Abstract
Allogeneic immune responses, which are initiated by dendritic cells (DCs) of both donor and host origins, remain a major obstacle in organ transplantation. Presentation of intact major histocompatibility complex (MHC) molecules by allogeneic DCs and allogeneic peptides by syngeneic DCs leads to complex allogeneic immune responses. This study reports a novel strategy designed to suppress both pathways. A stable DC line XS106 (A/J mouse origin) was transfected with CD95L cDNA and fused with splenic DCs purified from allogeneic BALB/c mice. The resulting “killer” DC-DC hybrids: (1) expressed CD95L and MHC class I and class II molecules of both A/J and BALB/c origins, while maintaining otherwise characteristic surface phenotypes of mature DCs; (2) inhibited MHC class I– and class II–restricted mixed leukocyte reactions between the parental strains by triggering apoptosis of alloreactive T cells; and (3) abolished delayed-type hypersensitivity responses of A/J (and BALB/c) mice to BALB/c-associated (and A/J-associated) alloantigens when injected intravenously into A/J (and BALB/c) mice. The onset of graft-versus-host disease in (BALB/c × A/J) F1 hosts receiving A/J-derived hematopoietic cell transplantation was suppressed significantly (P < .001) by killer DC-DC hybrid treatment. These results form both technical and conceptual frameworks for clinical applications of CD95L-transduced killer hybrids created between donor DCs and recipient DCs in the prevention of allogeneic immune responses following organ transplantation.
Introduction
Transplantation of hematopoietic organs (eg, bone marrow) into immunocompetent hosts induces the activation of alloreactive T cells of both recipient and donor origins, thus causing the host → graft reactions expressed clinically as graft rejection and the graft → host reactions manifested as graft-versus-host disease (GVHD), respectively. It has become evident that dendritic cells (DCs) play crucial roles in the initiation of both types of alloresponses.1,2 Host → graft reactions are mediated primarily by host T cells that recognize either intact donor major histocompatibility complex (MHC) molecules expressed on donor DCs (direct presentation) or donor-derived peptide antigens being presented by host DCs (indirect presentation). Likewise, donor T cells that recognize intact host MHC molecules on host DCs or host-derived peptide antigens presented by donor DCs function as main effector leukocytes for GVHD.3,4 Both CD4+ and CD8+ T cells are involved in the above bidirectional allogeneic immune responses, thus providing an additional level of complicity.5 6
Many therapies have been developed to prevent the onset of allogeneic immune responses after organ transplantation. For example, GVHD, a major complication after allogeneic bone marrow transplantation, has been prevented and treated clinically by conventional immunosuppressive agents and, in selected cases, by ex vivo removal of T cells from the donor inoculum before in vivo infusion.7,8 GVHD in experimental animals has been successfully treated by more innovative immunomodulatory strategies that are designed to: (1) trigger clonal anergy of effector T cells by blocking costimulatory molecules9-13; (2) control the expansion and differentiation of effector T cells by administration of recombinant cytokines or cytokine inhibitors7,14-17; or (3) interfere with effector T-cell trafficking by blocking adhesion molecules.18-20 Our ultimate goal is to develop a new strategy that is designed to selectively kill alloreactive T cells.
Recently, we have created “killer” DCs by introducing the CD95L cDNA into a fully mature DC line (XS106) derived from A/J mice.21 The resulting CD95L-transduced killer DC clone, when pulsed with ovalbumin (OVA), induced rapid apoptosis of OVA-reactive T cells and prevented the induction of delayed-type hypersensitivity (DTH) responses to OVA in syngeneic A/J mice. Likewise, contact hypersensitivity responses to dinitrofluorobenzene (DNFB) were suppressed almost completely by administration of DNFB-pulsed killer DCs. On the other hand, when administered into allogeneic BALB/c hosts, killer DCs inhibited only partially the host immune responses to A/J-associated MHC molecules. We have interpreted these results to suggest that killer DCs may deliver apoptotic signals only to the host T cells that recognize allo-MHC molecules via direct presentation. Thus, we have hypothesized that complex alloimmune responses may be suppressed more efficiently if one can create CD95L-transduced killer hybrids by fusing donor DCs and host DCs (Figure 1A).
Generation of killer DC-DC hybrids.
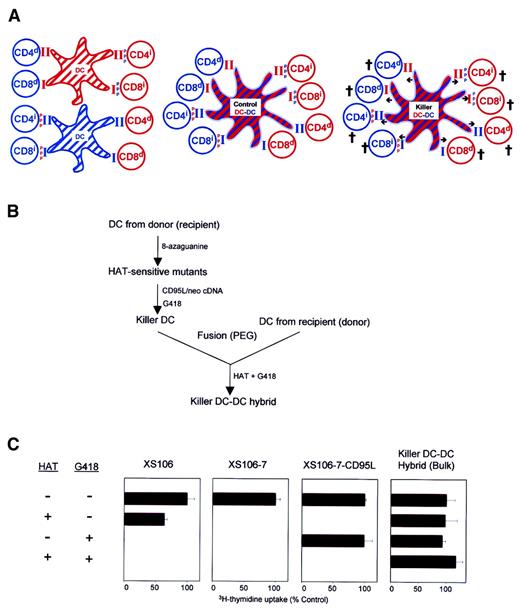
(A) The working model is illustrated in this schema. CD4+and CD8+ T cells from a recipient (blue) or a donor (red) recognize intact MHC molecules presented by allogeneic DCs (ie, direct presentation; indicated by d) or MHC-derived peptide antigens () presented by syngeneic DCs (ie, indirect presentation; indicated by i) (left panel). Control DC-DC hybrids expressing both recipient- and donor-derived MHC molecules should activate all of the above alloreactive T-cell populations (middle panel), whereas CD95L-transduced killer DC-DC hybrids expressing CD95L molecules (indicated by arrows) should deliver apoptotic signals to all T-cell populations (right panel). (B) Schematic flow chart of the methods developed for the generation of killer DC-DC hybrids (see text for details). (C) The indicated cell lines were examined for their proliferative potential in the presence of HAT and/or G418. Data are representative of 2 independent experiments, showing the means ± SD (n = 3) of the relative 3H-thymidine uptake (percentage of control) as compared with control culture in the absence of either HAT or G418.
Generation of killer DC-DC hybrids.
