Abstract
Thrombocytopenia developed in some individuals treated with a recombinant thrombopoietin (TPO), pegylated recombinant human megakaryocyte growth and development factor (PEG-rHuMGDF). Three of the subjects who developed severe thrombocytopenia were analyzed in detail to determine the cause of their thrombocytopenia. Except for easy bruising and heavy menses, none of these subjects had major bleeding episodes; none responded to intravenous immunoglobulin or prednisone. Bone marrow examination revealed a marked reduction in megakaryocytes. All 3 thrombocytopenic subjects had antibody to PEG-rHuMGDF that cross-reacted with endogenous TPO and neutralized its biological activity. All anti-TPO antibodies were immunoglobulin G (IgG), with increased amounts of IgG4; no IgM antibodies to TPO were detected at any time. A quantitative assay for IgG antibody to TPO was developed and showed that the antibody concentration varied inversely with the platelet count. Anti-TPO antibody recognized epitopes located in the first 163 amino acids of TPO and prevented TPO from binding to its receptor. In 2 subjects, endogenous TPO levels were elevated, but the TPO circulated as a biologically inactive immune complex with anti-TPO IgG; the endogenous TPO in these complexes had an apparent molecular weight of 95 000, slightly larger than the full-length recombinant TPO. None of the subjects had atypical HLA or platelet antigens, and the TPO cDNA was normal in both that were sequenced. Treatment of one subject with cyclosporine eliminated the antibody and normalized the platelet count. These data demonstrate a new mechanism for thrombocytopenia in which antibody develops to TPO; because endogenous TPO is produced constitutively, thrombocytopenia ensues.
Introduction
Thrombopoietin (TPO) is the key regulator of platelet production.1 TPO stimulates bone marrow progenitor cells to become megakaryocytes that in turn shed platelets. TPO is produced in a constitutive fashion in the liver, and its production is not increased in thrombocytopenic disorders such as immune thrombocytopenic purpura (ITP).2 In animals in which the TPO gene or the TPO receptor, c-Mpl, has been eliminated in a homozygous fashion, bone marrow megakaryocyte progenitors are reduced to 5% of normal and the platelet count to 10% to 15% of normal.3,4 Levels of white blood cells (WBCs) and red blood cells (RBCs) are not altered, but their bone marrow precursors are reduced to 30% to 40% of normal.5
Two recombinant TPOs have undergone extensive clinical testing. These are recombinant human TPO (rHuTPO), a glycosylated molecule identical in amino acid sequence to endogenous TPO, and pegylated recombinant megakaryocyte growth and development factor (PEG-rHuMGDF), a nonglycosylated molecule that contains the first 163 amino acids of endogenous TPO and is coupled to polyethylene glycol.1,6Both are potent stimulators of platelet production in humans and decrease the extent of thrombocytopenia associated with nonmyeloablative chemotherapy and may reduce the need for platelet transfusions.7 However, neither has any significant effect upon platelet recovery or the need for platelet transfusions in myeloablative chemotherapy settings such as bone marrow transplantation or induction chemotherapy for acute leukemia.8 PEG-rHuMGDF has been shown to increase the platelet count in individuals with human immunodeficiency virus thrombocytopenia9 and also to increase platelet donation by apheresis.10 Both recombinant TPOs improve the number of circulating peripheral blood progenitor cells that can be harvested for stem cell transplantation.11
In these clinical studies, both recombinant TPOs were safe and not associated with thrombosis. However, PEG-rHuMGDF was paradoxically associated with the development of persistent thrombocytopenia (platelet count ≤ 100 × 109/L) in 13 of 325 healthy volunteers who received 2 or 3 doses and in 4 of 650 oncology patients undergoing intensive nonmyeloablative chemotherapy who received multiple doses.6 We have analyzed 3 of the most severely thrombocytopenic subjects and found that the thrombocytopenia was due to the development of antibodies to PEG-rHuMGDF that cross-reacted with and neutralized endogenous TPO. Because TPO is produced in a constitutive fashion, thrombocytopenia ensued.
Materials and methods
Subjects
The 3 subjects studied were participants in clinical studies to assess the safety of PEG-rHuMGDF in healthy volunteers12or in cancer patients undergoing multiple cycles of chemotherapy.13 These studies were approved by the appropriate local institutional review boards for human subjects in accordance with the Helsinki protocol, and blood samples were collected under these guidelines. Large volumes of plasma from subjects no. 1 and 3 were obtained from therapeutic plasmapheresis treatments.
Four healthy subjects and one subject with severe aplastic anemia served as controls. Serum and platelet-rich plasma were prepared as previously described.14
Materials
PEG-rHuMGDF and rHuTPO were generous gifts from Amgen (Thousand Oaks, CA). Agarose-conjugated proteins A, G, and L, insoluble protein A, immunoglobulin G (IgG) subtype-specific antisera, and Western blotting reagents were obtained from Sigma (St Louis, MO) and Novex (San Diego, CA). Polyclonal, affinity-purified goat IgG versus human TPO was purchased from R & D Systems (Minneapolis, MN). Affinity-purified rabbit IgG versus human TPO was a generous gift from Kirin Pharmaceutical (Tokyo, Japan).
Clinical assays
Assays for antibody to platelet antigens were performed at the Blood Center for Southeastern Wisconsin (Milwaukee, WI) by indirect immunofluorescence by flow cytometry, antigen capture ELISA (ACE) using GPIIb/IIIa, ACE II using GPIb/IX, GPIV and class I HLA, modified ACE using GPIIb/IIIa, and GPIa/IIa. HLA and platelet phenotype were determined using both serology and polymerase chain reaction methods. DNA was harvested from whole blood samples from subjects and the TPO open reading frame sequenced by standard polymerase chain reaction methods using overlapping primers.
Immunochemical methods
TPO concentration was measured by using a sensitive enzyme-linked immunosorbent assay (ELISA) assay with a detection limit of 17 pg/mL. A total of 15 to 25 mCi/mg (555-925 mBq/mg)125I-rHuTPO was prepared as previously described14 and retained its biological activity. Immunoprecipitation was performed by adding agarose beads conjugated with protein A, G, or L to 20 to 50 μL serum samples and incubating at room temperature for 30 to 60 minutes followed by centrifugation at 3500g for 10 minutes. The isolated immunoprecipitate was washed once with phosphate-buffered saline (PBS) and the content of125I-rHuTPO measured in a gamma counter.
