Abstract
A retrospective analysis of granulocyte colony-stimulating factor (G-CSF)–mobilized peripheral blood mononuclear cell (G-PBMC) products harvested from healthy donors indicates significant variability in both the absolute number and relative proportion of CD34, CD3, and CD14 cells obtained. This report examined whether variations in the cellular composition of G-PBMC products correlated with clinical outcomes after myeloablative allogeneic transplantation. The numbers of CD34, CD3, and CD14 cells infused into 181 human leukocyte antigen (HLA)–identical sibling recipients were analyzed with respect to tempo of engraftment, acute graft-versus-host-disease (GVHD), clinical extensive chronic GVHD, overall survival, and disease relapse. Neither acute GVHD, overall survival, nor disease relapse was statistically significantly associated with CD34, CD3, or CD14 cell doses or the CD14 to CD3 ratio. CD3 and CD14 cell doses and CD14 to CD3 ratios did not correlate with the tempo of neutrophil and platelet engraftment. However, increasing CD34 cell numbers were significantly associated with accelerated neutrophil (P = .03) and platelet (P = .01) engraftment. Higher doses of CD34 cells (> 8.0 × 106/kg) were also associated with a significantly increased hazard of clinical extensive chronic GVHD (HR = 2.3, 95% confidence interval [CI] 1.4-3.7,P = .001), but neither CD3 nor CD14 doses were statistically significantly associated with chronic GVHD. It was concluded that CD34 cell dose in G-PBMC grafts appears to affect both the engraftment kinetics and the development of clinical extensive chronic GVHD in HLA-identical sibling recipients but without a demonstrable impact on survival, relapse, and acute GVHD. Given the morbidity associated with extensive chronic GVHD, efforts to further accelerate engraftment in HLA-matched sibling transplants by increasing CD34 cell number in G-PBMC products may be counterproductive.
Introduction
Successful engraftment of allogeneic hematopoietic stem cells and clinical outcomes of transplantation are functions of both stem and accessory cells present in the graft.1-3Granulocyte colony-stimulating factor (G-CSF)–mobilized peripheral blood mononuclear cells (G-PBMCs) are now increasingly used in place of bone marrow for allogeneic transplantation.4-8 Several reports indicate that the clinical outcome of stem cell transplantation in human leukocyte antigen (HLA)–identical siblings is improved by substituting G-PBMCs for marrow cells.5,7,9,10 G-PBMCs produce more rapid recovery of neutrophils and platelets than marrow grafts. The improved survival among patients with advanced leukemia appears to result from a decreased incidence of relapse but may also be a consequence of reduced transplant-related mortality.5,7It is not yet clear from these studies whether better survival would also be seen in patients with less advanced leukemias, although one preliminary report does suggest better survival in patients with less advanced diseases due to decreased transplant-related mortality.9 In most randomized studies comparing the use of G-PBMCs with marrow cells for allogeneic HLA-identical transplantation, the probability of acute graft-versus-host disease (GVHD) was similar in recipients of G-PBMCs and recipients of marrow grafts;6,7,11,12 however, there are conflicting data on the risks of chronic GVHD.6-8 13
The biologic basis for the improved survival observed after G-PBMC grafts is not clear but is likely related to differences in cellular composition between marrow and G-PBMC grafts. The administration of G-CSF to healthy donors and the harvesting technique of G-PBMCs with the use of continuous-flow blood separators results in a cellular composition of G-PBMCs that is not only quantitatively but also qualitatively different from marrow grafts.14-17Specifically, the number of CD3 cells can be more than 10-fold and the number of CD14 cells up to 100-fold higher in G-PBMCs compared with marrow. The CD14 cells express decreased levels of the T-cell costimulation molecule B7 and secrete an increased amount of interleukin-10, both of which serve to reduce T-cell responsiveness to alloantigen by reducing the ability of CD4 cells to utilize the CD28 signaling pathway.18 However, this effect depends on the CD14 to CD4 ratio.19 This ratio, as well as the number of CD34, CD14, and CD3 cells, differs significantly among G-PBMC products.16 20 We therefore hypothesized that variations in the cell composition of the G-PBMC products could lead to differences in immunologic properties, thereby affecting clinical outcomes after HLA-identical sibling hematopoietic stem cell transplantation.