(A) The working model is illustrated in this schema. CD4+and CD8+ T cells from a recipient (blue) or a donor (red) recognize intact MHC molecules presented by allogeneic DCs (ie, direct presentation; indicated by d) or MHC-derived peptide antigens () presented by syngeneic DCs (ie, indirect presentation; indicated by i) (left panel). Control DC-DC hybrids expressing both recipient- and donor-derived MHC molecules should activate all of the above alloreactive T-cell populations (middle panel), whereas CD95L-transduced killer DC-DC hybrids expressing CD95L molecules (indicated by arrows) should deliver apoptotic signals to all T-cell populations (right panel). (B) Schematic flow chart of the methods developed for the generation of killer DC-DC hybrids (see text for details). (C) The indicated cell lines were examined for their proliferative potential in the presence of HAT and/or G418. Data are representative of 2 independent experiments, showing the means ± SD (n = 3) of the relative 3H-thymidine uptake (percentage of control) as compared with control culture in the absence of either HAT or G418.
Materials and methods
Animals and cell lines
All mice (6- to 10-week-old females) were purchased from Jackson Labs (Bar Harbor, ME). Animal experiments were approved by the Institutional Review Board at the University of Texas Southwestern Medical Center and conducted according to the guidelines of the National Institutes of Health. The XS106 line is a stable DC line established from the epidermis of the A/J mouse, and its phenotypic and functional properties are described elsewhere.21,22 The parental XS106 line and its derivatives and hybrids were all maintained and expanded in the standard growth medium: complete RPMI 1640 supplemented with granulocyte-macrophage colony-stimulating factor and NS47 fibroblast culture supernatant.21 22
Generation of killer DC-DC hybrids
Hypoxanthine/aminopterin/thymidine (HAT)–sensitive clones were selected by culturing XS106 cells in the presence of 8-azaguanine (20 μg/mL; Sigma, St Louis, MO), followed by limiting dilution. A HAT-sensitive clone (XS106-7) was transfected with murine CD95L cDNA (pMKIT-C57BL/6/CD95L) or vector alone (pMKITneo)23 using a particle-mediated gene delivery device, and stably transfected clones were selected in the presence of 300 μg/mL G418 (Gibco BRL, Gaithersburg, MD). Subsequently, a CD95L-transduced clone (XS106-7–CD95L) and a vector-transfected clone (XS106-7–neo) were fused with splenic DCs isolated from BALB/c mice24 by using 50% polyethylene glycol 1500 (Roche Molecular Biochemicals, Indianapolis, IN). The cells were cultured in the double-selection medium containing 1× HAT and 300 μg/mL G418 and subjected to limiting dilution 10 days after fusion. All CD95L-transduced DC-DC hybrid clones tested (more than 20 clones) exhibited virtually the same phenotype, and one representative clone (clone 8) was used in this study. To test HAT and neomycin sensitivity, we cultured the cells (1 × 104 cells/well) in the above growth medium in the presence of HAT (0.1×) and/or G418 (300 μg/mL), pulsed them with 1 μCi/well (37 kBq) 3H-thymidine for 16 hours, and harvested them on day 5.
Reverse transcriptase–polymerase chain reaction and fluorescence-activated cell sorter analyses
To examine CD95L mRNA expression, we isolated total RNA from the parental XS106 line and its derivatives and hybrids and subjected it to reverse transcriptase–polymerase chain reaction (RT-PCR) using the primers 5′-GCAGAAGGAACTGGCAGAAC-3′ and 5′-TGAATACTGCCCCCAGGTAG-3′. Primers for β-actin were purchased from Clontech (Palo Alto, CA). PCR products were harvested within the linear range of amplification (30 cycles for CD95L and 25 cycles for β-actin) and analyzed as described previously.21 Surface expression of MHC molecules was examined with haplotype-specific monoclonal antibodies (mAbs) against MHC class I (H-2Kd or H-2Kk) and MHC class II (I-Ad or I-Ak) (BD Pharmingen, San Diego, CA). All other mAbs, including anti-CD95L (Kay-10), were also purchased from BD Pharmingen. Other conditions for fluorescence-activated cell sorter (FACS) analyses are described elsewhere.21 24
3H-thymidine release assay
Apoptosis of the Jurkat target and alloreactive T-cell targets was examined by the standard 3H-thymidine release assay.21 To generate alloreactive T cells, we immunized A/J (or BALB/c) mice by subcutaneous (SC) injection of BALB/c (or A/J) spleen cells (1 × 107 cells/animal), harvested the spleen 7 days later, and restimulated the spleen cells for 48 hours with γ-irradiated (20 Gy) BALB/c (or A/J) spleen cells. Actively proliferating cells were labeled with 3H-thymidine for the last 16 hours and used as targets. In some experiments, anti-CD95L mAb Kay-10 or isotype-matched control immunoglobulin (Ig) G2b (10 μg/mL) was added to the apoptosis assay.
Allo–mixed leukocyte reaction assays
In 2-way mixed leukocyte reaction (MLR), unfractionated spleen cells freshly isolated from A/J mice and BALB/c mice (1 × 105 cells/well) were cultured together for 5 days, and proliferative responses were measured by 3H-thymidine uptake in the last 16 hours. In one-way MLR, CD4+ or CD8+ T cells were purified from A/J (or BALB/c) mice using magnetic beads and cocultured (1 × 105 cells/well) with γ-irradiated (15 Gy) splenic DCs isolated from BALB/c (or A/J) mice (1 × 104 cells/well). CD95L-transduced or control DC-DC hybrids were added to the MLR (3 × 104 cells/well) after γ-irradiation.
Allo-DTH assay
Mice were immunized on the dorsal flank by SC inoculation of spleen cells (1 × 107 cells/animal) isolated from allogeneic mice on day 0 and challenged on day 7 at the right hind footpad by injecting the same antigens (1 × 107cells/animal). Footpad thickness was then measured on days 8 and 9 with a calipers-type engineer's micrometer by a third experimenter masked to the sample identity. The extent of swelling was calculated as the thickness of the right footpad (receiving spleen cells) minus the baseline thickness of the left footpad (receiving phosphate-buffered saline [PBS] alone). CD95L-transduced or control DC-DC hybrids were injected intravenously (IV) (1 × 106cells/injection/animal) on days −6, −4, 0, 3, and 6. In some experiments, these animals were rechallenged with the same spleen cells on days 14 and 60.