Anti-TPO IgG subtypes were measured by incubating 20 to 50 μL aliquots of subject sera with about 250 000 cpm of125I-rHuTPO followed by addition of biotinylated type-specific monoclonal antibody and immunoprecipitation with strepavidin-agarose. After the beads were washed, their125I-TPO content was counted in a gamma counter.
IgG was isolated from subject samples using a protein A affinity column followed by acid elution of the adherent IgG, pH adjustment, and concentration of the protein. Western blotting using the purified IgG was performed by native and sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) of 10 ng PEG-rHuMGDF or rHuTPO followed by transfer to nitrocellulose (Hybond-C, Amersham Pharmacia Biotech, Piscataway, NJ). The blots were incubated with purified IgG from thrombocytopenic or control subjects at 32 μg/mL for 2 hours at room temperature, washed, and incubated with peroxidase-conjugated antihuman IgG (Sigma) followed by detection by the ECL method (Amersham Pharmacia Biotech).
Endogenous TPO was isolated from sera from healthy, thrombocytopenic, or aplastic anemia subjects and from concentrated culture medium from a HepG2 cell line by passing 1 mL of filtered sample over a column of Sepharose G-50 coupled to polyclonal, affinity-purified antibody to rHuTPO. The column was washed extensively with equilibration buffer (50 mM Tris, 150 mM NaCl, pH 7.5) and the adherent protein eluted into 4 fractions of equal volume with Gentle Elution Buffer (Pierce, Rockford, IL), concentrated by ultrafiltration, and subjected to SDS-PAGE followed by Western blotting. This method recovered 100% of the TPO in the samples.
TPO bioassay
A BaF3-mpl cell line was developed15 and 4 × 104 cells incubated for 48 hours in 100 μL culture medium containing various concentrations of interleukin-3 (IL-3), rHuTPO, or PEG-rHuMGDF in the presence or absence of IgG fractions from healthy or thrombocytopenic subjects. The extent of cell growth was measured by assay with 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt.15
Assays for antibody to TPO
All patients were subjected to frequent qualitative screening ELISA assays for detection of antibody to TPO16 during their participation in these clinical studies.
A more sensitive and quantitative assay for TPO antibody was developed to assess the thrombocytopenic subjects.17 Quantitation of human anti-TPO antibody was based on the amount of125I-rHuTPO that coprecipitated with subject IgG following treatment with protein A or G. In brief, subject samples or rabbit standard were incubated with about 250 000 cpm of125I-rHuTPO and the total IgG content of the sample immunoprecipitated using insoluble protein A–agarose or protein G–agarose. After centrifugation, the pellet was washed with PBS, and its 125I-rHuTPO content was measured in a gamma counter. Test samples were compared with a standard curve generated with the rabbit anti-TPO antibody, and the amount of human anti-TPO antibody was expressed in micrograms per milliliter of the rabbit standard. This assay is reproducibly sensitive to 0.075 μg/mL rabbit anti-TPO IgG. Using 60 normal human serum samples, a normal cutoff (average + 3 SD for normal samples) of about 0.15 μg/mL has been established for this assay. This assay has a sensitivity of 100% and specificity of 96% for the clinical presence of antibody to TPO in humans.17
125I-rHuTPO competition binding assays
To measure the specificity of binding of IgG to TPO,125I-rHuTPO was incubated with subject serum in the presence of various concentrations of PEG-rHuMGDF, rHuTPO, or TPO peptides for 1 hour at room temperature. The TPO peptides tested contained the following TPO amino acid residues: 1-17, 12-28, 23-40, 34-50, 45-60, 55-70, 65-81, 75-92, 87-104, 99-116, 111-127, 122-138, 133-149, 144-159, 154-163, 20-28, 24-32, 28-36, 32-40, 109-117, 114-122, 117-125, and 121-129. After addition of protein G–agarose beads, the reaction was incubated for another hour. The beads were washed twice, and their 125I-rHuTPO content was measured in a gamma counter.
To measure the effect of subject IgG on TPO binding to its platelet receptor, 125I-rHuTPO was incubated with 300 μL platelet-rich plasma in the presence of serial dilutions of subject serum for 1 hour at room temperature. The platelets were collected by centrifugation, washed twice with PBS, and their125I-rHuTPO content measured. The supernatant was then collected, incubated with protein G–agarose beads for 1 hour at room temperature, the beads collected by centrifugation, washed, and their125I-rHuTPO content measured.
Results
Clinical histories
Subject no. 1.
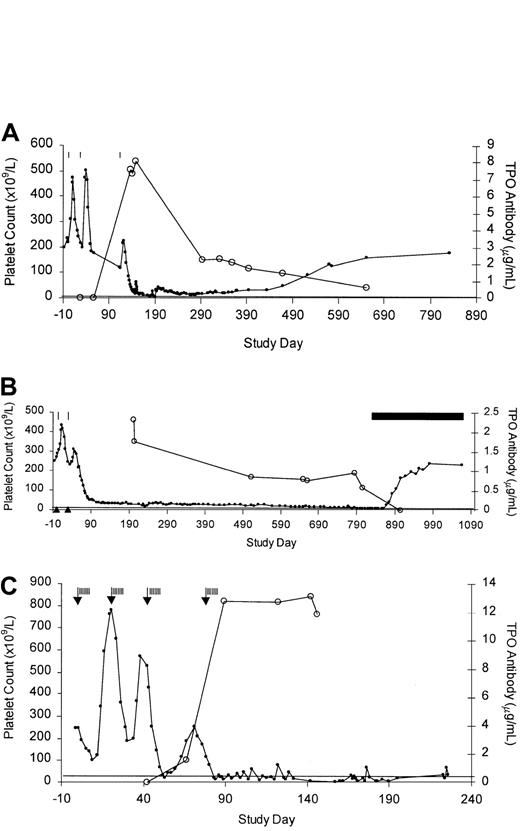
A previously healthy 49-year-old female volunteer received 3 injections of PEG-rHuMGDF at the times indicated in Figure1A. Her platelet count rose to an equal extent after the first 2 injections but rose much less after the third. On study day 133 the platelet count fell below 100 × 109/L, and the subject complained of several weeks of generalized muscle aches and intermittent mild headaches. A screening test indicated the possible presence of an antibody to PEG-rHuMGDF. On study day 149 she received a transfusion of single-donor platelets for a platelet count of 18 × 109/L, and her platelet counts 1 and 12 hours after transfusion were 61 × 109/L and 43 × 109/L, respectively. The erythrocyte sedimentation rate was 39 mm/h, and the hematocrit and WBC count were normal. There was no splenomegaly. On study day 153, prednisone at 60 mg daily was initiated and continued for 25 days with no change in platelet count. The subject received 30 g intravenous immunoglobulin (IVIG) on study days 157, 161, and 164 with no response. Throughout this period, the subject experienced occasional petechiae and ecchymoses but had no melena or hematuria. At a platelet count of 6 × 109/L on study day 180, she experienced a bout of gingival bleeding and epistaxis, and she underwent 6 plasmaphereses beginning on study day 185, complicated by staphylococcal sepsis. The platelet count rose to 42 × 109/L by study day 197 but then fell, and she subsequently received 3 transfusions of platelets for platelet counts below 10 × 109/L. Over the next 636 days, her platelet count gradually returned to normal. Currently (study day 833) she is well, with no sequelae, and has a normal platelet count.