To test this hypothesis, we asked whether there was a correlation between the cellular composition of G-PBMC grafts and clinical outcomes in 181 HLA-identical sibling recipients treated with myeloablative conditioning and G-PBMC transplantation for hematologic malignancies. In this study, we retrospectively analyzed CD34, CD3, and CD14 cell doses and the CD14 to CD3 ratio in G-PBMC grafts with respect to the tempo of engraftment, risks of acute and clinical extensive GVHD, disease relapse, and overall survival.
Patients, materials, and methods
Patients
The analysis included 181 patients who underwent G-PBMC transplantation from HLA-identical sibling donors for various hematologic malignancies between March 1996 and December 1999 at the Fred Hutchinson Cancer Research Center or the Veterans Affairs Medical Center in Seattle, WA. Patient characteristics are shown in Table1. Written informed consent was obtained from each patient and donor, and the institutional review board of the Fred Hutchinson Cancer Research Center, Seattle, WA, approved all protocols.
Donors
Donors were treated with G-CSF at a dose of 16 μg/kg per day. Leukaphereses were performed using a continuous-flow blood separator (CobeSpectra, Cobe Laboratories, Lakewood, CO) starting from day 4 of G-CSF treatment until more than 4 × 106/kg of CD34 cells/kg of recipient weight were collected. A single leukapheresis was sufficient in 147 donors (81%) to collect the target dose of CD34 cells (in one case 3.9 × 106 CD34 cells were collected), whereas 25 donors (14%) required 2 leukaphereses, and 2 donors (1%) required 3 leukaphereses. The target dose was not reached in 7 donors (4%) despite performing 2 or more leukaphereses.
G-PBMC cell subsets enumeration
Enumeration of CD34, CD14, and CD3 cells in each G-PBMC product was performed on a FACScan cytometer (Becton Dickinson, San Jose, CA) by the Cellular Therapy Laboratory, Fred Hutchinson Cancer Research Center. For each sample, 3 tubes were prepared and processed in parallel: (1) double staining with CD34 phycoerythrin (PE) (HPCA-2-PE; Becton Dickinson) and CD14 fluorescein isothiocyanate (FITC) (Leu-M3; Becton Dickinson); (2) double staining with Simultest CD3 PE and CD4 FITC (Leu 4/3A; Becton Dickinson); (3) matched isotype controls (Becton Dickinson). Viability of the specimen was determined by staining with 7-aminoactinomycin-D (7-AAD). Gates were set around forward versus side scatter and low 7-AAD staining to include only viable nucleated cells for further analysis. For CD34 determinations, additional gating to exclude CD14+ and high side scatter events was utilized. Equivalent gating on isotype controls was used for background subtraction. At least 100 000 total events were acquired on each sample.