Acute GVHD model
Spleen cells and lymph node cells isolated from A/J mice were combined at a ratio of 12.5:1 and cultured (5 × 106cells/mL) for 16 hours with γ-irradiated spleen cells (5 × 105 cells/mL) isolated from (BALB/c × A/J) F1 mice. The resulting ex vivo–activated leukocyte preparations were used as effector cells without further purification. (BALB/c × A/J) F1 recipients received whole-body γ-irradiation of 5 Gy and IV administration of graded numbers of effector cells (from 3 × 106 to 1 × 108 cells/recipient) on day 0. In the treatment panel, killer DC-DC hybrids were added to the above 16-hour ex vivo preactivation cultures (7.5 × 105cells/mL) and injected IV (1 × 106cells/injection/animal) into the recipients on days 0, 3, 5, and 7. The control panel received IV infusion of the effector cells activated in the absence of killer DC-DC hybrids and 4 IV injections of PBS alone. Animals in the treatment and control panels were housed in a randomly mixed manner. A third experimenter recorded survival and body weight every day (11 mice/panel). Three additional animals in each panel were killed on day 7 to examine histologic changes and cytotoxic T-lymphocyte (CTL) activities. For CTL assays, spleen cells individually prepared from 3 mice per panel were cultured for 5 days in complete RPMI in the absence of added cytokine with γ-irradiated (20 Gy) F1 spleen cells. These ex vivo–activated cells were then examined for cytotoxicity against fibroblast lines established from A/J mice (NS46 line) and BALB/c mice (NS47 line) in a standard 16-hour51Cr release assay.25
Statistical analyses
Animal experiments were conducted with 5 to 11 mice per panel, and in vitro experiments were performed with triplicate samples. Experimental results were analyzed for statistical significance by using a 2-tailed Student t test. The survival data were plotted by the Kaplan-Meier method and analyzed for statistical significance using the generalized Wilcoxon test and the log-rank test.
Results
Generation of killer DC-DC hybrids
We created killer DC-DC hybrids by using an A/J mouse–derived, mature DC line XS106.21 22 Considering the MHC haplotype of A/J mice (H-2Kk/H-2Dd/H-2Ld/I-Ak/I-Ek), we chose BALB/c mice (H-2Kd/H-2Dd/H-2Ld/I-Ad/I-Ed) as a fusion partner. When XS106 DCs were fused with splenic DCs purified from BALB/c mice, only small fractions (less than 5%) of the resulting cells expressed both A/J and BALB/c haplotypes, indicating relatively low frequencies for heterotypic DC-DC fusion (data not shown). To overcome this technical problem, we used a double-selection protocol (Figure 1B). Briefly, we first selected a HAT-sensitive XS106 mutant clone (XS106-7), introduced the CD95L and neomycin resistance genes into this DC clone, and established a stably transfected DC clone (XS106-7–CD95L) by limiting dilution. The XS106-7–CD95L DC clone was, indeed, HAT sensitive and G418 resistant, whereas the parental XS106 line was HAT resistant/G418 sensitive and the XS106-7 clone was HAT sensitive/G418 sensitive (Figure 1C). Subsequently, we fused the XS106-7–CD95L clone with splenic DCs freshly purified from BALB/c mice and selected hybrid clones in the presence of HAT and G418. In theory, only heterotypic hybrids should grow in this medium because nonfused XS106-7–CD95L cells and nonfused spleen DCs will be killed by HAT and by G418, respectively. In fact, many HAT-resistant/G418-resistant hybrid lines (in bulk) and clones (after limiting dilution) were readily generated by our protocol (Figure 1C).
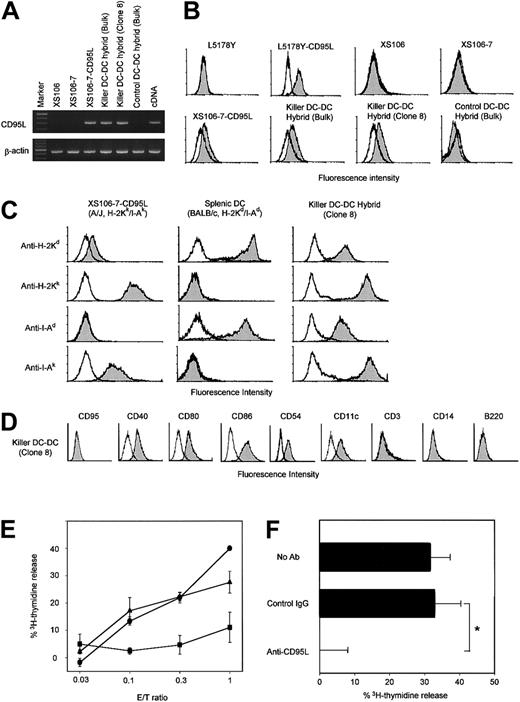
Neither the parental XS106 line nor the HAT-sensitive XS106-7 clone expressed CD95L mRNA at detectable levels (Figure2A). CD95L mRNA expression was detected in killer DCs (XS106-7–CD95L) and killer DC-DC hybrids, but not in control DC-DC hybrids transfected with vector alone. Likewise, surface CD95L expression was detected only on killer DCs and killer DC-DC hybrids (Figure 2B). All tested killer DC-DC hybrid lines and clones expressed both MHC class I and class II molecules derived from A/J mice (H-2Kk/I-Ak) and from BALB/c mice (H-2Kd/I-Ad), indicating high efficiency of our double-selection protocol (Figure 2C). Moreover, killer DC-DC hybrids expressed CD40, CD80, CD86, and CD54 at relatively high levels, thus maintaining the mature DC phenotype observed in the parental XS106 line (Figure 2D). Interestingly, killer DC-DC hybrids expressed CD11c, which was detected on splenic DCs but not on the parental XS106 line. Surface markers for T cells (eg, CD3), monocytes/macrophages (CD14), or B cells (B220) were absent from killer DC-DC hybrids. Control DC-DC hybrids were phenotypically indistinguishable from the killer DC-DC hybrids except for the lack of surface CD95L (data not shown). Consistent with the observation that killer DC-DC hybrids continued to proliferate in culture without undergoing suicidal death (Figure 1C), CD95 (a receptor of CD95L) was not detectable on their surfaces (Figure 2D).
Phenotypic and functional properties of killer DC-DC hybrids.
