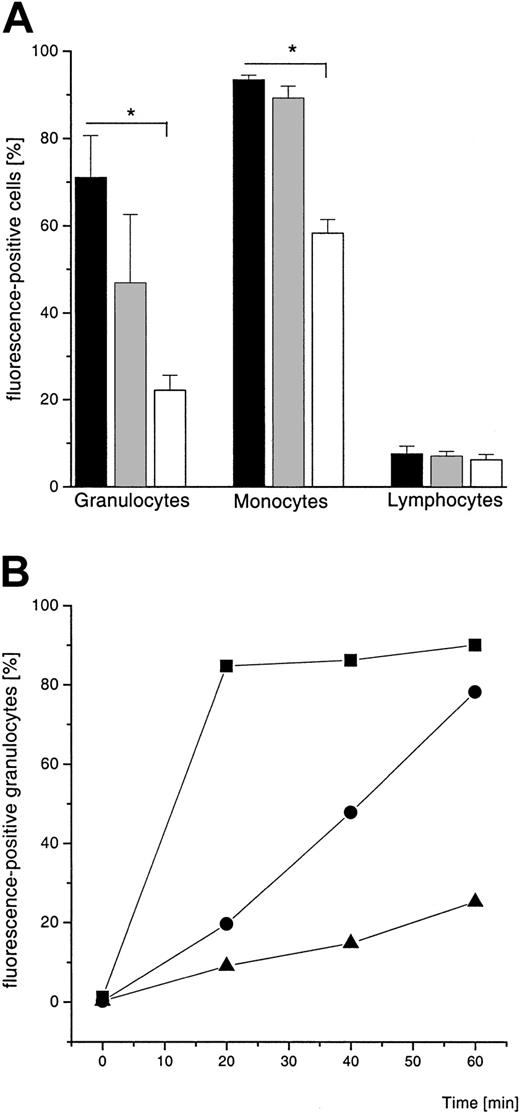
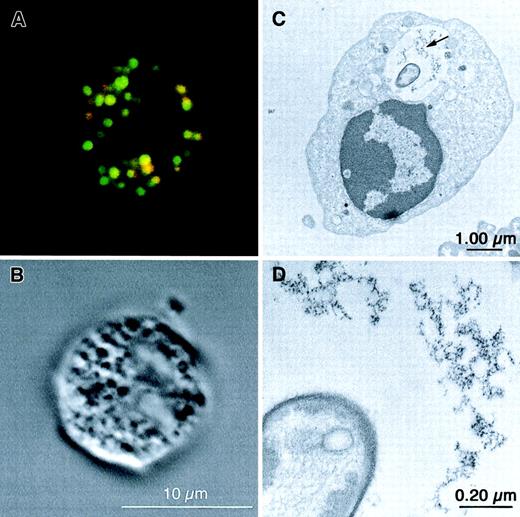
Flow cytometric analysis of the uptake of 2 fluorescence-labeled types of negatively charged liposome formulations (EPC:SPS:Chol and EPC:EPG:Chol; size: 160 ± 10 nm and 180 ± 10 nm, respectively) and one uncharged liposome type (EPC:Chol, size: 200 ± 10 nm) revealed that negatively charged liposomes are phagocytosed preferentially by granulocytes and monocytes (Figure1A). In contrast, less than 10% of the lymphocyte population showed liposome association. The uptake of liposomes by granulocytes was time-dependent (Figure 1B). Based on these analyses all further experiments were performed with negatively charged liposomes (EPC:SPS:Chol and EPC:EPG:Chol). Confocal microscopic analysis of subsequent incubation of granulocytes with S aureus (green) and liposomes (red, EPC:EPG:Chol) demonstrated that both are localized mainly inside the phagocyte. Color overlay (yellow) indicates the colocalization of bacteria and liposomes (Figure2A). With the electron microscopic analyses we can show that some of the bacteria are located inside the same vacuole with ferritin, derived from the uptake of ferritin-containing liposomes (Figure 2C,D). The negative control with plain liposomes failed to show the electron-dense structure (not shown). Both confocal laser microscopy and electron microscopy gave no evidence for extracellular membrane attachment of liposomes.
Flow cytometric analysis of charged and uncharged liposome formulations.
(A) FACS analysis of the uptake of rhodamine-B-PE–labeled liposomes by leukocytes in whole blood after 60 minutes. Shown is the percentage of fluorescence-positive cells (mean ± SD, n = 3). ▪ indicates EPC:SPS:Chol; ░, EPC:EPG:Chol (both 4:3:3 mole ratio and negatively charged); ■, EPC:Chol (7:3 mole ratio, uncharged). Results of 2-group t test are marked with asterisk; P < .001. (B) FACS analysis of the time-dependent uptake of rhodamine-B-PE–labeled liposomes by granulocytes (5 × 105 cells/mL). Liposomal lipid concentration is 1 mM. Values are given as percentage of the whole population, showing a single experiment of 3 independent experiments; ▪ indicates EPC:SPS:Chol; ●, EPC:EPG:Chol (both 4:3:3 mole ratio); ▴, EPC:Chol (70:30 mol%).
Flow cytometric analysis of charged and uncharged liposome formulations.
(A) FACS analysis of the uptake of rhodamine-B-PE–labeled liposomes by leukocytes in whole blood after 60 minutes. Shown is the percentage of fluorescence-positive cells (mean ± SD, n = 3). ▪ indicates EPC:SPS:Chol; ░, EPC:EPG:Chol (both 4:3:3 mole ratio and negatively charged); ■, EPC:Chol (7:3 mole ratio, uncharged). Results of 2-group t test are marked with asterisk; P < .001. (B) FACS analysis of the time-dependent uptake of rhodamine-B-PE–labeled liposomes by granulocytes (5 × 105 cells/mL). Liposomal lipid concentration is 1 mM. Values are given as percentage of the whole population, showing a single experiment of 3 independent experiments; ▪ indicates EPC:SPS:Chol; ●, EPC:EPG:Chol (both 4:3:3 mole ratio); ▴, EPC:Chol (70:30 mol%).