Platelet counts and anti-TPO antibody concentration.
The platelet count (closed circles) is inversely related to anti-TPO antibody concentration (open circles) in thrombocytopenic subjects no. 1 (A), 2 (B), and 3 (C). PEG-rHuMGDF treatment is indicated by vertical lines and chemotherapy by the arrows. Horizontal line denotes normal cutoff of assay for anti-TPO IgG. In panel B, the solid bar indicates cyclosporine administration and solid triangles denote negative anti-TPO antibody screening tests. The anti-TPO antibody titer on day 56 for subject no. 1 (A) is slightly above the cutoff value.
Platelet counts and anti-TPO antibody concentration.
The platelet count (closed circles) is inversely related to anti-TPO antibody concentration (open circles) in thrombocytopenic subjects no. 1 (A), 2 (B), and 3 (C). PEG-rHuMGDF treatment is indicated by vertical lines and chemotherapy by the arrows. Horizontal line denotes normal cutoff of assay for anti-TPO IgG. In panel B, the solid bar indicates cyclosporine administration and solid triangles denote negative anti-TPO antibody screening tests. The anti-TPO antibody titer on day 56 for subject no. 1 (A) is slightly above the cutoff value.
Subject no. 2.
A previously healthy 31-year-old female volunteer received 2 injections of PEG-rHuMGDF. Her platelet count rose after the first injection but rose less well after the second injection, and she developed a platelet count below 100 × 109/L on study day 73 (Figure 1B). A screening test for antibody to PEG-rHuMGDF was positive on study day 76. From study days 167 to 749, the platelet count gradually fell from 30 × 109/L to 13 × 109/L, and except for occasional petechiae and ecchymoses she was well and received no platelet transfusions. The spleen was not palpable. On study days 749 to 751 she received daily infusions of 31 g IVIG, which was complicated by anaphylaxis, but she had no rise in platelet count. Over 772 study days, the hematocrit dropped from 40.2 to 25.9 and, because of excessive menstrual bleeding at a platelet count of 11 × 109/L, she underwent an uncomplicated dilation and curettage without platelet transfusion on study day 777. Postoperatively the hematocrit rose to 32.6 with iron supplementation. Despite normal vitamin B12 and folic acid measurements on multiple determinations, the mean cell volume rose from normal (84.5-97.8 fL) to 115.9 fL with a mean cell hemoglobin of 40.7 pg/rbc (normal = 27.7-33.4 pg/rbc) by study day 832. Bone marrow chromosome analysis was normal. The prestudy WBC count of 5.7 × 109/L to 7.3 × 109/L also declined over this period to 3.3 × 109/L to 4.4 × 109/L. Cyclosporine (125 mg twice daily) was begun on day 832; on day 869 the platelet count was 37 × 109/L, and by day 919 the platelet count was 175 × 109/L. Cyclosporine was discontinued on day 1066 at a WBC count of 7.7 × 109/L, hematocrit 41, mean cell volume 103 fL, and platelets 235 × 109/L.
Subject no. 3.
A 61-year-old woman with stage IIIA non–small cell lung cancer and a history of scleroderma and Raynaud phenomenon was enrolled in a clinical trial13 in which she received 4 cycles of chemotherapy with paclitaxel (275 mg) and carboplatin (890 mg). Twenty-four hours after the administration of chemotherapy, she received PEG-rHuMGDF at a dose of 5 μg/kg subcutaneously daily for 7 days and filgrastim 5 μg/kg subcutaneously daily for 14 days for all 4 cycles. Her platelet count fell after each administration of chemotherapy but rebounded less well after each cycle. After the fourth chemotherapy cycle, the platelet count fell below 20 × 109/L on day 84. The hematocrit was 21 with an absolute neutrophil count of 0.6 × 109/L. With multiple RBC and platelet transfusions and filgrastim, her hematocrit and WBC count returned to a normal range, but the platelet count never rose above 20 × 109/L without platelet transfusions. A screening test for antibody to PEG-rHuMGDF was positive on study day 92. On study day 152 the platelet count was 2 × 109/L with an absolute neutrophil count of 6.4 × 109/L. Plasmapheresis was performed on day 148 with no improvement. Marked progression of cancer in the chest, abdomen, and pelvis was noted on study day 177. Brain metastases were subsequently irradiated, and the subject died on study day 226 due to disease progression.