(A) The indicated cell lines were examined for CD95L and β-actin mRNA expression by RT-PCR. cDNA encoding CD95L or β-actin was amplified to serve as a positive control. The data are representative of 3 independent experiments. (B) The indicated cell lines were stained with anti-CD95L mAb (filled histograms) or isotype-matched control mAb (open histograms). CD95L-transfected and nontransfected L5178Y cell lines were stained in parallel to serve as positive and negative controls, respectively. (C) Two parental DC preparations, the XS106-CD95L clone (A/J origin) and splenic DCs (BALB/c origin), and the resulting killer DC-DC hybrid were stained with the indicated haplotype-specific mAbs (filled histograms) or isotype-matched control IgG (open histograms). Modest cross-reactivity of anti–H-2Kd mAb (SF1-1.1) with the H-2Kk determinant (top left) has been reported previously.26 (D) Killer DC-DC hybrids (clone 8) were stained with mAbs against the indicated surface molecules (filled histogram) or control IgG (open histogram). (E) The killer DC-DC hybrid line (closed triangles) and clone 8 (closed circle), as well as the control DC-DC hybrid line (closed squares), were examined for their capacity to kill Jurkat targets in a standard 18-hour3H-release assay at the indicated E/T ratios. Data are representative of 3 independent experiments, showing the means ± SD of percentage specific lysis from triplicate samples. (F) The killer DC-DC hybrid clone 8 was tested for cytotoxicity against the Jurkat target at the E/T ratio of 1.0 in the presence of neutralizing mAb against CD95L (Kay-10) or control IgG. The data are representative of 2 independent experiments, showing the means ± SD (n = 3). (*P < .01; brackets indicate groups being compared.)
Phenotypic and functional properties of killer DC-DC hybrids.
(A) The indicated cell lines were examined for CD95L and β-actin mRNA expression by RT-PCR. cDNA encoding CD95L or β-actin was amplified to serve as a positive control. The data are representative of 3 independent experiments. (B) The indicated cell lines were stained with anti-CD95L mAb (filled histograms) or isotype-matched control mAb (open histograms). CD95L-transfected and nontransfected L5178Y cell lines were stained in parallel to serve as positive and negative controls, respectively. (C) Two parental DC preparations, the XS106-CD95L clone (A/J origin) and splenic DCs (BALB/c origin), and the resulting killer DC-DC hybrid were stained with the indicated haplotype-specific mAbs (filled histograms) or isotype-matched control IgG (open histograms). Modest cross-reactivity of anti–H-2Kd mAb (SF1-1.1) with the H-2Kk determinant (top left) has been reported previously.26 (D) Killer DC-DC hybrids (clone 8) were stained with mAbs against the indicated surface molecules (filled histogram) or control IgG (open histogram). (E) The killer DC-DC hybrid line (closed triangles) and clone 8 (closed circle), as well as the control DC-DC hybrid line (closed squares), were examined for their capacity to kill Jurkat targets in a standard 18-hour3H-release assay at the indicated E/T ratios. Data are representative of 3 independent experiments, showing the means ± SD of percentage specific lysis from triplicate samples. (F) The killer DC-DC hybrid clone 8 was tested for cytotoxicity against the Jurkat target at the E/T ratio of 1.0 in the presence of neutralizing mAb against CD95L (Kay-10) or control IgG. The data are representative of 2 independent experiments, showing the means ± SD (n = 3). (*P < .01; brackets indicate groups being compared.)
In vitro function of killer DC-DC hybrids
We next tested the potential of killer DC-DC hybrids to kill Jurkat targets that are known to express CD95 constitutively. Killer DC-DC hybrids, but not control DC-DC hybrids, induced apoptosis of this target (Figure 2E), and their cytotoxicity was blocked with neutralizing mAb against CD95L (Figure 2F), validating the functional activity of CD95L molecules on killer DC-DC hybrids. The observed cytotoxicity was modest, requiring relatively high effector-to-target (E/T) ratios of 0.3:1 to achieve 20% to 40% specific lysis in an 18-hour assay. Control DC-DC hybrids expressing MHC class I and class II molecules of both BALB/c and A/J origins induced marked proliferation of A/J-derived spleen cells as well as BALB/c-derived spleen cells (Figure 3A). By contrast, killer DC-DC hybrids failed to activate either A/J or BALB/c spleen cells, and anti-CD95L mAb restored their allostimulatory capacity. Thus, transduced expression of CD95L is fully responsible for the inability of killer DC-DC hybrids to activate alloreactive T cells.
In vitro impact of killer DC-DC hybrids on allogeneic immune responses.
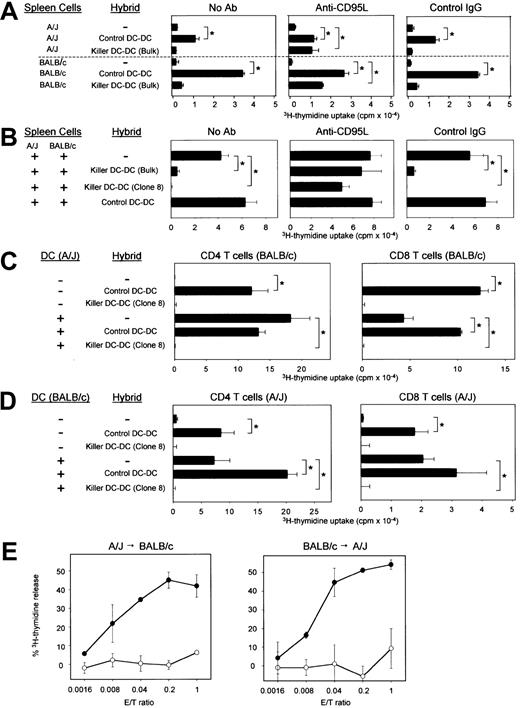
(A) Control or CD95L-transduced DC-DC hybrids were γ-irradiated and cocultured with unfractionated spleen cells isolated from A/J or BALB/c mice in the presence of PBS alone, anti-CD95L mAb (Kay-10), or control IgG. (B) γ-irradiated control or killer DC-DC hybrids were added to the 2-way MLR in which unfractionated spleen cells isolated from A/J and BALB/c mice were cocultured in the absence of γ-irradiation. (C,D) γ-irradiated control or killer DC-DC hybrids were added to the one-way MLR in which CD4+ or CD8+ T cells purified from the indicated strain were cocultured with γ-irradiated splenic DCs isolated from the indicated allogeneic strain. (E) Control DC-DC hybrids (open circles) or killer DC-DC hybrids (closed circles) were examined for their cytotoxicity against A/J T cells reactive to BALB/c-associated alloantigens (left panel) or BALB/c T cells reactive to A/J-associated alloantigens (right panel) in a standard 6-hour3H-release assay at the indicated E/T ratios. All of the data in this figure are representative of at least 2 independent experiments, showing the means ± SD from triplicate samples. (*P < .01; brackets indicate groups being compared.)