Localization of liposomes and
S aureus in granulocytes by confocal laser microscopy and electron microscopy. (A) Confocal laser microscopy. Granulocytes (1 × 106/mL) were incubated at 37°C for 15 minutes with 10 × 106 BODIPY-labeled (green) S aureus particles, followed by an incubation with 1 mM rhodamine-B-PE–labeled (red) liposomes (EPC:EPG:Chol, 4:3:3 mole ratio) for 20 minutes. Colocalization of S aureusand liposomes is suggested in several vacuoles appearing yellow (red-green overlay). (B) Confocal laser microscopy. Same granulocyte visualized by differential interference contrast (DIC) microscopy. (C) Electron microscopy. Isolated normal granulocytes (15 × 106/mL) were incubated with 15 × 107S aureus particles and 3 mM ferritin-containing liposomes (EPC:EPG:Chol, 4:3:3 mole ratio) at 37°C for 60 minutes. The arrow indicates the ferritin particles derived from liposomes. (D) Higher magnification of the ferritin-containing vacuole of the same cell as in panel C.
Localization of liposomes and
S aureus in granulocytes by confocal laser microscopy and electron microscopy. (A) Confocal laser microscopy. Granulocytes (1 × 106/mL) were incubated at 37°C for 15 minutes with 10 × 106 BODIPY-labeled (green) S aureus particles, followed by an incubation with 1 mM rhodamine-B-PE–labeled (red) liposomes (EPC:EPG:Chol, 4:3:3 mole ratio) for 20 minutes. Colocalization of S aureusand liposomes is suggested in several vacuoles appearing yellow (red-green overlay). (B) Confocal laser microscopy. Same granulocyte visualized by differential interference contrast (DIC) microscopy. (C) Electron microscopy. Isolated normal granulocytes (15 × 106/mL) were incubated with 15 × 107S aureus particles and 3 mM ferritin-containing liposomes (EPC:EPG:Chol, 4:3:3 mole ratio) at 37°C for 60 minutes. The arrow indicates the ferritin particles derived from liposomes. (D) Higher magnification of the ferritin-containing vacuole of the same cell as in panel C.
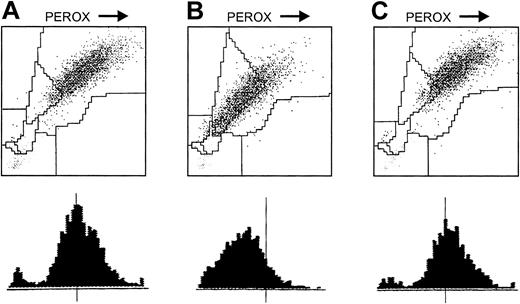
MPO activity
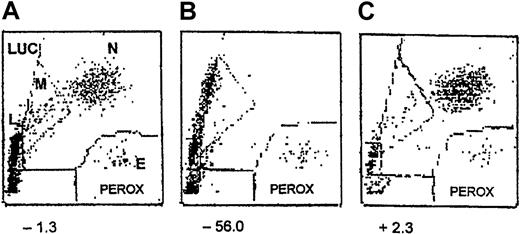
The basic requirement for the successful application of GOLs in CGD cells is the presence of an active MPO inside the NADPH oxidase-deficient (CGD) phagocytes. The MPXI of normal and CGD neutrophils from the patients of this study ranged between −10 and +10, indicating normal MPO activity (Figure3A,C). For comparison, an H3-leukogram of a person with MPO deficiency (MPXI = −56) is shown where the neutrophil cloud shifted to the left due to lack of MPO (Figure 3B).
Demonstration of MPO activity in CGD granulocytes in whole blood with the Bayer/Technicon H3 (leukogram).
(A) Whole blood of a healthy person (x-axis, peroxidase activity; y-axis, forward scattering). (B) Whole blood of a person with MPO deficiency. (C) Whole blood of a person with CGD. The MPXI is marked below; N indicates neutrophils; E, eosinophils; M, monocytes; L, lymphocytes; LUC, large unstained cells.
Demonstration of MPO activity in CGD granulocytes in whole blood with the Bayer/Technicon H3 (leukogram).
(A) Whole blood of a healthy person (x-axis, peroxidase activity; y-axis, forward scattering). (B) Whole blood of a person with MPO deficiency. (C) Whole blood of a person with CGD. The MPXI is marked below; N indicates neutrophils; E, eosinophils; M, monocytes; L, lymphocytes; LUC, large unstained cells.
