Abstract
The absolute content of CD34+ cells in the peripheral blood of 84 patients with myelofibrosis with myeloid metaplasia (MMM) and 23 patients with other Philadelphia-negative (Ph−) chronic myeloproliferative disorders (CMDs) was investigated. In MMM, the median absolute number of circulating CD34+cells was consistently high (91.6 × 106/L; range, 0-2460 × 106/L). Receiver operating characteristic curve analysis showed that 15 × 106/L as a decision criterion for CD34+cells produced an almost complete discrimination between MMM patients out of therapy and other Ph− CMDs (positive predictive value, 98.4%; negative predictive value, 85.0%). MMM patients with higher numbers of CD34+ cells had a significantly longer disease duration (P = .019) and higher spleen volume index (P = .014), liver volume (P = .000), percentage of circulating immature myeloid cells (P = .020), and percentage of myeloid blasts (P = .000). When CD34+ cells were correlated with the use of Dupriez risk stratification, CD34+ cells increased significantly from low-risk (median, 68.1 × 106/L) to intermediate-risk (median, 112.8 × 106/L) and high-risk patients (median 666.1 × 106/L) (F = 4.95;P = .009). When CD34+ cells were correlated with a severity score on the basis of both myeloproliferative and myelodepletive characteristics of the disease, only the myeloproliferation index was significantly associated with CD34+ cell level (F = 5.7;P = .000). Overall survival and interval to blast transformation from the time of CD34+ cell analysis were significantly shorter in patients with more than 300 × 106/L CD34+ cells (P = .005 and .0005, respectively). In conclusion, the absolute number of CD34+ circulating cells allows MMM to be distinguished from other Ph− CMDs; it is strongly associated with the extent of myeloproliferation and predicts evolution toward blast transformation.
Introduction
Chronic myeloproliferative disorders (CMDs) encompass a heterogeneous array of diseases that are due to somatic mutation and clonal proliferation of a pluripotent hematopoietic progenitor cell. The functional derangement of the malignant hematopoietic clone results in an increase of progenitor cells in the bone marrow and an increased number of circulating hematopoietic precursors, including pluripotent and committed progenitors.1-12 In myelofibrosis with myeloid metaplasia (MMM), a CMD characterized by bone marrow fibrosis and constitutive myeloid metaplasia,13 the number of circulating hematopoietic precursors has always been reported to be consistently high, with the mean levels being from 8- to 167-fold higher than those found in control subjects.1-9 When cells are measured by flow cytometry, the average CD34+ cell recovery from peripheral blood is higher in patients with MMM than in those with other Philadelphia-negative (Ph−) CMDs.10-12These observations suggest that the circulating pool of CD34+ cells in Ph− CMDs increases along with the proliferative capacity of the individual disease, and that this pool might have the potential to portray the proliferative patterns of such diseases. However, the absolute number of circulating CD34+ cells in MMM has not been quantified on a large scale, and the relationship between CD34+ cells and progression of the disease is not fully understood.
The purpose of this study was to define the levels of CD34+cells in a large, well-characterized population of patients with MMM. Moreover, we wished to assess the ability of the number of circulating CD34+ cells to distinguish MMM from other Ph−CMDs and to examine the relation between CD34+ cell number and progression of the disease, in particular the evolution toward blast transformation.
Patients, materials, and methods
Patients
Between February 1999 and November 2000, peripheral blood samples from 84 consecutive patients with MMM (57 males and 27 females; median age, 64 years; age range, 21-87 years) were studied. The samples were collected from 14 Italian centers participating in the Italian Registry for MMM.
The diagnosis of MMM was established according to the Italian Consensus Conference criteria,14 by which the diagnosis holds if diffuse bone marrow fibrosis is present and Philadelphia chromosome or BCR-ABL rearrangement in peripheral blood cells is absent. In addition, any 2 of the following criteria should be present when splenomegaly is present and any 4 when splenomegaly is absent: anisopoikilocytosis with teardrop erythrocytes, presence of circulating immature myeloid cells, presence of circulating erythroblasts, presence of clusters of megakaryoblasts and anomalous megakaryocytes in bone marrow sections, myeloid metaplasia.
Sixty-five patients were classified as having primary MMM and 19 as having secondary MMM (14 had post–polycythemia vera [PV] MMM, and 5 had post–essential thrombocythemia [ET] MMM). Twenty-two patients (26.2%) were studied at the time of diagnosis and before any therapy was started, while 62 were studied during the course of their disease. Sixty-seven patients (79.7%) were studied before the start of any cytoreductive treatment or when the cytoreduction had been stopped for at least 3 months. Seventeen patients (20.2%) were on hydroxyurea treatment at the time of the CD34+ analysis, and 8 (9.5%) had also had a splenectomy. To analyze the change in CD34+cells over time, blood was drawn from 7 patients at regular intervals over a period of at least 4 months.