In vitro impact of killer DC-DC hybrids on allogeneic immune responses.
(A) Control or CD95L-transduced DC-DC hybrids were γ-irradiated and cocultured with unfractionated spleen cells isolated from A/J or BALB/c mice in the presence of PBS alone, anti-CD95L mAb (Kay-10), or control IgG. (B) γ-irradiated control or killer DC-DC hybrids were added to the 2-way MLR in which unfractionated spleen cells isolated from A/J and BALB/c mice were cocultured in the absence of γ-irradiation. (C,D) γ-irradiated control or killer DC-DC hybrids were added to the one-way MLR in which CD4+ or CD8+ T cells purified from the indicated strain were cocultured with γ-irradiated splenic DCs isolated from the indicated allogeneic strain. (E) Control DC-DC hybrids (open circles) or killer DC-DC hybrids (closed circles) were examined for their cytotoxicity against A/J T cells reactive to BALB/c-associated alloantigens (left panel) or BALB/c T cells reactive to A/J-associated alloantigens (right panel) in a standard 6-hour3H-release assay at the indicated E/T ratios. All of the data in this figure are representative of at least 2 independent experiments, showing the means ± SD from triplicate samples. (*P < .01; brackets indicate groups being compared.)
We next determined whether killer DC-DC hybrids would inhibit the activation of alloreactive T cells in the 2-way MLR. Unfractionated spleen cells (containing T cells and DCs) from A/J mice and from BALB/c mice were cultured together in the absence of γ-irradiation. Robust proliferation observed in this system should represent complex alloresponses that occur in A/J → BALB/c and BALB/c → A/J directions, in MHC class I– and class II–restricted manners, and by direct and indirect presentation mechanisms. Proliferative responses in the 2-way MLR were inhibited almost completely by killer DC-DC hybrids, and their inhibitory potential was abrogated by anti-CD95L mAb (Figure3B). These results suggest that apoptotic signals delivered by killer DC-DC hybrids overrule the allostimulatory signals delivered by conventional DCs. CD4+ and CD8+ T cells purified from either BALB/c or A/J mice were both activated efficiently by control DC-DC hybrids, whereas killer DC-DC hybrids failed to activate any T-cell population (upper panels in Figure 3C,D). Splenic DCs purified from A/J or BALB/c mice induced marked proliferation of both CD4+ and CD8+ T cells isolated from allogeneic strains. When added to these one-way MLR systems, killer DC-DC hybrids inhibited T-cell activation almost completely in all tested combinations, illustrating their unique ability to suppress MHC class I– and class II–dependent bidirectional alloimmune responses (lower panels in Figure 3C,D).
Killer DC-DC hybrids killed BALB/c-reactive T-cell targets (derived from A/J mice) with extremely high efficiency, with 30% to 45% lysis achieved at E/T ratios of 0.04:1 to 0.2:1 in 6 hours (Figure 3E, left panel). A/J-reactive T-cell targets (derived from BALB/c mice) were lysed even more efficiently (Figure 3E, right panel). By contrast, control DC-DC hybrids failed to kill either target even at higher E/T ratios. These results document the potent ability of killer DC-DC hybrids to trigger apoptosis of alloreactive T cells.
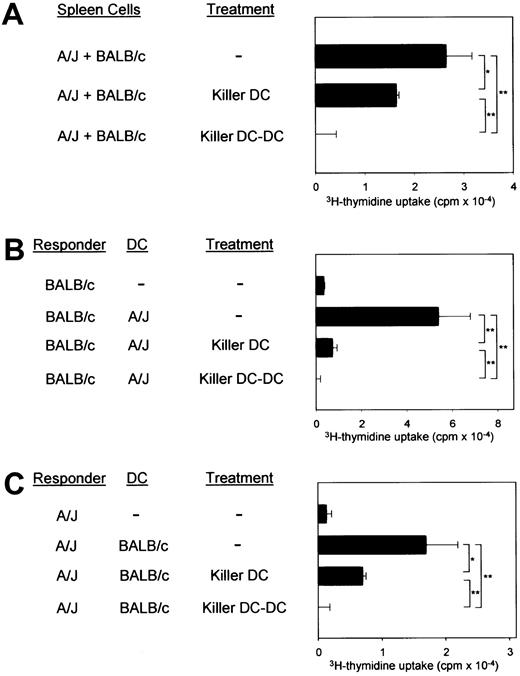
The CD95L-transduced XS106 cells expressing A/J-derived MHC molecules (killer DCs) suppressed the 2-way MLR between the A/J and BALB/c spleen cells only modestly (Figure 4A). In one-way MLR, killer DCs inhibited almost completely (more than 95%) BALB/c T-cell responses to A/J DCs (Figure 4B) and only partially inhibited (50% to 60%) A/J T-cell responses to BALB/c DCs (Figure4C). In all the MLR experiments, killer DC-DC hybrids were significantly more potent than killer DCs, producing complete inhibition in 2 opposing directions, signifying the advantage of the new technology.
Comparison between killer DCs and killer DC-DC hybrids.
(A) The killer DC clone (XS106-7–CD95L) and the killer DC-DC hybrid clone (clone 8) were γ-irradiated and then added to the 2-way MLR in which unfractionated spleen cells isolated from A/J and BALB/c mice were cocultured in the absence of γ-irradiation. (B,C) The same clones were added to the one-way MLR in which responder T cells isolated from the indicated strain were cocultured with splenic DCs purified from the indicated allogeneic strain. All data in this figure are representative of 2 independent experiments, showing the means ± SD (n = 3) of 3H-thymidine uptake. (*P < .05, **P < .01; brackets indicate groups being compared.)
Comparison between killer DCs and killer DC-DC hybrids.