Clinical assessment of thrombocytopenic subjects
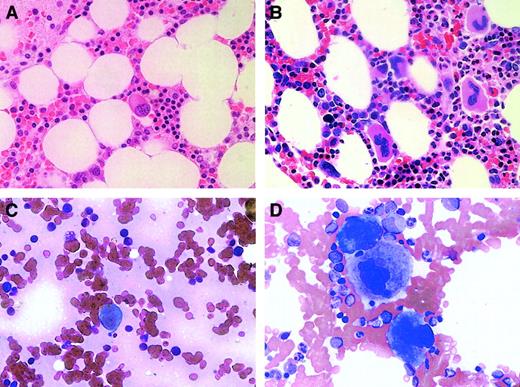
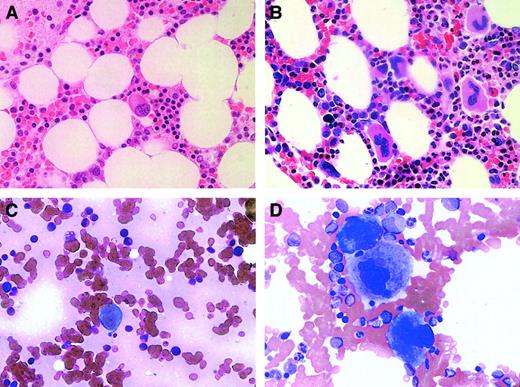
All 3 thrombocytopenic subjects underwent bone marrow examination (on days 142, 777, and 146 for subjects no. 1, 2, and 3, respectively), and all had a marked decrease in bone marrow megakaryocytes. The 2 nonchemotherapy subjects, no.1 and no. 2, had normal cellular marrows with no evidence of other hematologic disorders (and normal chromosome and flow cytometry analyses) but with megakaryocytes that were no more than 5% to 10% of that present in normal marrow (Figure2A). This is markedly decreased from what would have been seen in the marrow of a subject with ITP and a similar extent of thrombocytopenia (Figure 2B). Moreover, the megakaryocytes that were present were small and hypolobulated. The marrow of subject no. 3 was also devoid of significant numbers of megakaryocytes and showed postchemotherapy changes of hypocellularity (5%-10%) but with normal maturation of myeloid and erythroid lineages.
Bone marrow megakaryocytes were decreased in subjects with anti-TPO antibody.
Bone marrow biopsies (A,B) and aspirates (C,D) were examined at an original magnification of × 50 in subject no. 2 (A,C) and in a patient with ITP (B,D).
Bone marrow megakaryocytes were decreased in subjects with anti-TPO antibody.
Bone marrow biopsies (A,B) and aspirates (C,D) were examined at an original magnification of × 50 in subject no. 2 (A,C) and in a patient with ITP (B,D).
The immunologic status of these subjects was analyzed for host factors that might have contributed to the development of antibodies to TPO (Table 1). Only subject no. 3 had any history of autoimmune disorder. HLA class I and class II antigens and platelet antigens showed no consistent pattern. Four different tests for antiplatelet antibodies were used, and only one was positive: Subject no. 1 had a modest elevation in platelet-associated antibody that was consistent with prior pregnancy or transfusion.
To determine whether these subjects had a polymorphism of their TPO coding sequence, complementary DNA (cDNA) was prepared from leukocytes from subjects no. 1 and 2 and the TPO cDNA sequenced. Both showed a wild-type TPO coding sequence identical to that found in PEG-rHuMGDF and rHuTPO.
Assessment of TPO levels
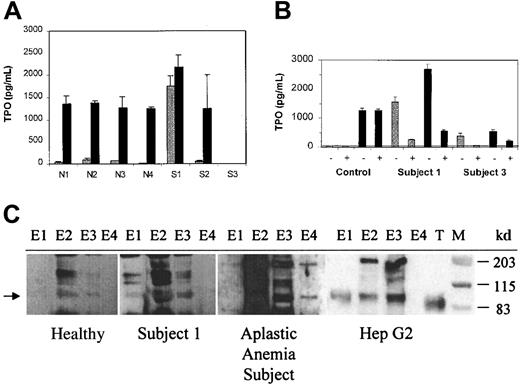
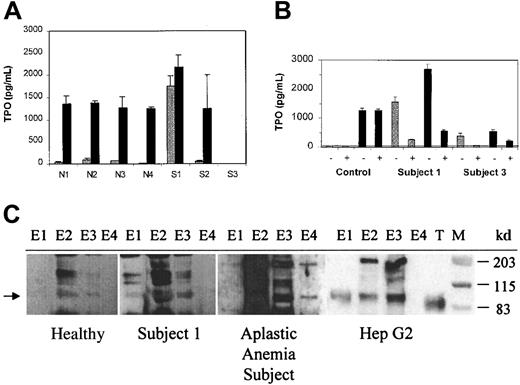
Median normal levels of endogenous TPO are approximately 179 pg/mL (range, 45-400 pg/mL).18 In patients with aplastic anemia and thrombocytopenia to the degree seen in the subjects studied, TPO levels range from 1500 to 2500 pg/mL.18 Thrombocytopenic subjects no. 2 and 3 had inappropriately normal or low levels of TPO in the circulation, but subject no. 1 had a marked elevation of TPO (Figure 3A).
Plasma from thrombocytopenic subjects contained antibody to TPO and circulating TPO-IgG complexes.
(A) TPO levels before (light bars) and after (dark bars) addition of rHuTPO. The TPO concentration was determined before and after the addition of 1500 pg/mL rHuTPO to serum samples from 4 healthy subjects (N1-N4) and from subjects no. 1, 2, and 3. (B) Endogenous TPO circulates as a TPO-IgG complex. The TPO concentration was determined before (−) and after (+) immunoprecipitation with protein A beads, both before (gray bars) and after (black bars) addition of 1500 pg/mL rHuTPO to serum samples from a healthy control subject and subjects no. 1 and 3. The horizontal line indicates the detection limit for the TPO ELISA assay. (C) The immune complexes from subject no. 1 contain full-length, endogenous TPO. Immune complexes were isolated and their TPO content assessed by SDS-PAGE and Western blot as described in “Materials and methods.” Endogenous TPO isolated from plasma from a healthy subject, plasma from an aplastic anemia subject, and conditioned medium from HepG2 cells is shown for comparison. For each sample, 4 equivalent sequential column elution fractions (E1-E4) were analyzed. Lanes containing rHuTPO (T) and molecular weight markers (M) are indicated. The arrow denotes the position of endogenous TPO.
Plasma from thrombocytopenic subjects contained antibody to TPO and circulating TPO-IgG complexes.
(A) TPO levels before (light bars) and after (dark bars) addition of rHuTPO. The TPO concentration was determined before and after the addition of 1500 pg/mL rHuTPO to serum samples from 4 healthy subjects (N1-N4) and from subjects no. 1, 2, and 3. (B) Endogenous TPO circulates as a TPO-IgG complex. The TPO concentration was determined before (−) and after (+) immunoprecipitation with protein A beads, both before (gray bars) and after (black bars) addition of 1500 pg/mL rHuTPO to serum samples from a healthy control subject and subjects no. 1 and 3. The horizontal line indicates the detection limit for the TPO ELISA assay. (C) The immune complexes from subject no. 1 contain full-length, endogenous TPO. Immune complexes were isolated and their TPO content assessed by SDS-PAGE and Western blot as described in “Materials and methods.” Endogenous TPO isolated from plasma from a healthy subject, plasma from an aplastic anemia subject, and conditioned medium from HepG2 cells is shown for comparison. For each sample, 4 equivalent sequential column elution fractions (E1-E4) were analyzed. Lanes containing rHuTPO (T) and molecular weight markers (M) are indicated. The arrow denotes the position of endogenous TPO.