Clinical outcomes
Clinical outcomes after transplantation that were considered included tempo of neutrophil and platelet engraftment; recoveries of neutrophils, monocytes, and lymphocytes within 100 days after transplantation; acute GVHD; clinical extensive chronic GVHD; overall survival; and disease relapse. Time to neutrophil engraftment was defined as the first of 3 consecutive days in which the absolute neutrophil count (ANC) exceeded 500/μL. Time to platelet engraftment was defined as the first of 7 consecutive days in which the platelet count exceeded 20 000/μL without transfusions. The absolute numbers of neutrophils, monocytes, and lymphocytes in patients after transplantation were calculated from the total number of white cells quantified by automated leukocyte counter (Sysmex E 2500, Kobe, Japan) and differential counts obtained from standard May-Grunwald-Giemsa–stained smears. One investigator graded the peak severity of acute GVHD according to previously published criteria.22 Patients were assessed for chronic GVHD after day +100 and graded by standard criteria.23
Statistical analysis
In order to assess the association of cell-type variables with outcome, non–cell-type variables were first examined using regression models. The non–cell-type variables examined included patient age at transplantation, patient/donor sex, patient/donor cytomegalovirus (CMV) serostatus, risk of disease (advanced vs less advanced), use of total body irradiation (TBI) for conditioning, presence of major ABO mismatch, and GVHD prophylaxis (methotrexate/cyclosporine [MTX/CSP] vs others). Non–cell-type variables that were significantly or suggestively associated with outcome were used to create a “base” model for each outcome. Once this base model was fit, each cell-type variable was added to assess the improvement to the base model. The significance of the improvement was measured by the likelihood ratio test. Each cell-type variable was modeled as a continuous variable, a log-transformed variable, and according to quartiles. Logistic regression was used for the outcome grades II-IV acute GVHD; Cox regression24 was used for the outcomes clinical extensive chronic GVHD, relapse, and survival, and linear regression was used for the engraftment outcomes among those who engrafted. The association of clinical extensive chronic GVHD with the hazard of relapse was examined by treating GVHD as a time-dependent covariate. Spearman rank correlation coefficient was used to estimate the correlation between cell types and to associate cell dose with time to engraftment. All P values are 2-sided and no adjustments were made for multiple comparisons; values between .01 and .05 should therefore be considered as suggestive of a difference rather than definitive evidence of a difference. P values from fitted regression models were derived from the Wald test. Cumulative incidence estimates were used to measure the probability of clinical extensive chronic GVHD and relapse.25 Probability of survival was estimated by the method of Kaplan and Meier.26
Results
Cellular composition of G-PBMC grafts
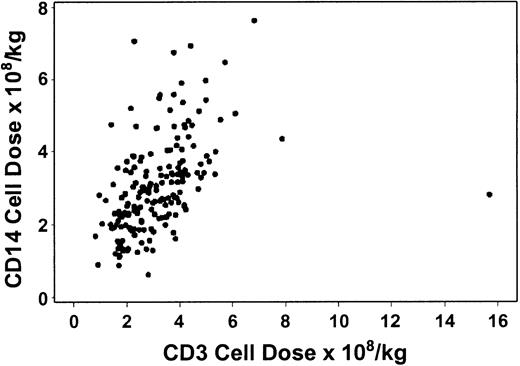
Table 2 shows the absolute numbers of CD34, CD3, and CD14 cells and CD14 to CD3 cell ratios in G-PBMC products. The correlation between CD34 and CD3 cells was suggestively significant, but the strength of the correlation was relatively low (R = .15, P = .04). Similarly, the correlation between CD34 and CD14 cells was statistically significant, but the strength of the association was relatively weak (R = .21, P = .005). On the other hand, the correlation between CD3 and CD14 cells was stronger (R = .58, P < .0001, Figure1). Table3 shows the average CD3 and CD14 cell doses as well as the average CD14 to CD3 ratio among the high and low CD34 dose groups.
The number of CD3 cells in G-PBMCs correlates with the number of CD14 cells.
There was modest correlation between the number of CD3 and CD14 cells in the graft (R = 0.58, P < .0001).
The number of CD3 cells in G-PBMCs correlates with the number of CD14 cells.
There was modest correlation between the number of CD3 and CD14 cells in the graft (R = 0.58, P < .0001).
Correlation between clinical outcomes and cellular composition of G-PBMC grafts
Engraftment.