(A) The killer DC clone (XS106-7–CD95L) and the killer DC-DC hybrid clone (clone 8) were γ-irradiated and then added to the 2-way MLR in which unfractionated spleen cells isolated from A/J and BALB/c mice were cocultured in the absence of γ-irradiation. (B,C) The same clones were added to the one-way MLR in which responder T cells isolated from the indicated strain were cocultured with splenic DCs purified from the indicated allogeneic strain. All data in this figure are representative of 2 independent experiments, showing the means ± SD (n = 3) of 3H-thymidine uptake. (*P < .05, **P < .01; brackets indicate groups being compared.)
In vivo impact of killer DC-DC hybrids
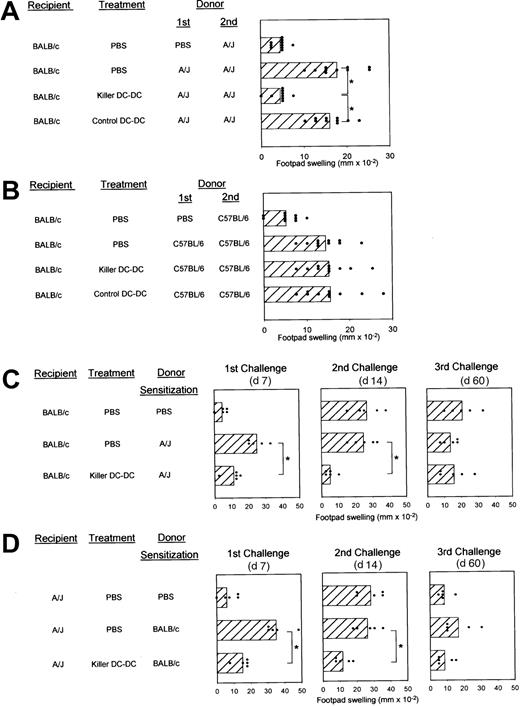
Allospecific DTH responses provide a relatively handy, fully quantitative, and reproducible assay system to study allospecific immune responses.27 28 BALB/c mice immunized with A/J spleen cells showed marked DTH responses upon challenge (Figure5A). This BALB/c → A/J response was inhibited almost completely by repeated injections of killer DC-DC hybrids before and after sensitization. In all 4 independent experiments, killer DC-DC hybrids induced 70% to 100% inhibition, whereas control DC-DC hybrids injected in the same protocol never caused significant inhibition. To test allospecificity, we immunized BALB/c mice with spleen cells isolated from C57BL/6 mice (H-2Kb/H-2Db/H-2Lb/I-Ab/I-Eb). As shown in Figure 5B, killer DC-DC hybrids (A/J × BALB/c) failed to affect allo-DTH responses to C57BL/6 spleen cells, formally excluding the possibility that suppressive effects induced by killer DC-DC hybrids might simply reflect nonspecific cytotoxicity of in vivo–administered CD95L molecules.
In vivo impact of killer DC-DC hybrids on allo-DTH responses.
(A,B) BALB/c mice (10 mice/panel/experiment) were immunized by SC injection of spleen cells derived from A/J (A) or C57BL/6 mice (B) on day 0 and challenged with the same antigens in the right hind footpad on day 7. These animals received IV injections of PBS alone, killer DC-DC hybrids (clone 8), or control DC-DC hybrids on days −6, −4, 0, 3, and 6. Data are representative of 2 independent experiments, showing the individual data points and the means ± SD of footpad swelling on day 8. (*P < .01; brackets indicate groups being compared.) (C,D) BALB/c mice (C) or A/J mice (D) (5 mice/panel/experiment) were initially immunized on day 0 by SC injection of spleen cells isolated from the indicated allogeneic strain or PBS alone. All animals were subsequently challenged with allogeneic spleen cells on days 7, 14, and 60. These animals were treated with IV injections of PBS alone or killer DC-DC hybrids (clone 8) on days −6, −4, 0, 3, and 6. Data are representative of 2 independent experiments, showing the individual data points and the means ± SD of footpad swelling at 24 hours after each challenge. (*P < .01; brackets indicate groups being compared.)
In vivo impact of killer DC-DC hybrids on allo-DTH responses.
(A,B) BALB/c mice (10 mice/panel/experiment) were immunized by SC injection of spleen cells derived from A/J (A) or C57BL/6 mice (B) on day 0 and challenged with the same antigens in the right hind footpad on day 7. These animals received IV injections of PBS alone, killer DC-DC hybrids (clone 8), or control DC-DC hybrids on days −6, −4, 0, 3, and 6. Data are representative of 2 independent experiments, showing the individual data points and the means ± SD of footpad swelling on day 8. (*P < .01; brackets indicate groups being compared.) (C,D) BALB/c mice (C) or A/J mice (D) (5 mice/panel/experiment) were initially immunized on day 0 by SC injection of spleen cells isolated from the indicated allogeneic strain or PBS alone. All animals were subsequently challenged with allogeneic spleen cells on days 7, 14, and 60. These animals were treated with IV injections of PBS alone or killer DC-DC hybrids (clone 8) on days −6, −4, 0, 3, and 6. Data are representative of 2 independent experiments, showing the individual data points and the means ± SD of footpad swelling at 24 hours after each challenge. (*P < .01; brackets indicate groups being compared.)
We next examined the time course and bidirectionality. Killer DC-DC hybrids suppressed DTH responses of BALB/c mice to A/J spleen cells after initial challenge on day 7 (left panels in Figure 5C) and even after the second challenge on day 14 (middle panels in Figure 5C). Significant inhibition became undetectable after the third challenge on day 60 (right panels in Figure 5C). The footpad-swelling responses detected in the PBS-sensitized control group at the second and third challenges (top panel in Figure 5C) most likely reflect successful sensitization by the injected A/J spleen cells at the first challenge. As observed in MLR experiments, killer DC-DC hybrids also suppressed allo-DTH responses of A/J mice to BALB/c spleen cells, documenting their unique property to inhibit bidirectional alloresponses (left panels in Figure 5D). Unresponsiveness in this direction (A/J → BALB/c) was again maintained until the second challenge (middle and right panels in Figure 5D).