To assess whether a factor neutralizing endogenous TPO might be present, a screening test was performed in which 1500 pg rHuTPO was added to 1 mL samples from each of the subjects and the total TPO concentration measured again. In 4 healthy individuals, the addition of 1500 pg rHuTPO increased the total TPO level from 57 ± 34 pg/mL to 1314 ± 67 pg/mL, as expected.14 Although the total TPO level in subject no. 2 rose by an extent similar to the healthy subjects, the total TPO concentration in subjects no. 1 and 3 rose much less or not at all, suggesting the presence of a neutralizing activity.
Presence of TPO-IgG immune complexes
To explore the etiology of the elevated endogenous TPO level in subject no. 1, a serum sample (day 469) was tested for its TPO content before and after treatment with beads conjugated with protein A. The protein A treatment specifically removed 90% to 93% of the IgG in the sample. As shown in Figure 3B, 87% of the endogenous TPO in subject no. 1 coprecipitated with the IgG. Upon addition of rHuTPO to a serum sample from subject no. 1, 81% of the total TPO coprecipitated with the IgG. Immunoprecipitation of IgG from normal control serum did not reduce the TPO content either before or after the addition of rHuTPO. In different serum samples from subject no. 3, the endogenous TPO content ranged from undetectable (Figure 3A) to modestly elevated (Figure 3B). When present in detectable amounts in subject no. 3, 85% of the endogenous TPO coprecipitated with IgG. These results suggest that endogenous TPO was circulating as a presumably inactive TPO-IgG complex in subject no. 1 and to a lesser extent in subject no. 3.
To determine whether the endogenous TPO in the immune complex in subject no. 1 contained full-length TPO or TPO degradation products, the immune complexes were collected and the associated TPO was purified on a TPO affinity column, electrophoresed, and subjected to Western blotting. As shown in Figure 3C, the immune complexes from subject no. 1 contained a TPO band migrating at a molecular weight of about 95 kd. This band was identical in size to the endogenous TPO purified from the serum of a subject with aplastic anemia who had a high endogenous TPO level and from a subject with a normal TPO level as well as from the conditioned media of a TPO-producing hepatoma cell line. Endogenous TPO is slightly larger than the rHuTPO that runs at about 90 kd. The relative intensities of the TPO bands for the healthy subject, subject no. 1, and the aplastic anemia subject reflect the differences in serum TPO concentrations: 135, 1552, and 1934 pg/mL, respectively.
Characterization of antibodies to TPO
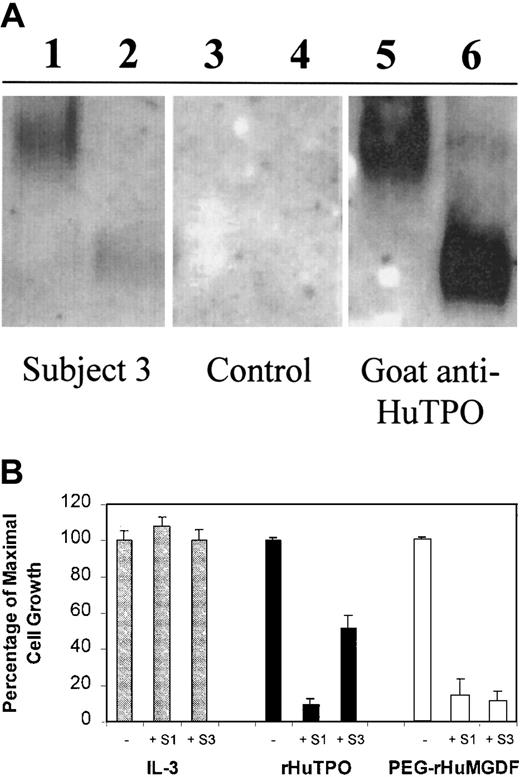
IgG from thrombocytopenic subjects bound and neutralized PEG-rHuMGDF and rHuTPO.
The IgG fraction was purified from subjects no. 1 and 3 and tested for its ability to bind to PEG-rHuMGDF and rHuTPO (Figure4). IgG from subject no. 3 bound to native rHuTPO and PEG-rHuMGDF on Western blots (Figure 4A) but did not bind to denatured and reduced rHuTPO or PEG-rHuMGDF (data not shown). Purified IgG from subject no. 1 as well as from healthy subjects repeatedly failed to bind to either native or denatured PEG-rHuMGDF or rHuTPO on Western blots.
IgG from thrombocytopenic subjects binds to TPO and neutralizes its biological activity.
(A) rHuTPO (lanes 2,4,6) and PEG-rHuMGDF (lanes 1,3,5) were subjected to native PAGE (10% Tris-glycine, pH 8.3, without SDS, heat denaturation, or reduction) and blotted with IgG from subject no. 3 (lanes 1,2), IgG from a control subject (lanes 3,4), or a polyclonal goat antibody to TPO (lanes 5,6). (B) BaF3-mpl cells were grown in the presence of varying concentrations of IL-3 (gray bars), rHuTPO (black bars), or PEG-rHuMGDF (white bars) in the absence (−) or presence (+) of IgG from subjects no. 1 or 3 and the effect on cell growth measured.
IgG from thrombocytopenic subjects binds to TPO and neutralizes its biological activity.
(A) rHuTPO (lanes 2,4,6) and PEG-rHuMGDF (lanes 1,3,5) were subjected to native PAGE (10% Tris-glycine, pH 8.3, without SDS, heat denaturation, or reduction) and blotted with IgG from subject no. 3 (lanes 1,2), IgG from a control subject (lanes 3,4), or a polyclonal goat antibody to TPO (lanes 5,6). (B) BaF3-mpl cells were grown in the presence of varying concentrations of IL-3 (gray bars), rHuTPO (black bars), or PEG-rHuMGDF (white bars) in the absence (−) or presence (+) of IgG from subjects no. 1 or 3 and the effect on cell growth measured.