At the time that blood was drawn for the measurement of CD34+ cells, the patients also had a complete blood count and a peripheral blood smear examination, and their spleen and liver measurements were taken. White blood cell count was corrected for the number of circulating erythroblasts. Circulating nucleated cells were classified as immature myeloid cells, erythroblasts, and blasts. Blasts were defined as undifferentiated cells with an immature nucleolated nucleus and basophilic cytoplasm with or without azurophilic granules.
The size of the spleen was measured by ultrasonography by measuring the length from the splenic tip to the costal margin in centimeters and by using the spleen index calculated by multiplying the length of the longitudinal axis by that of the transverse axis, the latter defined as the maximal width of the organ.15 Liver enlargement was measured as the distance from the right costal margin in centimeters.
Patients were assigned a prognostic score on the basis of the findings of Dupriez et al.16 A score of 0 was assigned to a hemoglobin concentration of more than 100 g/L and a white blood cell count between 4 × 109/L and 30 × 109/L; a score of 1 was assigned to either a hemoglobin concentration lower than 100 g/L or a white blood cell count more than 30 × 109/L or less than 4 × 109/L; and a score of 2 was assigned if both the hemoglobin and the white blood cell values were in those aberrant ranges.
Since there is no accepted definition of disease severity, we evaluated a severity score by indexing leukocytosis, thrombocytosis, and splenomegaly (myeloproliferation index) and anemia, leukopenia, and thrombocytopenia (myelodepletion index). There were 3 grades for splenomegaly or hepatomegaly in patients who had had a splenectomy (0, nonpalpable; 1, no more than 10 cm below the costal margin; 2, more than 10 cm below the costal margin); 2 grades for leukocytosis (0, white blood cell count between 4 × 109/L and 15 × 109/L; 1, white blood cell count exceeding 15 × 109/L); and 2 grades for thrombocytosis (0, platelet count between 150 × 109/L and 500 × 109/L; 1, platelet count exceeding 500 × 109/L), making the myeloproliferation index range from 0 to 4. There were 3 grades for anemia (0, hemoglobin level exceeding 120 g/L; 1, from 100 to 120 g/L; 2, lower than 100 g/L or under transfusion); 2 grades for leukopenia (0, white blood cell count between 4 × 109/L and 30 × 109/L; 1, white blood cell count lower than 4 × 109/L); and 2 grades for thrombocytopenia (0, platelet count between 150 × 109/L and 500 × 109/L; 1, platelet count lower than 150 × 109/L), making the myelodepletion index range from 0 to 4. The severity score ranged from 0 to 6.
Patients were followed up to the end of the study with a median follow-up of 11.5 months (range, 1-25 months), and death and evolution toward blast transformation were recorded. A diagnosis of blast transformation required the percentage of peripheral blood blasts to exceed 20% of the white blood cell count and/or the percentage of blasts in the bone marrow to exceed 40%.
For comparison, 20 patients with other Ph− CMDs were also studied (9 females and 11 males with a median age of 55.5 years; range, 21 to 66 years). They were categorized as having PV (6 cases), ET (6 cases), or atypical myeloproliferative disorders (AMDs) (8 cases). The diagnoses of PV and ET were established according to the Polycythemia Vera Study Group criteria17,18 after exclusion of MMM. For the diagnosis of AMD, we considered patients with documented clonal hematopoiesis (in females), who lacked the criteria established for the diagnosis of PV, ET, or MMM. As described in a previous paper,19 all the patients with AMD were under the age of 40 years, had mild signs of myeloproliferation, mild or no disease progression, and a strong tendency to develop thrombotic episodes. Of the patients with other Ph− CMDs, 5 were studied at diagnosis and 12 from 1 to 12 years after diagnosis and before any therapy had been started or when therapy had been stopped for at least 3 months. Three patients had had a splenectomy because of spent phase of the disease (2 PV patients) or because of symptomatic splenomegaly (1 AMD patient).
Control samples were obtained from 21 healthy individuals (15 males and 6 females; median age, 65 years).
All peripheral blood samples were collected after obtaining informed consent.