Intracellular GO activity after uptake of GOLs by CGD granulocytes
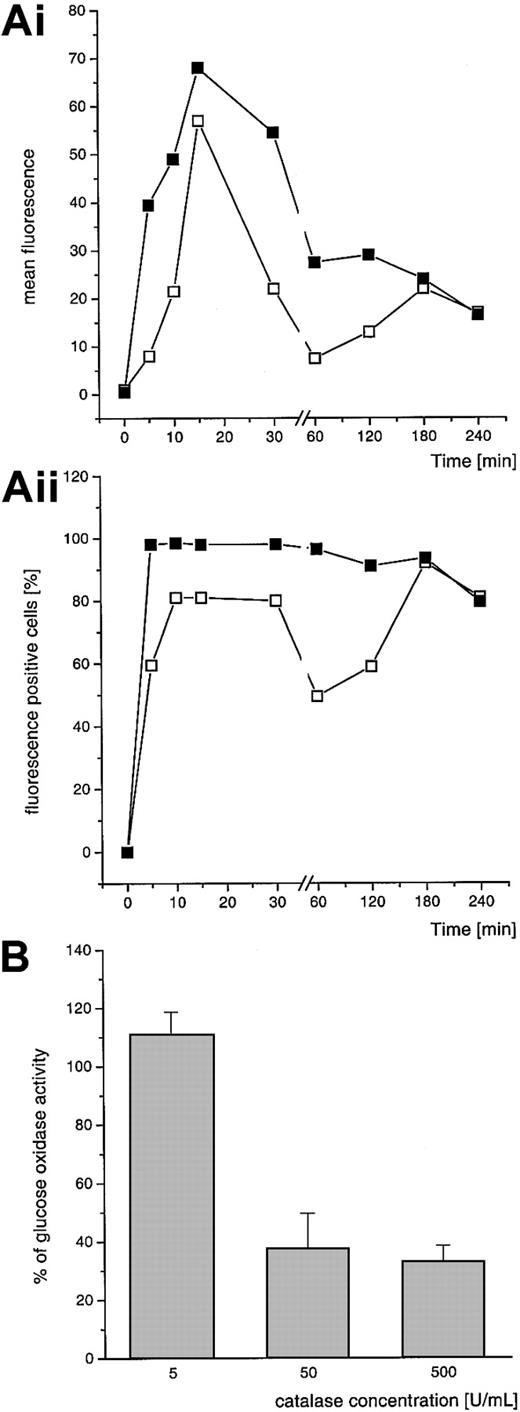
The detection of H2O2-positive granulocytes with the DHR assay revealed that the amount of fluorescence-positive cells was stable for 180 minutes. The mean fluorescence of intracellular DHR oxidation began to decrease after 15 to 30 minutes (Figure 4A). The extracellular addition of catalase (50 U/mL) diminished the H2O2 concentration outside the granulocytes. There was no additional decrease in DHR-detectable H2O2 production with higher concentrations of catalase (Figure 4B). The amount and the mean fluorescence of DHR+ cells by catalase addition decreased over 180 minutes. After this time no further loss was observed. Furthermore, the DHR assay revealed that there are still H2O2-producing CGD granulocytes after 8 hours (75% fluorescence-positive cells at 8 hours after loading the cells with GOL (EPC:EPG:Chol; mean, n = 2). Results are shown only for EPC:EPG:Chol liposomes, but those for EPC:SPS:Chol were comparable.
DHR oxidation by GOL-loaded CGD granulocytes.
(A) (Ai indicates mean fluorescence; Aii, fluorescence-positive cells.) Cells were preincubated with GOLs (EPC:EPG:Chol, both 4:3:3 mole ratio, 1 mM) in PBS at 37°C for 15 minutes. Nonincorporated liposomes were washed out and the incubation was followed for different time periods (5-240 minutes) in PBS plus glucose. Subsequently, DHR was added. Diffusion of extracellular H2O2 was reduced by addition of catalase (50 U/mL) at time point 0 of the cellular incubation. Mean of 2 independent experiments with CGD granulocytes of 2 different patients; ▪ indicates fluorescence without catalase; ■, fluorescence with addition of catalase. (B) Effect of different concentrations of catalase over 30 minutes of incubation. Values are expressed as percentage of remaining DHR oxidation of CGD cells under the influence of catalase; mean of 3 independent experiments with granulocytes of different donors, mean ± SD.
DHR oxidation by GOL-loaded CGD granulocytes.
(A) (Ai indicates mean fluorescence; Aii, fluorescence-positive cells.) Cells were preincubated with GOLs (EPC:EPG:Chol, both 4:3:3 mole ratio, 1 mM) in PBS at 37°C for 15 minutes. Nonincorporated liposomes were washed out and the incubation was followed for different time periods (5-240 minutes) in PBS plus glucose. Subsequently, DHR was added. Diffusion of extracellular H2O2 was reduced by addition of catalase (50 U/mL) at time point 0 of the cellular incubation. Mean of 2 independent experiments with CGD granulocytes of 2 different patients; ▪ indicates fluorescence without catalase; ■, fluorescence with addition of catalase. (B) Effect of different concentrations of catalase over 30 minutes of incubation. Values are expressed as percentage of remaining DHR oxidation of CGD cells under the influence of catalase; mean of 3 independent experiments with granulocytes of different donors, mean ± SD.
HOCl production
Native CGD cells, which are unable to produce HOCl due to their NADPH oxidase defect, produced comparable amounts of HOCl to those of normal granulocytes when treated with GOLs (Figure5). Cells were preincubated with GOLs, the unbound liposomes were washed out, and the incubation was continued in glucose-containing medium for 30 minutes. When the incubation was carried out without removal of GOLs, the amount of produced HOCl was, of course, higher in both cell types (for SPS: 180 ± 36 and 167.5 ± 35.5 nmoles/30 min; for EPG: 184.5 ± 26.5 and 138.5 ± 55.5 nmoles/30 min, normal and CGD, respectively). Therefore at least 15% of the HOCl production within CGD cells resulted from incorporation of GOLs (Figure 5). No HOCl could be detected in the presence of 1 mM l-methionine, a scavenger of HOCl. There was no significant difference for both liposome formulations.
HOCl production by normal and CGD granulocytes (5 × 106/mL).
Cells were preincubated with GOLs (EPC:SPS:Chol and EPC:EPG:Chol, both 4:3:3 mole ratio, 1 mM) at 37°C for 15 minutes. Nonincorporated liposomes were washed out and the incubation was followed for 30 minutes in PBS plus glucose. Negative controls were performed by addition of l-methionine [1 mM]. Mean ± SD of 2 independent experiments in duplicate. ▪ indicates normal; ░, CGD.
HOCl production by normal and CGD granulocytes (5 × 106/mL).