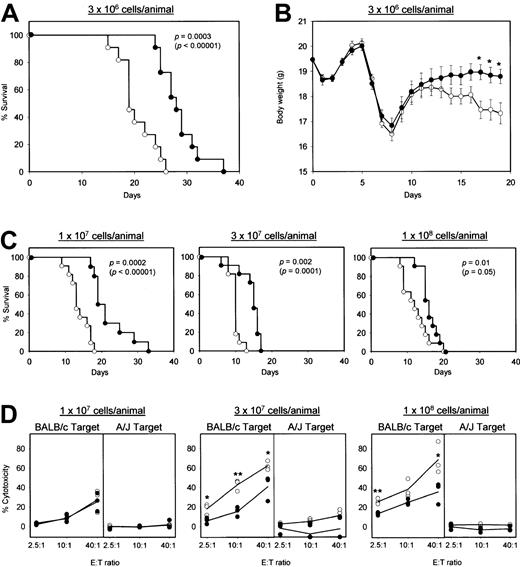
As an initial step toward clinical application, we assessed the preclinical efficacy of killer DC-DC using a standard GVHD model. In this model, spleen cells and lymph node cells isolated from A/J mice were infused into sublethally irradiated (BALB/c × A/J) F1 mice, leading to the activation of donor T cells (H-2a) that recognized MHC molecules of the H-2d haplotype on the F1 hosts (H-2d/a).13 To optimize the frequency of H-2d–reactive T cells, we first cultured the donor cell preparations for 16 hours with γ-irradiated F1 spleen cells. Infusion of these ex vivo–activated hematopoietic cells produced progressive body-weight loss and histologic changes (prominent lymphocyte infiltration and tissue destruction) in the small intestine, skin, and liver. None of these features were observed after γ-irradiation alone or γ-irradiation plus hematopoietic cell transplantation from syngeneic F1 mice (data not shown). The F1 hosts receiving 3 × 106 A/J-derived hematopoietic cells per animal all died within 26 days in the control panel, whereas their survival was prolonged significantly by killer DC-DC hybrid treatment (Figure6A). Moreover, the same treatment reduced the extent of body-weight loss significantly (Figure 6B). Notably, killer DC-DC hybrid treatment remained effective even when the hosts received 3-fold, 10-fold, or 30-fold excess numbers (1-10 × 107 cells/host) of effector cells above the threshold number (3 × 106 cells/host) required for inducing GVHD (Figure 6C). The F1 hosts receiving relatively high loads of A/J-derived hematopoietic cells exhibited marked CTL activities against the H-2d targets, but not the H-2atargets, and killer DC-DC hybrid treatment suppressed significantly the generation of such allospecific CTL activities (Figure 6D). It should be noted, however, that the impact of killer DC-DC hybrids was partial, consistent with their impact on the survival of the F1 host animals.
Efficacy of killer DC-DC treatment in acute GVHD model.
(A-C) Spleen cells and lymph node cells isolated from A/J mice were ex vivo activated overnight with γ-irradiated spleen cells isolated from (BALB/c × A/J) F1 mice in the presence of killer DC-DC hybrids (closed circles) or PBS alone (open circles). The resulting effector cells were infused IV at the indicated cell numbers into γ-irradiated (BALB/c × A/J) F1 host animals (11 animals/panel). The hosts received IV injections of killer DC-DC hybrids (closed circles) or PBS alone (open circles) on days 0, 3, 5, and 7. The data are representative of 2 independent experiments, showing the survival curves plotted by the Kaplan-Meier method, with P values calculated based on the generalized Wilcoxon test and the log-rank test (in parentheses) (A,C), and the means ± SEM of body weight in live animals (B). (*P < .05 between the killer DC-DC hybrid panel and the PBS control panel.) (D) Three additional animals in each panel receiving killer DC-DC hybrids (closed circles) or PBS alone (open circles) were killed on day 7 and examined for allospecific cytotoxicity in a standard 16-hour 51Cr release assay. Allospecificity was examined by comparing cytotoxicity against BALB/c-derived NS47 targets versus A/J-derived NS46 targets. The data are representative of 2 independent experiments, showing the cytotoxicity observed for individual animals in each panel (the means from triplicate samples) and the mean values from the 3 animals per panel. (*P < .05 and **P < .01 between the killer DC-DC hybrid panel and the PBS control panel.)
Efficacy of killer DC-DC treatment in acute GVHD model.
(A-C) Spleen cells and lymph node cells isolated from A/J mice were ex vivo activated overnight with γ-irradiated spleen cells isolated from (BALB/c × A/J) F1 mice in the presence of killer DC-DC hybrids (closed circles) or PBS alone (open circles). The resulting effector cells were infused IV at the indicated cell numbers into γ-irradiated (BALB/c × A/J) F1 host animals (11 animals/panel). The hosts received IV injections of killer DC-DC hybrids (closed circles) or PBS alone (open circles) on days 0, 3, 5, and 7. The data are representative of 2 independent experiments, showing the survival curves plotted by the Kaplan-Meier method, with P values calculated based on the generalized Wilcoxon test and the log-rank test (in parentheses) (A,C), and the means ± SEM of body weight in live animals (B). (*P < .05 between the killer DC-DC hybrid panel and the PBS control panel.) (D) Three additional animals in each panel receiving killer DC-DC hybrids (closed circles) or PBS alone (open circles) were killed on day 7 and examined for allospecific cytotoxicity in a standard 16-hour 51Cr release assay. Allospecificity was examined by comparing cytotoxicity against BALB/c-derived NS47 targets versus A/J-derived NS46 targets. The data are representative of 2 independent experiments, showing the cytotoxicity observed for individual animals in each panel (the means from triplicate samples) and the mean values from the 3 animals per panel. (*P < .05 and **P < .01 between the killer DC-DC hybrid panel and the PBS control panel.)
Discussion
The present study introduces an entirely new immunosuppressive strategy that is designed to selectively eliminate alloreactive effector T cells. Killer DC-DC hybrids expressed functionally active CD95L and MHC class I and class II molecules of both parental strains, induced rapid apoptosis of alloreactive T cells, and inhibited bidirectional activation of alloreactive CD4+ and CD8+ T cells between the parental strains. Upon in vivo administration, killer DC-DC hybrids inhibited allo-DTH responses, delayed the onset of acute GVHD, and suppressed significantly the generation of allospecific CTL activities.