Twelve patients never reached a sustained neutrophil count of more than 500/μL and died at a median of 9.5 days (range, 2-46 days) after the transplantation. Thirty-seven patients failed to reach a sustained platelet count of more than 20 000/μL before returning to the care of the referring physician; 35 of these 37 died at a median of 27 days (range, 2-251 days) after the transplantation. Average CD34, CD3, and CD14 cell doses infused were similar among patients who did and did not reach neutrophil and platelet engraftment (data not shown). Neutrophil engraftment (> 500/μL) occurred between 10 and 33 days (median 16 days), whereas unsupported platelet counts of more than 20 000 were achieved between 7 and 39 days (median 14 days). No non–cell-type variables were statistically significantly associated with time to neutrophil or platelet recoveries. Among patients who reached a sustained neutrophil count of more than 500/μL, an increased CD34 cell dose was suggestively associated with a shorter time to neutrophil recovery, although the strength of the association was relatively weak (R = .15, P = .06; Figure2). The median (mean) day of neutrophil recovery by quartiles of CD34 cells was 16 (17.2), 16 (15.7), 16 (15.3), and 15 (16.0), respectively. Among patients who achieved a sustained platelet count of more than 20 000/μL, an increased CD34 cell dose was also suggestively associated with a faster time to platelet recovery, although the association was not strong (R = .18,P = .03; Figure 3). The median (mean) day of platelet recovery by quartiles of CD34 cells was 14 (15.8), 14 (14.8), 13 (14.8), and 13.5 (13.2), respectively. In contrast, neither CD3 cell dose, CD14 cell dose, nor CD14 to CD3 cell ratio was statistically significantly associated with the tempo of neutrophil (Table 4) or platelet engraftment (Table 4). In addition, none of the other cell type variables (CD3 cell dose, CD14 cell dose, CD14 to CD3 ratio) qualitatively changed the association between CD34 cell dose and time to neutrophil and platelet engraftment (data not shown). Table5 summarizes the proportion of patients who achieved sustained neutrophil and platelet engraftment according to CD34 cell dose.
Neutrophil recovery is associated with CD34 cell number.
There was a relatively weak association between CD34 cell dose in G-PBMC grafts from HLA-identical sibling donors and time to neutrophil engraftment among patients (n = 169) who reached a sustained ANC of more than 500/μL (R = .15, P = .06). Closed circles represent patients who engrafted; open circles represent patients who did not engraft before dying. Vertical lines separate first through fourth quartiles of CD34 cells.
Neutrophil recovery is associated with CD34 cell number.
There was a relatively weak association between CD34 cell dose in G-PBMC grafts from HLA-identical sibling donors and time to neutrophil engraftment among patients (n = 169) who reached a sustained ANC of more than 500/μL (R = .15, P = .06). Closed circles represent patients who engrafted; open circles represent patients who did not engraft before dying. Vertical lines separate first through fourth quartiles of CD34 cells.
Platelet recovery is associated with CD34 cell number.
There was a relatively weak association between CD34 cell dose in G-PBMC grafts from HLA-identical sibling donors and time to platelet engraftment among patients (n = 144) who reached a sustained platelet count of more than 20 000/μL before dying or returning to the care of the referring physician (R = .18, P = .03). Closed circles represent patients who engrafted before dying or being sent home; open circles represent patients who did not engraft before dying or being sent home. Vertical lines separate first through fourth quartiles of CD34 cells.
Platelet recovery is associated with CD34 cell number.
There was a relatively weak association between CD34 cell dose in G-PBMC grafts from HLA-identical sibling donors and time to platelet engraftment among patients (n = 144) who reached a sustained platelet count of more than 20 000/μL before dying or returning to the care of the referring physician (R = .18, P = .03). Closed circles represent patients who engrafted before dying or being sent home; open circles represent patients who did not engraft before dying or being sent home. Vertical lines separate first through fourth quartiles of CD34 cells.
Acute GVHD.
Acute GVHD grading information was missing for 8 patients at the time of analysis. Among the remaining 173, 117 (68%; 95% CI, 61%-75%) developed grades II-IV GVHD. Thirty-five patients (20%; 95% CI, 14%-26%) developed grades III-IV GVHD. Among non–cell-type variables, patient/donor CMV serostatus and patient age were suggestively associated with the probability of grades II-IV acute GVHD. After controlling for these factors, none of the cell-type variables was statistically significantly associated with the probability of acute GVHD (Table 4). Table 5 summarizes the proportion of patients who developed grades II-IV acute GVHD according to CD34 cell dose.
Clinical extensive chronic GVHD.