Immunophenotype analysis
Cells in EDTA-anticoagulated blood were counted, and their phenotype was determined by flow cytometry analysis. Cells were incubated for 30 minutes on ice with 10 mL fluorescein isothiocyanate (FITC)–, phycoerythrin (PE) peridinin chlorophyll protein (PerCP)–, and laser dye styryl (LDS751)–conjugated monoclonal antibodies. The following antibody combinations were used: CD45-FITC/CD34-PE/LDS751 and CD38-FITC/CD34-PE/CD45-PerCP. Each fluorescence analysis included a double-negative isotype control (immunoglobulin G1[IgG1]–FITC/IgG1-PE). Directly conjugated monoclonal antibody and isotype controls were obtained from Becton Dickinson Immunocytometry Systems (San Jose, CA). Ammonium chloride lysing solution (prepared by the Pharmacology Department, IRCCS Policlinico San Matteo) was added to lyse the red blood cells. The analysis was completed according to the cell-gating guidelines recommended by the International Society of Hematotherapy and Graft Engineering as previously described.20 Briefly, the cells were first gated into region 1 (R1) so that the CD45+ cells could include all nucleated white blood cells and exclude red blood cells, nucleated red blood cells, platelets, and cellular debris. CD45+ events in R1 were then analyzed for CD34 staining, and positive events were gated into region 2 (R2). Events defined by R1 and R2 were analyzed on a dot plot describing light scatter versus CD45 staining and on a second dot plot describing the light-scattering characteristics of the cells. The number of CD34+ cells meeting all fluorescence and light-scatter criteria was derived from the number of events representing specific staining, as determined by CD45-FITC/CD34-PE/LDS751 staining, minus the number of events representing nonspecific staining, as determined by the CD45-FITC/isotype-PE control. For the calculation of absolute CD34+ cells, the corrected number of CD34+cells was divided by the average total number of CD45+events from the CD45 FITC/CD34-PE/LDS751 staining; this value was multiplied by the absolute leukocyte count. A minimum number of 100 CD34+ events and 100 000 CD45+ events were collected for the CD34+ cell quantification by flow cytometry. The CD34+ population was divided into CD34+CD38− and CD34+CD38+ subpopulations.
With this method, a between-assays coefficient of variation of 13.07% was obtained.21
Statistical methods
Results were considered statistically significant whenP < .05. Skewed variables were logarithmically transformed before entering a parametric analysis. Comparisons between groups were performed by the Mann-Whitney U test or chi-square test when appropriate. Associations between patient characteristics (covariates) were assessed for pairs of numerical variables by the Spearman correlation, and for categorical and continuous variables by Wilcoxon-Mann-Whitney statistics. The optimal cutoff point for CD34+ cell number for discriminating between MMM and other Ph− CMDs was sought by constructing receiver operating characteristic (ROC) curves, which were generated by calculating the sensitivities and specificities of data at several predetermined cutoff points.22 Logistic regression was used to assess the ability of the patients' characteristics to predict the number of CD34+ cells in the peripheral blood. We examined the following covariates: sex, age at test examination, white blood cell count corrected for circulating erythroblasts, hemoglobin concentration, platelet count, percentage of immature myeloid cells in peripheral blood (excluding blasts), percentage of circulating erythroblasts, presence and number of circulating blasts, and spleen and liver sizes. Multivariate logistic models were obtained by performing a backward elimination with a cutoff ofP = .05, and then allowing any variable previously deleted to enter the final model if it was P < .05. Survival analysis and time to blast transformation curves were drawn by means of the Kaplan-Meier procedure. Patients were censored from the analysis of blast transformation risk factors if, at the time of CD34+examination, their circulating blasts exceeded 10%. Multivariate analysis was performed on MMM patients to investigate independent variables predicting blast transformation rate. The following variables were analyzed: hemoglobin level, white blood cell count, circulating blasts (both as a percentage of white blood cell count and as an absolute value), time from diagnosis to examination, having had a splenectomy, or having received prior cytotoxic therapy, CD34 and CD34+CD38− expression. The relative importance of each of the variables was estimated by means of the Cox proportional regression model. All computations were performed with Statistica software (Statsoft, Tulsa, OK).
Results
Patient characteristics
The hematological and clinical characteristics of the population of MMM patients studied are summarized in Table1. CD34+ cell analysis was performed at a median of 24 months (range, 0-204 months) after the diagnosis of MMM. Risk stratification according to the Dupriez-based prognostic scoring system showed a predominance of low- and intermediate-risk classes (85% of the patients). The severity score of the disease based on spleen size and hematological parameters measured at the time of CD34+ cell analysis ranged from 0 to 6, with a median value of 3. The myeloproliferation index (range, 0-4) had a median value of 3 and was no higher than 1 in 39 patients (46.4%), while the myelodepletion index (range, 0-4) had a median value of 2 and was no higher than than 1 in 34 patients (40.5%).
The level and phenotype profile of CD34+ cells in the peripheral blood of MMM patients
The median absolute number of circulating CD34+ cells in the overall population of MMM patients was 91.6 × 106/L (range, 0-2460 × 106/L), 360 times higher than in healthy subjects (median, 0.25 × 106/L; range, 0.15-0.35 × 106/L). As shown in Table 2, there was no significant difference in the median CD34+ cell level between patients on chemotherapy and patients out of therapy. Likewise, there was no significant difference between primary MMM and post-PV or post-ET MMM. The highest median number of CD34+ cells was found in patients who had had a splenectomy (n = 8), in whom the median value was 616.2 × 106/L as compared with 232.2 × 106/L in patients who had not had a splenectomy (P = .016).