Is the killer DC-DC hybrid technology potentially applicable to the prevention of acute GVHD in human patients? Short-term DC lines can be readily generated by culturing CD34+ progenitors or CD14+ monocytes from human peripheral blood in the presence of selected cytokines,29,30 and transfection efficiency for DCs has been improved dramatically by the use of viral vectors.31 Thus, we believe it is technically feasible to generate killer hybrids between the donor DCs and recipient DCs. In the present study, donor hematopoietic cells were activated ex vivo with recipient antigen-presenting cells in the presence of killer DC-DC hybrids. Guinan et al12 recently used a similar protocol to prevent acute GVHD in leukemia patients receiving bone marrow transplantation from MHC-mismatched donors. Before infusion, the donor bone marrow cells were cultured with γ-irradiated recipient's mononuclear cells in the presence of CTLA4-Ig, an agent known to trigger T-cell anergy by blocking the CD80/CD86-dependent costimulatory pathway. Although this ex vivo treatment regimen failed to completely prevent the onset of acute GVHD even in combination with conventional immunosuppressive and conditioning therapies, it significantly reduced the frequency and disease severity. Our strategy differs from this CTLA-Ig strategy most notably in the terminal fate of alloreactive effector T cells, that is, apoptosis versus anergy, respectively. Nevertheless, our results together with the above clinical observations provide conceptual and technical frameworks for clinical applications of the killer DC- DC technology.
With regard to safety, we considered liver toxicity as a potential risk because massive apoptosis of hepatocytes has been induced experimentally by systemic administration of agonistic anti-CD95 mAb.32 None of the more than 300 animals that had been treated with killer DC-DC hybrids, except for those in the GVHD experiments, died during the experimental periods. Consistent with our previous observations after killer DC treatments,21 we detected no significant changes in the liver histology or serum levels of aspartate aminotransferase or alanine aminotransferase after administrations of killer DC-DC hybrids (data not shown). Thus, the risk of acute liver cytotoxicity appears to be limited, perhaps reflecting the relatively low CD95L expression detected on killer DC-DC hybrids. In this regard, killer DC-DC hybrids killed alloreactive T-cell targets much more efficiently than the Jurkat target, and they failed to suppress allo-DTH responses to irrelevant MHC antigens (ie, C57BL/6 spleen cells). We interpret these observations to suggest that killer DC-DC hybrids require signal 1 (ligation of the MHC-peptide complex with relevant T-cell receptor complex on the targets) and perhaps signal 2 (coupling of the costimulatory molecules with corresponding ligands on the targets) to exert their maximal cytotoxicity. If so, the resulting target specificity may serve as a safety mechanism to minimize uncontrolled tissue damage caused simply as a consequence of exogenous CD95L administration.
We previously reported the potential of CD95L-transduced XS106 cells (ie, killer DCs) to prevent DTH responses to foreign protein antigens and contact hypersensitivity responses to reactive haptens in an antigen-specific manner.21 More recently, Min et al33 extended these observations by introducing the CD95L gene into bone marrow–derived murine DCs of BALB/c origin and testing the impact on allogeneic graft rejection. These investigators were able to induce hyporesponsiveness to BALB/c-associated alloantigens and prolong the survival of BALB/c-derived cardiac grafts in C57BL/6 mice by injecting CD95L-transfected DCs repeatedly. Zhang et al34 35 also reported that T-cell–mediated immune responses to foreign antigens as well as alloantigens (assessed by MLR) were suppressed by using CD95L-transfected macrophages. On the other hand, our attempt to prolong the survival of A/J-derived skin grafts by repeated injections of killer DC-DC hybrids into BALB/c recipients has not been successful thus far. Considering that the same protocol abrogated the bidirectional allo-DTH responses almost completely, one may argue that threshold numbers of alloreactive T cells required for skin graft rejection are much smaller than those required for DTH responses. Alternatively, these observations may imply the inability of killer DC-DC hybrids to eliminate effector T cells that recognize unique, tissue-specific minor antigens.
CD95L is thought to contribute to immune privilege in the eye, testis, and some cancers by inducing CD95-mediated apoptosis of infiltrating cells.36,37 Genetically engineered CD95L expression in the allografts has been shown to induce graft immunoprotection in some reports.38-40 On the other hand, CD95L expression on graft tissues was frequently found to induce inflammation (mostly neutrophil infiltration) and allograft rejection, perhaps reflecting the chemotactic potential of soluble CD95L and/or caspase-mediated processing and secretion of interleukin (IL)-1β and IL-18.41-44 Our strategy is unique in that CD95L was transduced in DC-DC hybrids to trigger selective apoptosis of alloreactive T cells.
As described in “Introduction” and illustrated in Figure 1A, transplantation immunology is highly complex, being mediated by diverse T-cell subsets that recognize donor- or host-derived alloantigen, in an MHC class I– or class II–restricted manner, and by a direct or indirect presentation mechanism. Vector-transfected control DC-DC hybrids expressed MHC class I and class II molecules of both parental strains and induced robust activation of alloreactive CD4+and CD8+ T cells isolated from either strain. Although our primary objective was to study the outcome of transduced expression of CD95L, the DC-DC hybrid technology per se may be applicable to the development of other strategies to prevent allogeneic immune responses. Conventional DCs have been converted into tolerogenic antigen-presenting cells by exposure to ultraviolet B irradiation,45 pretreatment with IL-10,46 or transfection with viral IL-10, transforming growth factor (TGF)-β1, or CTLA4-Ig cDNA.47-50 Moreover, TGF-β1–transduced DCs generated from B10 bone marrow delayed the rejection of B10-derived cardiac grafts in C3H recipients,51 and CTLA4-Ig–transduced DC line D2SC/1 (established originally from BALB/c mice) prolonged the survival of BALB/c-derived islet transplants in C57BL/6 recipients.50 Thus, it is tempting to speculate that the hybrids created between donor DCs and host DCs may serve as a more appropriate “vector” to deliver immunosuppressive signals (eg, TGF-β1 or CTLA4-Ig) to diverse effector T-cell subsets that are involved in allogeneic immune responses.
We thank Dr Michael Bennett for his intellectual input to the GVHD experiments, Julie Loftus and Dale Edelbaum for their technical assistance, and Pat Adcock for her secretarial assistance.
Supported by National Institutes of Health grants (RO1-AI46755, RO1- AR35068, RO1-AR43777, RO1-AI43232) and by Centre de Recherches et d' Investigations Épidermiques et Sensorielles (CE.R.IES.) Award (A.T.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hiroyuki Matsue, Department of Dermatology, University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd, Dallas, TX 75390-6069; e-mail: hiroyuki.matsue@utsouthwestern.edu.