Cells were preincubated with GOLs (EPC:SPS:Chol and EPC:EPG:Chol, both 4:3:3 mole ratio, 1 mM) at 37°C for 15 minutes. Nonincorporated liposomes were washed out and the incubation was followed for 30 minutes in PBS plus glucose. Negative controls were performed by addition of l-methionine [1 mM]. Mean ± SD of 2 independent experiments in duplicate. ▪ indicates normal; ░, CGD.
Killing of S aureus
The bactericidal activity of GOL-treated granulocytes was investigated by killing of S aureus. Untreated CGD granulocytes (without liposome addition) showed no reduction of bacterial growth after 1 hour (98% ± 22%; n = 3) compared to normal controls, and only a slight decrease after 2 hours (89% ± 24%; n = 3; Figure 6). Normal granulocytes reduced the bacterial growth to 75% ± 22% after 1 hour and to 69% ± 14% after 2 hours of incubation. Interestingly, CGD cells preincubated with GOLs (nonincorporated liposomes were removed with the supernatant after 15 minutes by washing the cells with PBS) showed the most effective and significant killing of S aureus after 2 hours (65% ± 13% for EPC:SPS:Chol and 66% ± 7% for EPC:EPG:Chol, respectively) compared to untreated CGD control. Incubation of bacteria alone with GOLs showed no reduction of their growth.
Killing of
S aureus by normal and CGD granulocytes. Cells (5 × 105) were incubated at 37°C with S aureus (5 × 105) with and without liposome treatment. GOLs (EPC:SPS:Chol, panel A, and EPC:EPG:Chol, panel B, both 4:3:3 mole ratio, lipid concentration 1 mM) were mixed with granulocytes. Incubation was carried out in the continuous presence of GOLs or after a 15-minute preincubation and subsequent removal of nonphagocytosed GOLs before bacteria were added. Values were set as 100% at the start (0 hours). Shown are the mean values of 3 independent experiments in duplicates (CV [coefficient of variation] < 0.27); ▪ indicates normal granulocytes; ●, CGD granulocytes (both without liposome treatment); ▴, liposomes; ▾, CGD granulocytes with continuous incubation of liposomes; ♦, CGD granulocytes preincubated with liposomes. Results of 2-groupt test are marked with asterisk; P < .05.
Killing of
S aureus by normal and CGD granulocytes. Cells (5 × 105) were incubated at 37°C with S aureus (5 × 105) with and without liposome treatment. GOLs (EPC:SPS:Chol, panel A, and EPC:EPG:Chol, panel B, both 4:3:3 mole ratio, lipid concentration 1 mM) were mixed with granulocytes. Incubation was carried out in the continuous presence of GOLs or after a 15-minute preincubation and subsequent removal of nonphagocytosed GOLs before bacteria were added. Values were set as 100% at the start (0 hours). Shown are the mean values of 3 independent experiments in duplicates (CV [coefficient of variation] < 0.27); ▪ indicates normal granulocytes; ●, CGD granulocytes (both without liposome treatment); ▴, liposomes; ▾, CGD granulocytes with continuous incubation of liposomes; ♦, CGD granulocytes preincubated with liposomes. Results of 2-groupt test are marked with asterisk; P < .05.
Effect of liposomes on degranulation
Granulocytes that are confronted with phagocytosable liposomes may react with degranulation. For detection of degranulation, release of MPO from azurophilic granules was measured16 using the Bayer ADVIA120. Incubation of isolated granulocytes with cytochalasin B/NfMLP for 30 minutes resulted in a clear shift of the granulocyte cloud to the left, indicating an intense release of MPO. In contrast, no shift was observed after incubation with plain liposomes and GOLs (Figure 7).
Influence of liposomes on degranulation of granulocytes.
Granulocytes were incubated with DMSO (A), with cytochalasin B (0.5 μg/mL)/NfMLP (10−6 M) diluted in DMSO, positive control (B), and with plain liposomes (EPC:EPG:Chol, 1 mM lipid) (C) at 37°C for 30 minutes. Degranulation of MPO from azurophilic granules of neutrophils was measured by registration of the shift of the granulocyte cloud from right to left on the x-axis (decrease of MPO in single neutrophils). Plain EPC:SPS:Chol liposomes and GOLs gave the same results, and incubation with DMSO for 30 minutes had no influence on the signals compared to freshly prepared granulocytes (data not shown).
Influence of liposomes on degranulation of granulocytes.
Granulocytes were incubated with DMSO (A), with cytochalasin B (0.5 μg/mL)/NfMLP (10−6 M) diluted in DMSO, positive control (B), and with plain liposomes (EPC:EPG:Chol, 1 mM lipid) (C) at 37°C for 30 minutes. Degranulation of MPO from azurophilic granules of neutrophils was measured by registration of the shift of the granulocyte cloud from right to left on the x-axis (decrease of MPO in single neutrophils). Plain EPC:SPS:Chol liposomes and GOLs gave the same results, and incubation with DMSO for 30 minutes had no influence on the signals compared to freshly prepared granulocytes (data not shown).