When cells were double-stained with anti-CD34 and anti-CD38, the median percentage of CD34+ cells in MMM that also expressed CD38 was 66%, but the range was from 23% to 99%. Sixty-four percent of the cases had more than 60% CD38+ cells.
Differentiation between MMM and other Ph−CMDs
The median number of CD34+ cells in other Ph− CMDs was 18 times lower than in patients with MMM (Table 2). This number was independent of the clinical progression of the diseases: the median number of CD34+ cells in patients studied at disease diagnosis (n = 5) was 5.15 × 106/L (range, 3.2-8.0 × 106/L); the median for patients studied during the course of their disease (n = 12) was 8.25 × 106/L (range, 3.7-26.9 × 106/L); and the median for patients who had had a splenectomy (n = 3) was 5.0 × 106/L (range, 4.4-6.7 × 106/L).
To study the power of CD34+ cell number to discriminate between MMM and other Ph− CMDs, we used only cases out of therapy. ROC curve analysis showed that when the upper level of CD34+ cells in Ph− CDM (26.9 × 106/L) was used as the discriminatory cutoff between MMM and Ph− CMDs, the sensitivity and specificity were 87.7% and 100%, respectively. Lowering the level of CD34+ cells to 15 × 106/L as the discriminatory criterion significantly increased the sensitivity of CD34+ cells to 95.4%, while the specificity decreased slightly to 94.4%. Using this cutoff facilitated nearly complete discrimination of the Ph− CMDs and MMM (positive predictive value, 98.4%; negative predictive value, 85.0%).
Correlation between circulating CD34+ cells and disease characteristics in MMM
The analysis of correlation between circulating CD34+cells and disease characteristics was restricted to MMM patients who were studied out of cytoreductive treatment (n = 67). In this series, 3 patients had a post-PV or post-ET MMM. There was no significant correlation between CD34+ cells in peripheral blood and patients' age, sex, hemoglobin concentration, or platelet count. By contrast, on comparing CD34+ cell levels with duration of disease, as measured from the time of diagnosis to the time of sample collection and analysis, we found a significant direct correlation (R = 0.28; P = .019). In 21 patients whose blood was drawn at diagnosis, the median number of CD34+cells was 81.9 × 106/L (range, 2.04-349.4 × 106/L), lower than in patients (n = 45) whose blood was drawn more than 4 months after diagnosis (median CD34+ cell n = 305.6 × 106/L; range, 4.3-2460 × 106/L; P = .04). This suggests that CD34+ count increases as MMM progresses. According to the regression model, a value of 200 × 106/L CD34+ cells had to be expected after 36 months from diagnosis.
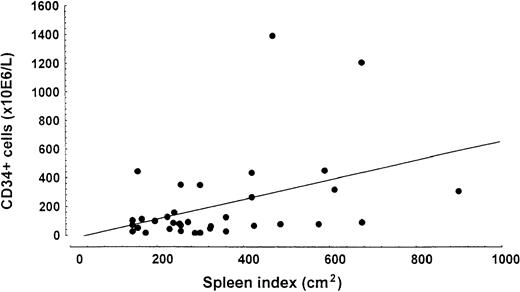
Patients with higher numbers of CD34+ cells had a significantly higher spleen volume index (R = 0.41;P = .014) (Figure 1) and spleen size measurement in centimeters (R = 0.41;P = .002), liver volume (R = 0.51;P = .000), percentage of immature myeloid cells (R = 0.31; P = .020), and percentage of circulating myeloid blasts (R = 0.68;P = .000). Patients with blasts in peripheral blood (n = 22) had a median CD34+ level of 282.8 × 106/L (range, 55.8-2460 × 106/L) whereas patients without blasts (n = 36) had a median of 69.5 × 106/L (range, 2.04-722.5 × 106/L). The median number of CD34+ cells in patients whose spleen was enlarged more than 10 cm from the costal margin (n = 19) was 301.0 × 106/L (range 14.4-2460 × 106/L) whereas this level was 70.0 × 106/L (range 2.04-1204.0 × 106/L) in patients with a smaller spleen volume (n = 47).
Relationship between spleen index (cm2) and number of CD34+ cells in peripheral blood.
Linear regression analysis demonstrated a significant correlation (R = 0.41; P = .014).
Relationship between spleen index (cm2) and number of CD34+ cells in peripheral blood.
Linear regression analysis demonstrated a significant correlation (R = 0.41; P = .014).
A multiple linear regression was performed by forward selection of the above significantly correlated variables. The analysis yielded myeloid blasts and spleen volume as independent predictors of CD34+cells with an adjusted R2 of 52%.