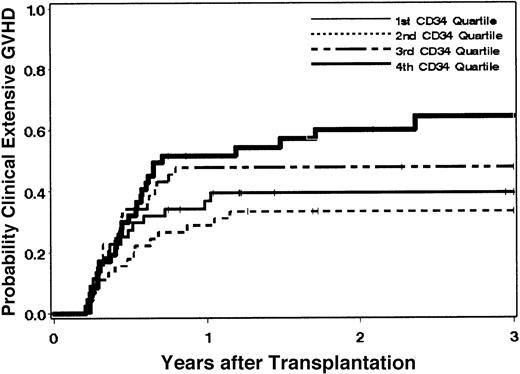
Clinical extensive chronic GVHD developed in 81 patients at a median of 156 days (range, 76-861 days) after the transplantation. The cumulative incidence estimate of the probability of clinical extensive chronic GHVD among all 181 patients at 3 years was 46% (95% CI, 39%-54%). One hundred nine patients developed clinical chronic GVHD for a cumulative incidence estimate of the probability at 3 years of 61%. Among 126 patients who survived to day 80 without relapse, the estimate of the probability of clinical extensive GVHD at 3 years was 64% (95% CI, 56%-73%). Among non–cell-type variables, age at transplantation, patient/donor sex, and risk of the disease were suggestively associated with the hazard of extensive chronic GVHD when all patients were included in the analysis. After controlling for these factors, increased CD34 cell dose was statistically significantly associated with an increased hazard of clinical extensive chronic GVHD (P = .01, log-transformed CD34 dose). In comparing “high” versus “low” CD34 doses (defined arbitrarily as above and below the median of 8.0 × 106 cells/kg), the hazard of clinical extensive chronic GVHD was increased among patients who received high CD34 doses compared with those given low doses (adjusted HR = 2.3; 95% CI, 1.4-3.7; P = .001). Figure4 shows the probability of clinical extensive chronic GVHD according to quartiles of CD34 cells. After controlling for variables contained in the base model, neither CD3 nor CD14 cell dose or CD14 to CD3 ratio showed any suggestion of an association with the hazard of clinical extensive chronic GVHD (Table4). In addition, none of the other cell variables qualitatively changed the association between CD34 and the hazard of clinical extensive chronic GVHD (data not shown). Table 5 summarizes the proportion of patients who developed clinical extensive chronic GVHD according to CD34 cell dose.
Chronic GVHD is associated with CD34 cell dose.
The probability of extensive chronic GVHD after conventional transplantation of G-PBMCs from HLA-identical siblings is increased in patients receiving higher CD34 cell doses.
Chronic GVHD is associated with CD34 cell dose.
The probability of extensive chronic GVHD after conventional transplantation of G-PBMCs from HLA-identical siblings is increased in patients receiving higher CD34 cell doses.
Relapse.
Thirty-eight patients had a recurrence of their underlying disease. The cumulative incidence estimate of relapse at one year was 21% (95% CI, 15%-27%). Only stage of disease at the time of the transplantation (advanced vs nonadvanced) was statistically significantly associated with the hazard of relapse among the non–cell-type variables examined. After controlling for stage of disease, none of the cell-type variables showed a statistically significant association with the hazard of relapse (Table 4). This lack of association appeared to be relatively consistent according to disease type (data not shown). In addition, the presence of clinical extensive chronic GVHD showed no suggestion of being associated with a decreased hazard of relapse (adjusted HR = 1.6; 95% CI, 0.7 to 3.6; P = .25). The presence of clinical (limited or extensive) chronic GVHD also showed absolutely no suggestion of being associated with a decreased hazard of relapse (adjusted HR = 2.2; 95% CI, 0.9 to 5.4;P = .08). Table 5 summarizes the proportion of patients who relapsed according to CD34 cell dose.
Survival.