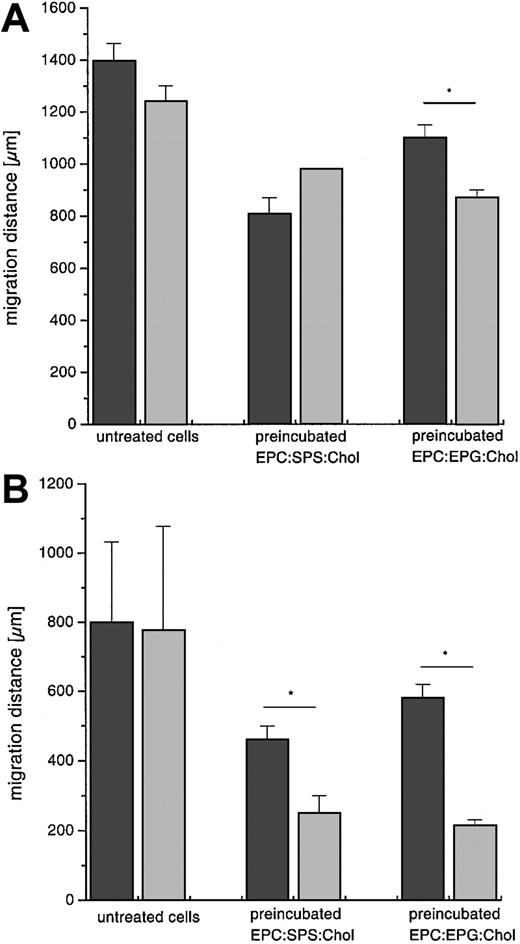
Chemotaxis after GOL treatment
The effect of GOL treatment of normal and CGD cells was tested by their migration toward chemotactic stimuli such as NfMLP (Figure8A) or LTB4 (Figure 8B). The preincubation of granulocytes with GOLs resulted in a significant decrease of the migration distance. Nevertheless, granulocytes retained motility toward the chemotactic stimuli after ingestion of GOLs. Statistical analysis revealed differences after preincubation of CGD cells with liposomes compared to normal cells (as indicated with the asterisk in Figure 8).
Chemotaxis assay of normal and CGD granulocytes.
Cells (1 × 106) migrated with and without GOL treatment toward NfMLP, 1 μM (A), and LTB4, 1 μM (B). Granulocytes were preincubated at 37°C for 15 minutes with liposomes (EPC:SPS:Chol and EPC:EPG:Chol, both 4:3:3 mole ratio, 1 mM), then washed and assayed. Values are given as mean ± SD, n = 3. ▪ indicates normal; ░, CGD. Results of 2-group t test are marked with asterisk; P < .05.
Chemotaxis assay of normal and CGD granulocytes.
Cells (1 × 106) migrated with and without GOL treatment toward NfMLP, 1 μM (A), and LTB4, 1 μM (B). Granulocytes were preincubated at 37°C for 15 minutes with liposomes (EPC:SPS:Chol and EPC:EPG:Chol, both 4:3:3 mole ratio, 1 mM), then washed and assayed. Values are given as mean ± SD, n = 3. ▪ indicates normal; ░, CGD. Results of 2-group t test are marked with asterisk; P < .05.
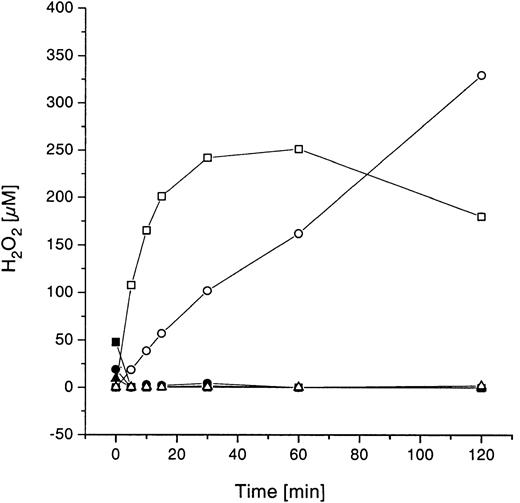
H2O2 concentration and methemoglobin formation in whole blood samples after incubation with GOLs
Possible side effects of GOL treatment in whole blood samples (glucose concentration, 3.1-6.4 mM) were investigated by measuring the net concentration of H2O2 (production minus degradation) and generation of methemoglobin as a response parameter to this potential oxidative stress. A wide range of GO concentrations (0.002, 0.2, and 2 U/mL or 0.01, 1, and 10 mM lipid) encapsulated in EPC:SPS:Chol and EPC:EPG:Chol liposomes showed generally the same results concerning H2O2 occurrence. The results shown in Figure 9 are exemplary only for EPS:SPS:Chol liposomes. To differentiate between H2O2 production and degradation, parallel incubations were carried out in PBS containing 5.6 mM glucose (physiologic concentration). H2O2 measurements (Figure 9) showed a time- and a dose-dependent production of H2O2 in PBS/glucose, where no H2O2 degradation is possible. In contrast, H2O2 could not be detected in whole blood samples, even at the highest GO concentration (2 U/mL).
Time-dependent H2O2concentration in PBS/glucose and whole blood during incubation with GOLs.
Whole blood and PBS/glucose, respectively, were incubated with different concentrations of GOLs (EPC:SPS:Chol, 4:3:3 mole ratio; 2, 0.2, and 0.002 U/mL GO; 10, 1, 0.01 mM total lipid concentration). Shown are the means of 3 unrelated experiments with different healthy donors (CV < 0.27); ▪ indicates whole blood with liposomes (10 mM lipid; 2 U/mL GO); o, PBS/Gluc plus GOLs (10 mM lipid; 2 U/mL GO); ●, whole blood with liposomes (1 mM lipid; 0.2 U/mL GO); ○, PBS/Gluc plus GOLs (1 mM lipid; 0.2 U/mL GO); ▴, whole blood with liposomes (0.01 mM lipid; 0.002 U/mL GO); ▵, PBS/Gluc plus GOLs (0.01 mM lipid; 0.002 U/mL GO).
Time-dependent H2O2concentration in PBS/glucose and whole blood during incubation with GOLs.
Whole blood and PBS/glucose, respectively, were incubated with different concentrations of GOLs (EPC:SPS:Chol, 4:3:3 mole ratio; 2, 0.2, and 0.002 U/mL GO; 10, 1, 0.01 mM total lipid concentration). Shown are the means of 3 unrelated experiments with different healthy donors (CV < 0.27); ▪ indicates whole blood with liposomes (10 mM lipid; 2 U/mL GO); o, PBS/Gluc plus GOLs (10 mM lipid; 2 U/mL GO); ●, whole blood with liposomes (1 mM lipid; 0.2 U/mL GO); ○, PBS/Gluc plus GOLs (1 mM lipid; 0.2 U/mL GO); ▴, whole blood with liposomes (0.01 mM lipid; 0.002 U/mL GO); ▵, PBS/Gluc plus GOLs (0.01 mM lipid; 0.002 U/mL GO).