An analysis of the more immature CD34+ cells, ie, CD34+CD38− cells, for the correlation with disease features, found that these increased with the increasing number of circulating blasts, but that the blasts correlated better with the whole population of CD34+ cells.
When the number of circulating CD34+ cells was correlated with risk stratification according to the Dupriez prognostic scoring system on the basis of white blood cell count and severity of anemia, the number of CD34+ cells increased significantly from low-risk (n = 29; median, 68.1 × 106/L; range, 2.04-448.9 × 106/L) to intermediate-risk (n = 30; median, 112.8 × 106/L; range, 5.0-1700 × 106/L) and high-risk patients (n = 7; median, 666.1 × 106/L; range, 14.4-2460 × 106/L) (F = 4.95;P = .009).
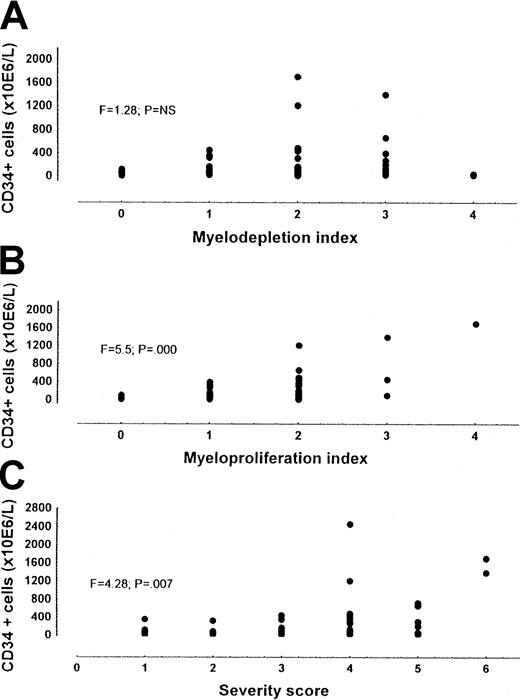
When the number of CD34+ cells were correlated with the severity score on the basis of both myeloproliferative and myelodepletive characteristics of the disease, the number of CD34+ cells proved to be associated with the severity score (F = 4.28; P = .007), but a wide range of CD34+ cell values was evidenced for any severity considered (Figure 2). To account for this variability, we separated the contribution given to the score by the myeloproliferation index (spleen size, leukocytosis, and thrombocytosis) and that given by the myelodepletion index (anemia, thrombocytopenia, and leukopenia). Only the myeloproliferation index was significantly associated with CD34+ cell number (F = 5.7; P = .000) (Figure 2).
CD34+ cells in patients with MMM out of therapy.
Panel A shows the myelodepletion index; panel B, the myeloproliferation index; and Panel C, the severity score. While CD34+ cell number was significantly correlated with the severity score and the myeloproliferation index, CD34 +cell number was not correlated with the myelodepletion index.
CD34+ cells in patients with MMM out of therapy.
Panel A shows the myelodepletion index; panel B, the myeloproliferation index; and Panel C, the severity score. While CD34+ cell number was significantly correlated with the severity score and the myeloproliferation index, CD34 +cell number was not correlated with the myelodepletion index.
CD34+ and response to therapy
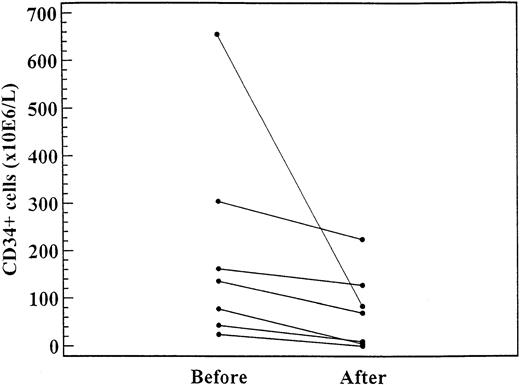
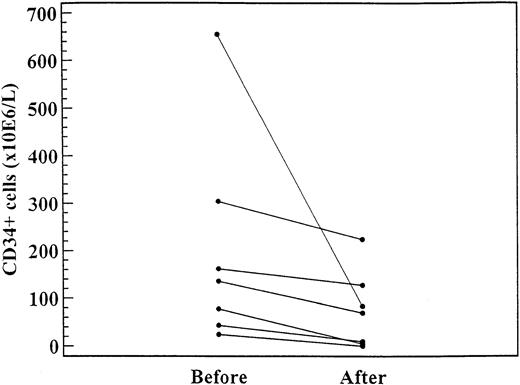
As shown in Figure 3, in 7 patients the CD34+ cell levels were significantly lower after 2 to 4 months of treatment (daily dose range, 1000-1500 mg) with hydroxyurea (median, 135.3 × 106/L; range, 24.4-655.0 × 106/L) than before treatment (median, 223.2 × 106/L; range, 0-223 × 106/L;P = .02).