The purified IgG fractions were tested for their ability to inhibit the biological activity of TPO. A BaF3-mpl cell line15dependent upon either IL-3 or TPO grew maximally in the presence of IL-3, rHuTPO, or PEG-rHuMGDF. The IgG fractions from subjects no. 1 and 3 had no inhibitory effect on the growth of these cells when stimulated by IL-3 (Figure 4B). However, in the presence of rHuTPO, IgG from subject no. 1 reduced maximal cell growth by 90% and IgG from subject no. 3 reduced cell growth by approximately 48%. When the cells were grown in the presence of PEG-rHuMGDF, IgG samples from both subjects reduced cell growth by 86% to 89%. IgG from healthy subjects did not affect the growth of cells in the presence of any of these hematopoietic growth factors.
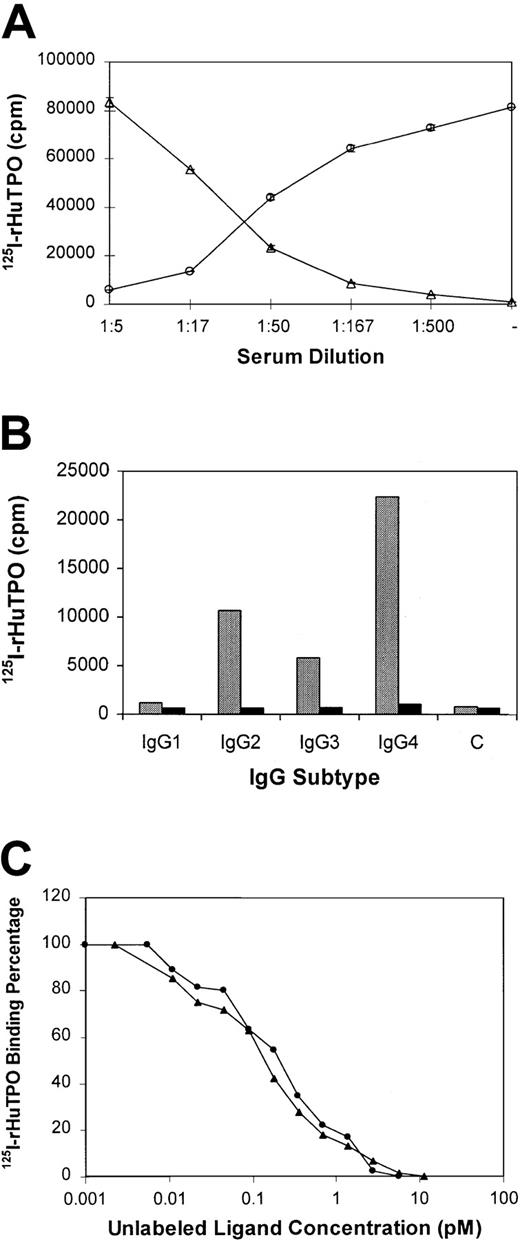
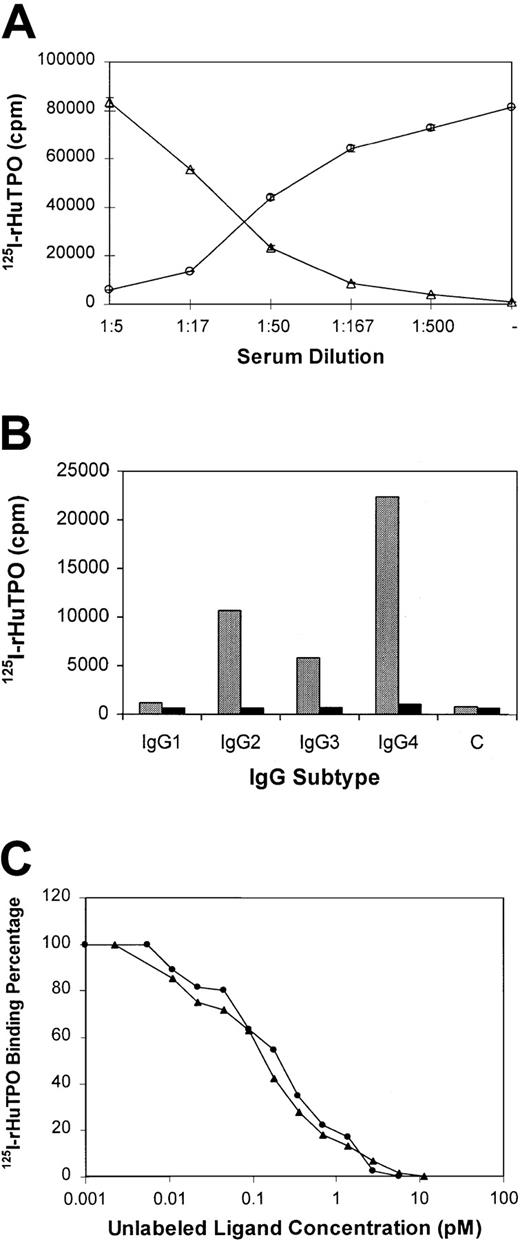
Antibody to TPO neutralized the biologic activity of rHuTPO by preventing the binding of rHuTPO to its receptor.
125I-rHuTPO was added to platelet-rich plasma from healthy individuals and its binding to the platelet TPO receptor measured. As shown in Figure 5A, in the absence of serum from subject no. 3, all the 125I-rHuTPO bound to platelets and none could be immunoprecipitated from the plasma as a complex with IgG. However, upon addition of increasing amounts of serum from subject no. 3, the extent of platelet binding was reduced to 5% and the extent of IgG-associated 125I-rHuTPO rose to 95%.
Anti-TPO IgG prevents TPO from binding to its receptor and is directed toward epitopes in the first 163 amino acids of TPO.
(A) 125I-rHuTPO was added to normal platelet-rich plasma and incubated without (−) or with various dilutions of citrated plasma from subject no. 3. The amount (± SD) of platelet-associated (circles) and IgG-associated (triangles) 125I-rHuTPO was then determined as described in “Materials and methods.” (B) IgG subtypes of antibody to TPO. Serum from subject no. 3 (gray bars) or a healthy subject (black bars) was incubated with 125I-rHuTPO followed by addition of IgG subtype-specific antisera and immunoprecipitation. IgG subtype-specific sera was omitted from the control (“C”) lane. (C) PEG-rHuMGDF and rHuTPO compete equally for binding to anti-TPO antibody. Plasma from subject no. 3 was incubated with 125I-rHuTPO in the absence or presence of various concentrations of unlabeled rHuTPO (circles) or PEG-rHuMGDF (triangles) and the amount of 125I-rHuTPO present in the immunoprecipitate measured.