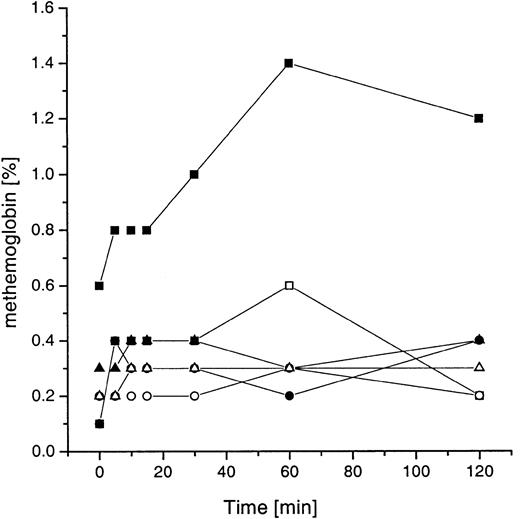
As a consequence, methemoglobin generation in whole blood (Figure10) was absent using 0.002 and 0.2 U/mL GO. Only slightly elevated values were observed after incubation with the extremely high concentration of GO (2 U/mL).
Methemoglobin formation in whole blood during incubation with GOLs.
Values are shown for SPS liposome-types (EPC:SPS:Chol, 4:3:3 mole ratio, lipid concentration 1 mM). Whole blood and PBS/glucose, respectively, were incubated with different concentrations of GOLs (2, 0.2, and 0.002 U/mL GO; 10, 1, 0.01 mM total lipid concentration). Shown are the means of 3 unrelated experiments with different healthy donors (CV < 0.27); ▪ indicates whole blood with liposomes (10 mM lipid; 2 U/mL GO); ■, PBS/Gluc plus GOLs (10 mM lipid; 2 U/mL GO); ●, whole blood with liposomes (1 mM lipid; 0.2 U/mL GO); ○, PBS/Gluc plus GOLs (1 mM lipid; 0.2 U/mL GO); ▴, whole blood with liposomes (0.01 mM lipid; 0.002 U/mL GO); ▵, PBS/Gluc plus GOLs (0.01 mM lipid; 0.002 U/mL GO).
Methemoglobin formation in whole blood during incubation with GOLs.
Values are shown for SPS liposome-types (EPC:SPS:Chol, 4:3:3 mole ratio, lipid concentration 1 mM). Whole blood and PBS/glucose, respectively, were incubated with different concentrations of GOLs (2, 0.2, and 0.002 U/mL GO; 10, 1, 0.01 mM total lipid concentration). Shown are the means of 3 unrelated experiments with different healthy donors (CV < 0.27); ▪ indicates whole blood with liposomes (10 mM lipid; 2 U/mL GO); ■, PBS/Gluc plus GOLs (10 mM lipid; 2 U/mL GO); ●, whole blood with liposomes (1 mM lipid; 0.2 U/mL GO); ○, PBS/Gluc plus GOLs (1 mM lipid; 0.2 U/mL GO); ▴, whole blood with liposomes (0.01 mM lipid; 0.002 U/mL GO); ▵, PBS/Gluc plus GOLs (0.01 mM lipid; 0.002 U/mL GO).
Discussion
Granulocytes provide a first line of defense against invading pathogens such as bacteria and fungi. Once activated (ie, through phagocytosis), granulocytes produce ROS, which play a central role in the destruction of ingested microorganisms.17 In the phagocytes of patients with CGD, the production of ROS is disturbed due to a defect in NADPH oxidase,18 whereas MPO is active in azurophilic granules.11 This was demonstrated again for the CGD granulocytes and monocytes used in this study.
Despite long-term administration of antibiotics and antimycotics in patients with CGD, the risk of life-threatening infections is high.1 Above all, the most difficult complications are caused by fungi (eg, Aspergillus species), a variety of gram-negative enteric bacilli, and catalase-positive bacteria (eg,S aureus)19 20 able to destroy endogenous H2O2. This hampers CGD granulocytes in HOCl production using their own MPO.
In the late 1970s, the idea of replacing defective H2O2 production after phagocytosis of GO encapsulated in liposomes was first described.10Encouraging results could not be followed up because of insufficient liposome technology at that time. In this study we asked whether GOLs could initiate HOCl production and consequently reconstitute the killing ability of CGD cells against S aureus after their uptake by leukocytes.
For this purpose, we tested different liposome formulations for their specific uptake by phagocytic leukocytes. We demonstrate here specific uptake of negatively charged liposomes (EPC:SPS:Chol and EPC:EPG:Chol) by phagocytes (granulocytes and monocytes). Only a low percentage of fluorescence-positive lymphocytes after incubation with liposomes was observed. The charge of the liposome membrane proved to be important for phagocytosis. Uncharged liposomes (EPC:Chol) showed a significantly lower percentage of fluorescence-positive cells compared to the other liposome formulations, especially for granulocytes. The importance of surface charge, specific headgroups, and liposome size for monocytes and macrophages has been demonstrated by many investigators.21-23 Based on these results, monocytes and granulocytes were proven to be ideal target cells for EPC:SPS:Chol/EPC:EPG:Chol liposomes. Related to these results are reports showing rapid uptake of conventional negatively charged liposomes by macrophages of the liver and the reticuloendothelial system (RES).7 24 For many drug-targeting applications this feature of conventional liposomes might be disadvantageous. However, in the case of CGD, formulation of conventional negatively charged liposomes now provides an excellent source for GO delivery to the phagocytes.