CD34+ cell levels in 7 patients before and after 4 to 6 months of treatment with hydroxyurea (daily dose range, 1000-1500 mg).
The difference was significant (P = .02).
CD34+ cell levels in 7 patients before and after 4 to 6 months of treatment with hydroxyurea (daily dose range, 1000-1500 mg).
The difference was significant (P = .02).
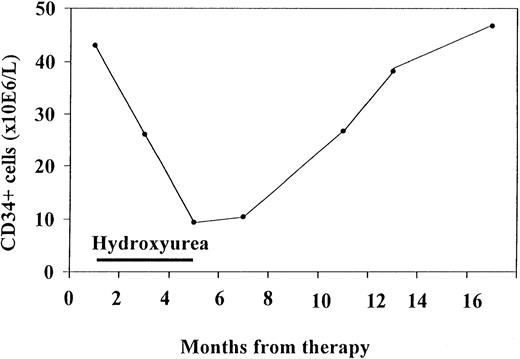
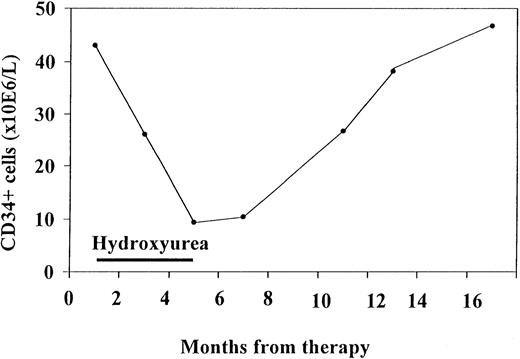
In one patient, consecutive blood samples were analyzed (Figure4). This patient was followed up for 1 year and had mildly increased levels of CD34+ cells at the first examination. He responded to hydroxyurea treatment, with a decrease in spleen volume of more than 30% and a decrease in CD34+ cells down to 16% of the initial value. Both CD34+ cells and spleen volume remained low for 2 months after termination of treatment. When spleen volume began to increase again, CD34+ cells levels also rose. Thus, CD34+ values in this patient tended to fluctuate in accordance with the spleen volume as an effect of therapy.
Consecutive CD34+ cells measurements in one patient who initially responded to hydroxyurea treatment.
CD34+ values tended to fluctuate in accordance with the effect of therapy.
Consecutive CD34+ cells measurements in one patient who initially responded to hydroxyurea treatment.
CD34+ values tended to fluctuate in accordance with the effect of therapy.
Prognostic value of circulating CD34+ cell number in MMM
By the follow-up, of the 84 patients with MMM studied, 13 (15.5%) had died. We compared the survival from the time of CD34+cell measurement according to whether the number of CD34+cells exceeded or fell below their median value (91.6 × 106/L). The overall survival was not significantly different in high CD34+ patients compared with low CD34+ patients. However, since it was possible that the relationship between CD34+ cell number and survival was more complicated, we used Martingale residual plots to examine the specific nature of this relationship. These plots suggested that as the number of CD34+ cells continued to increase to about 300 × 106/L, the difference in survival continued to increase. Hence, we compared survival in patients who had CD34+ levels above (n = 24) and below 300 × 106/L (n = 60). The death rate was 40% in the former group and 5.1% in the latter, and the difference in survival was significant (log-rank test, P = .005).
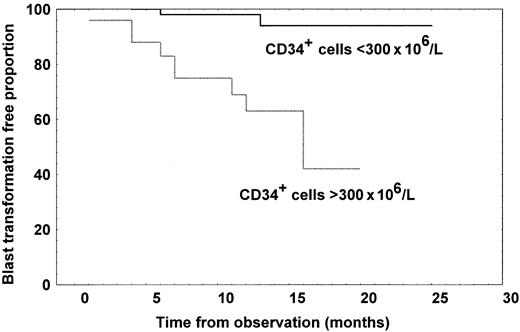
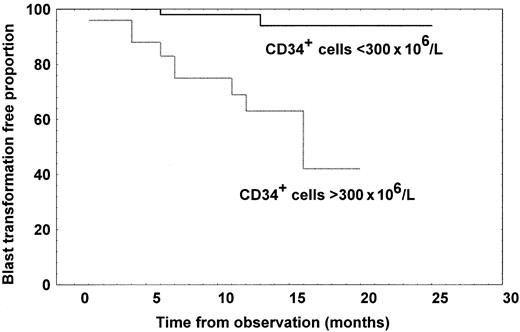
Of the 84 patients with MMM studied, 12 (14.3%) developed blast transformation, which was the cause of death in 8. After excluding one patient who had more than 10% of blast cells in peripheral blood at the time of CD34+ analysis, we compared the time to blast transformation according to whether the number of CD34+cells were greater or fewer than 300 × 106/L CD34+ cells. The blast transformation rate was 40% in the group with a high CD34+ cell count, but 3.4% in the group with a low count, and the difference in time to blast transformation was highly significant, as shown in Figure5 (log-rank test,P = .0005). Patients with high CD34+ cell count had a 50% probability of developing blast transformation at 11 months from the evaluation.