Anti-TPO IgG prevents TPO from binding to its receptor and is directed toward epitopes in the first 163 amino acids of TPO.
(A) 125I-rHuTPO was added to normal platelet-rich plasma and incubated without (−) or with various dilutions of citrated plasma from subject no. 3. The amount (± SD) of platelet-associated (circles) and IgG-associated (triangles) 125I-rHuTPO was then determined as described in “Materials and methods.” (B) IgG subtypes of antibody to TPO. Serum from subject no. 3 (gray bars) or a healthy subject (black bars) was incubated with 125I-rHuTPO followed by addition of IgG subtype-specific antisera and immunoprecipitation. IgG subtype-specific sera was omitted from the control (“C”) lane. (C) PEG-rHuMGDF and rHuTPO compete equally for binding to anti-TPO antibody. Plasma from subject no. 3 was incubated with 125I-rHuTPO in the absence or presence of various concentrations of unlabeled rHuTPO (circles) or PEG-rHuMGDF (triangles) and the amount of 125I-rHuTPO present in the immunoprecipitate measured.
IgG was the only antibody type that bound to TPO.
Serum samples from all 3 subjects were incubated with125I-rHuTPO and immunoprecipitated with protein G, which removes only IgG. Upon removal of the IgG–protein G complexes by centrifugation, the supernatant was then immunoprecipitated with protein L, which binds not only IgG but also IgM, IgA, IgD, and IgE. All of the 125I-rHuTPO was immunoprecipitated by the protein G and none by the protein L at any time point measured (data not shown), indicating that all of the antibodies to TPO were IgG.
The IgG subtype of the antibody to TPO was analyzed using subtype-specific monoclonal antibodies. As shown for subject no. 3, most of the anti-TPO antibody in this subject was IgG4 (Figure 5B, Table 1). This is in marked contrast to the normal IgG distribution in humans (Table 1). For subject no. 1 there was an increased amount of IgG4 but less than that seen in subject no. 3 (Table 1).
Anti-TPO antibody bound to epitopes within the first 163 amino acids of TPO.
Subject serum samples were incubated with 125I-rHuTPO along with various amounts of unlabeled rHuTPO or PEG-rHuMGDF and the IgG-TPO complexes immunoprecipitated with protein A. As shown in Figure 5C, both unlabeled rHuTPO and PEG-rHuMGDF competed equally for antibody binding, suggesting that only the first 163 amino acids of TPO contained the epitopes recognized. A series of shorter peptides within this region failed to compete for binding and suggested that more complex, nonlinear epitopes were involved. Identical studies were also performed with sera from subjects no. 1 and 2 with the same results.
Time course of anti-TPO antibody development in subjects.
Using a sensitive and specific assay for IgG antibody to TPO, the time course of appearance of the antibodies was quantified in all 3 subjects (Figure 1). In none of the subjects was antibody detected prior to the injection of PEG-rHuMGDF, and none showed any IgM antibody response. In subject no. 1 (Figure 1A), trace amounts of antibody first appeared on day 56 when the platelet count had just started to fall below baseline, was markedly elevated by day 137 at a platelet count of 53 × 109/L, and was maximal at day 147 at a platelet count of 21 × 109/L. The antibody concentration decayed over the next 686 study days, and with resolution of the thrombocytopenia no more antibody to TPO was detectable in the serum of this subject. For subject no. 2 (Figure 1B), the elevated antibody level, first quantified on day 202, slowly declined and following treatment with cyclosporine rapidly disappeared. For subject no. 3 (Figure 1C), measurable amounts of antibody were first detected on day 71 when the platelet count was 152 × 109/L, became markedly elevated when the platelet count failed to rise after the third cycle of chemotherapy, and remained at a very high plateau thereafter during the entire course of her thrombocytopenia despite plasmapheresis on day 148. Notably, the concentration of antibody varied greatly among the subjects: For the same degree of thrombocytopenia, subject no. 2 had a maximal antibody titer of approximately 2.1 μg/mL versus 8 μg/mL for subject no. 1 and 13 μg/mL for subject no. 3.
Discussion
Thrombocytopenia occurred unexpectedly in healthy volunteers and cancer chemotherapy patients treated with PEG-rHuMGDF. Thrombocytopenia developed after as little as 2 injections in the subjects studied here, and antibody to TPO was detected as early as 56 days after the initial injection. Except for easy bruising and increased menstrual bleeding, the subjects had no major bleeding episodes. Although there was a transient response to plasmapheresis in 1 subject, none of the subjects responded to corticosteroids or IVIG, and the 2 who received platelet transfusions had appropriate increases in the platelet count. Two subjects have recovered, and 1 died of her cancer. None of the 3 subjects had any unusual HLA or platelet phenotype, and all lacked evidence for antiplatelet antibody. Although polymorphism of the open reading sequence of TPO has so far not been described, the TPO cDNA from 2 subjects was sequenced and found to be normal.
All 3 subjects had a marked decrease in bone marrow megakaryocytes like that seen in homozygous knockout mice deficient in TPO3 or its receptor.4 As in those knockout animals, the megakaryocytes were small, hypolobulated, and had scant cytoplasm. Although formal platelet kinetic studies could not be performed in the subjects, their bone marrow appearance was consistent with decreased bone marrow production of platelets and differed distinctly from that in ITP. On average, the nadir platelet counts in all 3 subjects were 6% to 8% of baseline, somewhat lower than the 10% to 15% of normal found in the knockout animals. In one subject the WBC and RBC values also became modestly decreased. Assays could not be performed to see if these subjects had reduced levels of myeloid and erythroid progenitor cells, as has been reported for the knockout mice.5
The thrombocytopenia was temporally related to the appearance of antibody to TPO, and the antibody disappeared in both subjects who recovered. Small amounts of antibody to TPO could be detected in 2 of the subjects when the platelet count fell below baseline and before it decreased below 100 × 109/L. In general, the antibody level in any one subject was inversely proportional to the platelet count, but between subjects there was a variation in peak titer despite similarly low platelet counts.