The intracellular location of GOLs after incubation with granulocytes and the subsequent intracellular production of H2O2 and HOCl was indicated by several independent experiments. The flow cytometry data showed a preferential uptake of GOLs by phagocytes. If the binding to the outside of the cell membrane were nonspecific, a similar pattern would be expected for phagocytes as well as for lymphocytes.
Additional details about the localization of GOL and S aureus inside the granulocytes were obtained by confocal laser microscopy. These studies strongly suggest that GOLs are taken up via phagocytosis. After preincubation of granulocytes with S aureus and subsequent addition of liposomes, we observed an intracellular localization of bacteria and liposomes, reflecting their close intracellular contact. Some liposomes and bacteria seem to be colocalized inside the phagolysosomes (yellow spots). Previously, Ho and Huang showed the phagocytosis of liposomes followed by a fusion of the phagosome with lysosomes inside the cells by electron microscopy.25 In our electron microscopic analyses using ferritin-containing liposomes,26 we found vacuoles where bacteria and ferritin were localized together, suggesting a fusion of liposomes with the phagosome.
Uptake and intracellular activity of GOLs was further evidenced by DHR 123 oxidation through GO-derived H2O2.27,28 CGD granulocytes showed no detectable DHR oxidation after stimulation with phorbol-myristate-acetate. However, the DHR oxidation was already detectable after only 5 minutes of incubation with GOLs. Addition of catalase decreased the intracellular concentration of H2O2 to some extent. This may be due to diffusion of intracellularly produced H2O2 into the extracellular space, thereby scavenged by catalase before penetrating other cells.9 Experiments in which normal or MPO-deficient granulocytes were mixed with CGD granulocytes showed that H2O2 diffused through the membrane and could be used by CGD cells for the HOCl production and consequently for microbicidal killing.29,30 As shown in Figure 4, intracellular DHR oxidation decreased after 15 to 30 minutes under our experimental conditions. This may be due to the antioxidative capacity within the granulocyte, namely, by catalase or glutathione (GSH)-peroxidase,31 or proteolytic degradation. However, the evidence that H2O2-positive cells could be detected even after 8 hours indicates that GO is rather stable. To further evaluate the stability of GO, enzyme activity was analyzed in HBSS at acidic pH, in the presence of MPO, in supernatants of granulocytes after degranulation with cytochalasin B/NfMLP, and in extracts of granulocytes. In each of these systems, GO activity was not reduced after a 2-hour incubation period at 37°C compared to the control system (GO in HBSS, pH 7.4; data not shown). Time-dependent stability and activity of GO is certainly related to the different localizations of GO within the granulocytes (in phagolysosomes, liposomes encapsulated in the cytoplasm or released into the cytoplasm). Therefore, although the contact is closest in phagolysosomes, a colocalization of bacteria and GO is not a mandatory supposition for optimal activity, because degradation of GO may be faster due to presence of proteolytic enzymes.
The H2O2 produced in vacuoles after entry of glucose into the GOLs or after release of GO could easily diffuse into bacteria-containing phagolysosomes. Within these phagolysosomes HOCl can be produced for bacterial killing, provided that H2O2 was able to escape degradation by catalase or GSH-peroxidase. Generally, H2O2 can easily penetrate through liposomal bilayers. The permeability coefficient for passive H2O2 permeation through liposomal membranes is very high (approximately 10−2cm/s)32 and is in the same order as for O2(approximately 0.5 × 10−2 cm/s).33Therefore, the liposomal and cellular membranes are negligible diffusion barriers for both agents.
In contrast, compared to an active membrane transport, the passive glucose permeation through liposomal membranes is some orders of magnitude lower (about 5 × 10−8 cm/s).34In the presence of 1 mM or 5.6 mM glucose, the enzyme activity was reduced through liposomal entrapment by a factor of about 80 compared with the same free GO concentration, indicating that the glucose permeation through the liposome membranes is the rate-limiting step for H2O2 production (data not shown). Nevertheless, our results indicate sufficient liposomal release of H2O2 from liposomes. Furthermore, granulocytes increase glucose uptake after activation,35 and on decomposition of liposomes followed by GO release, H2O2 production is even enhanced.
Our investigations confirmed that CGD granulocytes have normal MPO activity. Therefore, CGD granulocytes treated with GOLs demonstrated normal production of HOCl. Compared to the direct incubation of GOLs with CGD granulocytes, the amount of produced HOCl was still 15% after removal of nonincorporated GOLs, indicating that a considerable part of GO is taken up by the granulocytes.
The degranulation assay using the ADVIA120 flow cytometry system36 did not show evidence that MPO is degranulated under liposomal influence. However, it cannot be completely excluded that a small amount of HOCl is produced outside the cell because of some extracellular membrane binding of GOLs and a small spontaneous release of MPO. HOCl is a very strong oxidant and uncontrolled production in the extracellular space can be harmful. Therefore, possible side effects are the focus of current studies.
We demonstrated an enhanced and effective killing of S aureus by CGD cells in the presence of GOLs. The successful killing of S aureus by CGD granulocytes treated with GOL is the consequence of restored H2O2 and HOCl production.
In general, CGD cells show a normal response toward chemotactic agents.37 38 We found that granulocytes treated with GOLs were still able to migrate along NfMLP and LTB4 gradients. The general decrease in chemotactic activity of GOL-treated granulocytes might be due to the oxidative stress mediated by H2O2.