Kaplan-Meier curves representing time to blast transformation for patients with MMM divided according to whether they had at least 300 × 106/L CD34+ cells (n = 24) or fewer than 300 × 106/L (n = 60).
The difference in time to blast transformation was significant (log-rank test, P = .0005).
Kaplan-Meier curves representing time to blast transformation for patients with MMM divided according to whether they had at least 300 × 106/L CD34+ cells (n = 24) or fewer than 300 × 106/L (n = 60).
The difference in time to blast transformation was significant (log-rank test, P = .0005).
Since there is a possible correlation between CD34+ cell number and disease characteristics that may influence both survival and blast transformation, we used multivariate analysis to assess the influence on prognosis of each of the following: the number of CD34+ cells; the CD34+CD38− cell level; hemoglobin level; white blood cell count; circulating blasts, both as a percentage of white blood cell count and as an absolute value; time from diagnosis to examination; having had a splenectomy; and having received prior cytotoxic therapy. Time from diagnosis to CD34+ cell examination and the CD34+ cell number remained significantly associated with survival (P = .026 and P = .013, respectively), while only CD34+ cell number remained associated with time to blast transformation (P = .005).
Discussion
CD34 is a surface antigen present on 1% to 3% of human bone marrow cells and on 0.05% of nucleated circulating cells. It serves as a marker for identifying and separating hematopoietic stem and progenitor cells because it is not found on fully differentiated, or mature, hematopoietic cells.23 The main finding in this study was that the median number of CD34+ cells in peripheral blood in a large, well-defined population of patients with MMM is 360 times higher than in a healthy population and 18 to 30 times higher than in a selected population of patients with other Ph− CMDs.
Since Ph− CMDs consist of a heterogeneous array of disorders without specific criteria for the diagnosis of individual diseases (which remains one of exclusion), with transitional forms and with the possibility of the evolution of one entity into another, we considered how the number of circulating CD34+ cells might be used to differentiate MMM from other Ph− CMDs (ie, PV, ET, and AMD) as identified according to the traditional clinical and hematological criteria. It was evident from the findings of this study, that CD34+ cell number allowed an almost compete discrimination between MMM and other Ph− CMDs when samples were taken from patients out of cytoreductive therapy. In fact, in the latter group of disorders, studied either at diagnosis, when the progression of the disease featured an enlarged spleen and a spent disease, or after splenectomy, the number of CD34+circulating cells were only slightly increased (always fewer than 30 × 106/L). This was in agreement with values reported in the literature, which were constantly in the range of 1 × 106/L to 18.1 × 106/L.10 11 By using ROC analysis, we were able to detect that a threshold of 15 × 106/L CD34+ cells could discriminate MMM patients out of cytoreductive treatment from patients with other Ph− CMDs with a 98.4% positive and an 85% negative predictive value. Since 26% of our samples from MMM patients were obtained at diagnosis, a consistently increased number of circulating CD34+ cells could be considered a valuable indicator of the presence of a myelofibrosis and myeloid metaplasia process.
By staining the CD34+ cells with other markers of differentiation, we found that the percentage of CD34+cells in MMM that are CD38+ is widely variable, ranging from 23% to 99%. CD38 is a transmembrane molecule expressed heterogeneously during hematopoietic cell differentiation. Most human immature hematopoietic cells with high potential for self-renewal and multilineage differentiation express low levels of CD38 or no detectable CD38 at all.24 Thus, CD34+ cells that leave the bone marrow of MMM patients display different levels of differentiation.
We have examined CD34+ measurements and how they correlate with hematological and clinical disease features to determine their usefulness as a clinical marker of disease progression. It was apparent that, on average, the number of circulating CD34+ cells tended to increase during progression of the disease. Moreover, the number of CD34+ cells reflected the number of immature myeloid and erythroid cells released into the peripheral blood, in particular, myeloid blasts. Patients with blasts in the peripheral blood had, on average, 4 times more CD34+ cells than patients without blasts. This agrees with data obtained in MDS,25 in which the existence of circulating blasts of at least 1% was accompanied by a high number of CD34+ cells compared with the number in patients with fewer than 1% blasts. Finally, the increasing CD34+ cell count was a determinant of splenomegaly progression. Together these observations establish that CD34+ cell number is related to the progression of the disease process and is a marker of myeloid metaplasia.