Even using a very sensitive assay, no IgM response could be detected at early time points. All of the antibody to TPO was IgG with an increased relative amount of IgG4, a subtype that fails to fix complement and is associated with autoimmune phenomena and immune complex formation. This predilection for IgG4 has previously been reported for autoantibody formation against factor VIII 19 and IL-1α.20
The antibody bound and neutralized TPO as demonstrated in several ways. First, excess TPO antibody could be inferred in 2 subjects when the predicted rise in TPO concentration was not seen after addition of known amounts of rHuTPO to serum samples. Second, purified IgG fractions from 2 subjects neutralized more than 90% of the TPO biological activity using a TPO-dependent cell line. Finally, using an assay directly detecting the ability of IgG to bind to125I-rHuTPO, all subjects had IgG that specifically bound125I-rHuTPO.
The anti-TPO IgG from subject no. 3 bound both rHuTPO and PEG-rHuMGDF but only in their native forms. The lack of binding to denatured and reduced proteins suggested that simple, linear epitopes were not involved. IgG from subject no. 1 did not bind to TPO reproducibly on Western blots, probably due to the lesser titer of antibody present or the fact that much of the antibody existed as an IgG-TPO complex. Because PEG-rHuMGDF contains only the first 163 of the 332 amino acids in rHuTPO, the finding that rHuTPO and PEG-rHuMGDF both competed equally for antibody binding suggests that all antigenic determinants were located within the first 163 amino acids.
In 2 subjects the endogenous TPO level was inappropriately low for the degree of thrombocytopenia and reflected the binding of IgG to endogenous TPO and its subsequent removal by the reticuloendothelial system. However, endogenous TPO was persistently elevated in subject no. 1 but circulated as a presumably inactive IgG-TPO immune complex. The TPO component of the immune complex had a molecular weight identical to that found in endogenous TPO isolated from sera from healthy and aplastic anemia subjects. These data also serve to demonstrate for the first time that endogenous TPO has a molecular weight of about 95 kd, slightly higher than the approximate 90 kd rHuTPO made in Chinese hamster ovary cells and used in other clinical studies.
Antibody to PEG-rHuMGDF occurred much more frequently in the immunocompetent healthy volunteers (13 of 325) than it did in the immunocompromised chemotherapy patients (4 of 650). A similar finding has been observed following the administration of molgramostim, a nonglycosylated granulocyte-macrophage colony-stimulating factor (GM-CSF) produced in Escherichia coli.21Although naturally occurring antibody to GM-CSF is rare, 95% of immunocompetent patients who received molgramostim developed antibody to GM-CSF whereas virtually no immunocompromised patients did.
In clinical studies with rHuTPO in more than 500 cancer chemotherapy patients, thrombocytopenia attributed to anti-TPO antibodies has not yet been reported despite the appearance of a partially neutralizing antibody in one subject.7,22 This may be due to the native structure of rHuTPO unlike the modified PEG-rHuMGDF. A second possible reason for the lack of thrombocytopenia with rHuTPO in these clinical studies was that rHuTPO was always given intravenously whereas PEG-rHuMGDF was always given subcutaneously.6 In experiments in rats, the subcutaneous but not intravenous injection of recombinant PEG-ratMGDF caused thrombocytopenia. Because the TPOs are potent stimulators of dendritic cells (J.L., unpublished observations, 1999), one possibility is that subcutaneous PEG-rHuMGDF has acted as an adjuvant for itself by stimulating antigen-presenting cells.
Platelet production may be especially sensitive to the appearance of antibody to TPO because TPO is constitutively synthesized in the liver and its rate of production does not rise during thrombocytopenia. Platelet production is regulated by a simple feedback loop whereby the TPO level is controlled directly by the circulating platelet count23; the addition of an antibody-mediated clearance system would markedly alter this homeostatic mechanism. This is in contrast to RBC production where renal synthesis of erythropoietin (EPO) can be markedly increased and might be less sensitive to antibody to EPO.24 Only rare cases of pure red cell aplasia due to naturally occurring antibody to EPO have been described.25,26 Equally uncommon are antibodies formed against recombinant human EPO.27
Low levels of antibody to G-CSF have been found in 11% of healthy adults and in 13% of cord blood samples but with no effect on the neutrophil count.28 These autoantibodies rose after administration of G-CSF but lacked clinical significance. This is in contrast to the aforementioned antibodies formed after the administration of molgramostim that reduced the half-life and biological effect of the recombinant drug but did not affect baseline WBC production.21
The finding that antibody to TPO can cause significant thrombocytopenia has several major implications. First, it illustrates the wide variation in the rate of antibody formation to recombinant polypeptide therapeutics. Antibodies to recombinant EPO and G-CSF have been uncommon in patients treated with these hematopoietic growth factors and have rarely caused cytopenias. However, antibodies to recombinant α-interferon, factor VIII, and human insulin have been associated with clinical problems.20
Second, these studies describe a new mechanism for thrombocytopenia in humans. Because TPO is constitutively produced, the appearance of a neutralizing antibody to TPO reduces platelet production by 95% and is similar to animals in which the TPO gene has been knocked out or in which autoantibodies to TPO have been generated.29,30Although iatrogenic in the subjects described here, one spontaneous case of thrombocytopenia due to antibody to TPO has been described.31 Both this patient and our subject no. 2 responded to cyclosporine, which supports the use of this therapy.32 Whether other cases of acquired amegakaryocytic thrombocytopenia are due to antibody to TPO is unknown; loss of function mutations of the TPO receptor33 is a cause of congenital amegakaryocytic thrombocytopenia.
Finally, the long-term complications of persistent antibody to TPO on other lineages may be of concern. Subject no. 2 developed a reversible macrocytic anemia and a low WBC count as well as the marked thrombocytopenia. Because pluripotential stem cells and progenitors of all lineages depend on TPO to prevent apoptosis, depletion of early progenitor cells and concomitant pancytopenia might ensue, as has been seen in some children born with amegakaryocytic thrombocytopenia due to a defective TPO receptor.33 However, mice deficient in TPO or its receptor are thrombocytopenic but have normal levels of RBCs and WBCs despite reduced progenitor cells of all lineages.3 4
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
David J. Kuter, Hematology/Oncology Unit, COX 640, Massachusetts General Hospital, 100 Blossom St, Boston, MA 02114; e-mail: kuter.david@mgh.harvard.edu.