As already stated above in the context of MPO release and potential extracellular HOCl production, an important point for a potential clinical application of GOLs for in vivo treatment of CGD is the identification of potential side effects and the question whether the antioxidative system of whole blood could cope with the enhanced oxidative stress. According to our investigations in whole blood samples, this is obviously the case. Using GOLs containing up to 2 U/mL GO, the continuously generated H2O2 could be successfully removed. Decrease of glucose under these in vitro conditions was moderate (data not shown). Furthermore, it can be assumed that in a dynamic in vivo situation (circulating blood with compensatory regulation during potential decrease of blood glucose) the glucose consumption is negligible. Our results show that methemoglobin levels never exceeded the normal range between 0.8% (nonsmoker) to 2.7% (smoker) except at very high concentrations of GO encapsulated in liposomes (approximately 2 U/mL, 10 mM total lipid). The 1 mM lipid concentration (0.2 U/mL) used in all experiments showed almost no methemoglobin elevation, which can be explained by the high antioxidative capacity of whole blood. Samoszuk and colleagues39 40 reported a higher formation of methemoglobin in mice after injection of free GO (1 U/g body weight); its generation was strongly reduced by lower concentrations of free GO.
With regard to liver abscesses being a severe complication in CGD, a rapid elimination of conventional negatively charged liposomes by liver and spleen could be a further advantage for bactericidal activity of GOLs. In addition to interferon-γ prophylaxis, which is thought to augment more oxidant-independent antimicrobial pathways,41,42 the stem cell transplantation20 and gene therapy43,44protocols for the curative treatment of CGD disease, the application of GOLs could support conventional therapy, because it is already used in a variety of drug applications.45,46 The fact that CGD granulocytes contain normal MPO activity should allow bacterial killing even if only a small amount of GO-generated H2O2 reaches phagolysosomal MPO because of the strong antimicrobial effect of HOCl.47 This additional strategy may provide a novel therapeutic approach in treatment of CGD patients. It will be the work of the ongoing project to perform further in vitro and in vivo experiments for possible clinical application.
We thank Thérèse Bruggmann and Dr Mathias Höchli (Laboratory for Electron Microscopy, Zurich) for the preparation of electron microscopy and confocal laser microscopy, and Prof Reinhard Seger (University Children's Hospital, Zurich) for critical reading of the manuscript.
Supported by the Reinhold-Beitlich-Stiftung and the fortüne-program (project 717-0-0), Tübingen, Germany, and the Schweizerische Vereinigung für Angeborene Immundefekte (SVAI), Zürich, Switzerland.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Claudia E. Gerber, University Children's Hospital, Department of Hematology and Oncology, Hoppe-Seyler-Str 1, D-72076 Tübingen, Germany; e-mail:claudia.gerber@med.uni-tuebingen.de.

![Fig. 5. HOCl production by normal and CGD granulocytes (5 × 106/mL). / Cells were preincubated with GOLs (EPC:SPS:Chol and EPC:EPG:Chol, both 4:3:3 mole ratio, 1 mM) at 37°C for 15 minutes. Nonincorporated liposomes were washed out and the incubation was followed for 30 minutes in PBS plus glucose. Negative controls were performed by addition of l-methionine [1 mM]. Mean ± SD of 2 independent experiments in duplicate. ▪ indicates normal; ░, CGD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/10/10.1182_blood.v98.10.3097/5/m_h82211737005.jpeg?Expires=1769978514&Signature=GZn7R8pTMvhVyq~y7uyaQXFcz1O7Ac1X1zPgIgQu1RO1y5-mioXF7UwdXCACDm-ed7QlAkcwxOAf9H26IDhpnvH2KXiOLWMpeomwE30WPi6o44xy5mVwP3vG5jh5QGO~dYSKTVzs-5Zl0Ez5Xalf9KdHa2XyW-S8~yRAZIBnqQLCCYyubQbommOZuXWL8Tzz-ukK3HlgVxvntPfS4zOFK~OplhiYHKUUnnjqYf1TaeI9aZR~9e9JdaVelhRIsRqbIZDNHuw-NM7y4-skEy39UHqGTXvDkn8AL~15PTvvDvxyBciBvZ8Mpf2xz~Z-6IzfyRHJN4Ilguj42D4lm~loKg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Killing of. / S aureus by normal and CGD granulocytes. Cells (5 × 105) were incubated at 37°C with S aureus (5 × 105) with and without liposome treatment. GOLs (EPC:SPS:Chol, panel A, and EPC:EPG:Chol, panel B, both 4:3:3 mole ratio, lipid concentration 1 mM) were mixed with granulocytes. Incubation was carried out in the continuous presence of GOLs or after a 15-minute preincubation and subsequent removal of nonphagocytosed GOLs before bacteria were added. Values were set as 100% at the start (0 hours). Shown are the mean values of 3 independent experiments in duplicates (CV [coefficient of variation] < 0.27); ▪ indicates normal granulocytes; ●, CGD granulocytes (both without liposome treatment); ▴, liposomes; ▾, CGD granulocytes with continuous incubation of liposomes; ♦, CGD granulocytes preincubated with liposomes. Results of 2-groupt test are marked with asterisk; P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/10/10.1182_blood.v98.10.3097/5/m_h82211737006.jpeg?Expires=1769978514&Signature=DyKW8qkLNGlte0JjWAUDO-cZkB75xt8Wc-C4cDxO3JrM6xQJB6~7UckO0iPZw7BsGf3tdoIZedbaKGQCX67nQl67fuyxcednoBPRag9BeB9dv08kYFTt5b0mifUgAVeDzw2xklLHMhl1CfG79jFNJg3RJ7Ft6V8ubZFTB8vjqpwweYLdMOwXBlguF25s8qviWnZ0RbZv7crLJmoalUZupvmi1Dv0C15U4CGd83fORP3tlmTnKt1qKQktxXOeoDUCplT7yYndZR3JYt0sc90N8PsU2uPJ7DrpkoIoznhLfXQ0HmlZG9eiz7pxCvglIFMMIPV3GKIRxK70mhT4qa5hbA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)