In this study, we also examined whether CD34+ cell levels could be useful as a follow-up parameter. During hydroxyurea treatment, the standard cytoreductive treatment in MMM, CD34+ cell number was significantly lower than before treatment. To obtain more detailed information on the validity of the CD34+ levels during and after treatment, we analyzed a patient over a long period. There was a tendency for CD34+ cell levels to decrease in response to effective treatment. The data suggest that the blood levels of CD34+ may fluctuate in accordance with the tumor burden and that CD34+ is a candidate in the search for markers of disease activity.
The possible prognostic relevance of measuring circulating CD34+ cells in hematologic malignancies has been appreciated in MDS.25,26 In the current study, we show that the extent of increased CD34+ cells in patients with MMM correlates with the Dupriez prognostic score and overall survival. However, the most relevant prognostic correlation we found in this study was between the number of circulating CD34+ cells and the development of blast transformation. Patients presenting more than 300 × 106/L CD34+ cells in peripheral blood have a 50% probability of developing blast transformation by 11 months. This prognostic factor was independent of other known predictors of blast transformation, such as hemoglobin level and the number of morphologically recognizable circulating blasts. This is in line with the results obtained in MDS, in which the presence of circulating CD34+ cells is associated with progression to acute myeloid leukemia and a shorter survival.26
Despite the foregoing, the role of CD34+ in the progression of MMM remains complex. In our whole population of MMM patients, disease progression, circulating myeloid cells, and spleen volume taken together justified only 52% of the variance in the number of CD34+ cells in the peripheral blood. This would suggest either that other, hidden factors intervene in determining the extent of circulating CD34+ or that MMM patients are heterogeneous in terms of the mechanisms of hematopoietic stem cell release from the bone marrow. The study of the relation to the severity of the disease contributed to clarification of this issue. In the absence of validated criteria for disease severity, we correlated the number of circulating hematopoietic cells to a construct of disease severity that included spleen size and hematological parameters. We showed that there was no relationship between CD34+ cells and the severity of disease, when indexed by anemia, thrombocytopenia, or leukopenia, while the correlation was strict when severity was indexed by leukocytosis, thrombocytosis, and splenomegaly. This documents that the release of hematopoietic stem cells from the bone marrow is higher in patients with a myeloproliferative pattern of disease than in patients with a myelodepletive pattern. Moreover, the demonstration that the number of circulating CD34+ cells better reflects the myeloproliferative characteristic of MMM than the myelodepletive pattern could be used to add to histological,27clinical,13 or erythrokinetic28 features in the search for criteria for classifying the disease. Since a correlation between marrow angiogenesis and myeloproliferation has been reported,29 an important contribution could be to assess the influence increased angiogenesis exerts on the release of CD34+ cells from the bone marrow.
In conclusion, we have shown a pronounced elevation of the number of circulating CD34+ cells in MMM in contrast to other Ph− CMDs. The cytometric analysis of circulating CD34+ cells can easily be performed at initial diagnosis and during the clinical course and allows reliable differentiation of MMM from other Ph−CMDs subsets. The prospective monitoring of CD34+ cells could be of clinical importance to disease outcome and, in particular, the early detection of blast transformation of the disease.
Members of the Italian Registry for MMM who contributed with cases to this study (person responsible, department, center city, listed by alphabetical order): Gerli Giancarla, Divisione di Medicina I, Ospedale San Paolo, Milan; Cirasino Lorenzo, Divisione Medica “Vergani,” Ospedale Ca' Granda Niguarda, Milan; Colonna Alberto, Divisione di Medicina Interna, Ospedale “F. Ferrari,” Casarano, Lecce; Comotti Benedetto, UO Medicina Interna e Servizio Ematologia-Oncologia, Policlinico San Pietro, Ponte San Pietro, Bergamo; Custodi Pietro, Medicina Interna, Ospedale San Biagio, Domodossola, Verbania. D'Arco Alfonso, Divisone Medicina Generale, Sezione Ematologia, Cava dei Tirreni, Salerno; Giordano Monica, Servizio di Oncologia Medica, Ospedale Sant'Anna, Como; Minetti Bruno, Divisione di Medicina, Ospedale Civile San Biagio, Clusone, Bergamo; Morandi Sergio, Ematologia, Istituti Ospedalieri, Cremona; Negri Maria, Divisione di Medicina Interna, Ospedale Civile di Voghera, Pavia; Pogliani Enrico, Divisione di Ematologia, Unità TMO, Ospedale San Gerardo, Monza; Scrinzi Luigi, UO Medicina Interna, Ospedale Civile San Bonifacio, Verona; Turpini Chiara, Divisione di Medicina Generale, Fondazione Maugeri, Pavia.
Supported by funds from the Italian Association for Cancer Research (AIRC).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Giovanni Barosi, Laboratorio di Informatica Medica, IRCCS Policlinico San Matteo, Viale Golgi 19, 27100 Pavia, Italy; e-mail: barosig@smatteo.pv.it.