A total of 94 deaths had occurred by the time of final analysis. The Kaplan-Meier estimate of survival among all 181 patients at 3 years was 44% (95% CI, 36%-52%) with a median follow-up among surviving patients of 831 days (range, 84-1871 days). Among the non–cell-type variables, disease risk, patient/donor sex, and patient/donor CMV serostatus were statistically significantly associated with the hazard of mortality. After controlling for these factors, none of the cell-type variables was statistically significantly associated with the hazard of mortality (Table 4). Moreover, none of the cell-type variables was statistically significantly associated with the hazard of nonrelapse mortality (data not shown). Table 5 summarizes the proportion of patients who died according to CD34 cell dose.
Discussion
In the current study, we have confirmed initial observations14 27 that the number of cells with selected cell subtypes given in G-PBMC grafts was highly variable. This was most likely caused by differences in the G-CSF–induced mobilization of CD34 and accessory cells in healthy donors or differences in the starting basal levels of those cell types in the donors and, in some cases, the disproportion between donor and recipient weights. Variations in CD34, CD3, and CD14 cell numbers in G-PBMC grafts allowed us to analyze the impact of dose of selected cell subtypes on various clinical outcomes after HLA-identical hematopoietic stem cell transplantation.
The role of CD3 cells in the development of acute and chronic GVHD as well as in relapse after conventional bone marrow transplantation has been clearly established.1,28 In the current study, however, we found no statistically significant association between CD3 cell dose and the probability of acute GVHD. This is consistent with initial observations suggesting that the risk of acute GVHD is not increased after G-PBMC transplants, despite the infusion of 1 to 2 logs more T cells compared with marrow grafts.5,29 The lack of correlation between CD3 cell dose and risk of acute GVHD might be explained by the diminished alloresponsiveness of T cells in G-PBMC grafts.30-32 Alternatively, a hypothesis originally generated by Kernan et al33 would suggest that once the initial threshold of clonogenic T cells has been exceeded, a further increase in T-cell numbers does not necessarily translate into more acute GVHD for a given degree of genetic disparity between donor and recipient. Support for this hypothesis comes from the clinical studies of T-cell–depleted marrow grafts. In those studies, a threshold dose of approximately 105 residual CD3 cells/kg was identified, above which there was no correlation between the risk of acute GVHD and CD3 cell dose.33 34
We also found no statistically significant correlations between CD14 cell dose and clinical outcomes except for association of higher CD14 cell dose with higher average monocyte counts within 100 days after the transplantation. Interestingly, patients who received G-PBMC products with the highest CD14 to CD3 ratios had higher average lymphocyte counts within 100 days after the transplantation. Those findings suggest that CD14 cells may indeed play a role in the immune recovery after G-PBMC transplantations.
We were specifically interested in whether the inhibitory effect of CD14 cells on T-cell functions observed in vitro31affected the probability of acute GVHD. The apparent lack of correlation between the CD14 to CD3 cell ratio and acute GVHD may argue that this was only an in vitro epiphenomenon. However, in vitro studies showed that the inhibitory effect of monocytes on T-cell function depended on the ratio of CD14 to CD3 cells and reached its maximum when the number of monocytes was equal or higher than lymphocytes.31 In the current study, most of the patients (85%) received grafts with approximately equal or higher numbers of CD14 to CD3 cells. Therefore, there were too few patients receiving G-PBMCs with low CD14 to CD3 ratios to estimate the impact of relatively low proportions of CD14 cells. Studies using partial T-cell depletion of G-PBMCs could answer this question.
A relationship between CD34 cell dose and transplantation outcome was first shown in marrow autografts in which 1 × 106 of CD34 cells/kg was identified as a safe minimum dose for engraftment.35 In the current study, increased CD34 cell dose was also suggestively associated with faster hematopoietic engraftment. However, this association might be of limited biologic significance since in the current study the difference between the median days of neutrophil engraftment for patients receiving a high (> 12 × 106/kg) and lower (4 × 106/kg-8 × 106/kg) CD34 cell dose was only 1 day, and there was no difference in days to platelet engraftment. This would be consistent with the observation made originally by Gianni et al that increasing the progenitor cell dose above a certain threshold had a limited potential to further accelerate neutrophil recovery.36
We did not observe a statistically significant association between CD34 cell dose and probability of acute GVHD. This stands in contrast to the results recently reported by Przepiorka et al.37 However, in that study, the association between CD34 cell dose and risk of acute GVHD depended on the type of postgrafting immunosuppression. The short course of methotrexate and cyclosporine, which was given to most current patients as GVHD prophylaxis, was not used in that study. Therefore, the differences in GVHD prophylaxis regimens may explain the apparent discrepancy between these 2 studies.
In the current study, an increased CD34, but not CD3, cell dose was statistically significantly associated with an increased hazard of developing clinical extensive chronic GVHD. Comparable results were previously reported in abstract form by Przepiorka et al.38 Cell dose is a continuous variable, and as such it is difficult to identify a precise CD34 cell dose above which the risk of extensive chronic GVHD is markedly increased. However, both studies suggest that patients receiving CD34 cell dose in excess of 8 × 106/kg have a higher risk of developing extensive chronic GVHD. This is an important finding since chronic GVHD limits the quality of life in long-term transplantation survivors. Additionally, our study suggests that the variations in CD34 cell doses given to G-PBMC recipients may explain some of the conflicting data reported so far on the risk of chronic GVHD after G-PBMC transplantations.6-8 13
In the current study, there was no suggestion that CD34 cell dose or extensive chronic GVHD was statistically significantly associated with a decreased risk of relapse. These results differ from previously published studies that reported an inverse association between chronic GVHD and risk of relapse39,40 suggesting that the graft-versus-leukemia effect carried by G-PBMC grafts is not further improved when clinical symptoms of extensive chronic GVHD develop. Recently reported results from the International Bone Marrow Transplant Registry also suggest that increased severity of chronic GVHD did not correlate with a reduced risk of relapse among HLA-identical siblings receiving conventional hematopoietic cell transplants.41
Variations in CD34, CD3, and CD14 cells in G-PBMC grafts did not statistically significantly affect overall survival. This finding suggests that improved survival observed after G-PBMC compared with marrow grafts is unlikely to be further improved by “mega-doses” of G-PBMCs. Potential benefits of slightly faster engraftment related to an increased CD34 cell dose would appear to be offset by the increasing risk of extensive chronic GVHD, which has been reported to be more severe and more difficult to treat in G-PBMC than bone marrow recipients.42 Our results suggest that the risk of clinical extensive chronic GVHD can be lowered among patients who receive unmodified G-PBMC grafts by controlling the number of CD34 cells. CD34 cell doses of more than 4 × 106/kg and less than 8 × 106/kg may lead to a decreased hazard of developing clinical extensive chronic GVHD, compared with doses in excess of 8 × 106/kg, without compromising time to hematopoietic recovery or affecting relapse rate or survival. In fact, if time to death or chronic GVHD is analyzed as a composite end point, the conclusions for the chronic GVHD end point drawn with respect to CD34 remain qualitatively the same. Studies of the various differentiation pathways for CD34 cells in vitro and in vivo might yield insights to elucidate the pathophysiology of this association with chronic GVHD.
We thank Nancy Anderson, Beth Macleod, David Yadock, and the dedicated staff of the Fred Hutchinson Cancer Research Center Cellular Therapy Laboratory for performing all flow cytometry staining and analyses. The authors thank Chris Davis for outstanding help in data retrieval. We are very grateful to Helen Crawford, Bonnie Larson, and Karen Carbonneau for their secretarial support. We also appreciate the work of the staff of the Fred Hutchinson Cancer Research Center who provided the care to the patients.
Supported in part by grants DK51417, CA18029, CA18221, DK56465, HL54881, HL36444, and CA15704 from the National Institutes of Health, Department of Health and Human Services (DHHS), Bethesda, MD. J.M.Z is a postdoctoral fellow from the Department of Hematology, University Medical School, Gdañsk, Poland, and is also a recipient of the International Fellowship for Young PhDs, awarded by the Foundation for Polish Science, Warsaw, Poland.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Beverly Torok-Storb, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave North, D1-100, PO Box 19024, Seattle, WA, 98109-1024; e-mail: btorokst@fhcrc.org.