Abstract
Treatment of leukemia by myeloablative conditioning and transplantation of major histocompatibility complex (MHC)–mismatched stem cells is generally avoided because of the high risk of graft rejection or lethal graft-versus-host disease (GVHD). This study shows that MHC-incompatible cells can engraft stably after nonmyeloablative conditioning with immunosuppressive chemotherapy and low-dose total body irradiation (TBI). Long-term mixed hematopoietic chimerism, clonal deletion of donor-reactive T cells, and bidirectional cytotoxic T-cell tolerance were achieved by transplanting MHC-mismatched marrow cells into recipients conditioned with pretransplantation fludarabine or cyclophosphamide (Cy), 50 to 200 cGy TBI on day −1, and Cy 200 mg/kg intraperitoneally on day 3. In this model, long-term donor chimerism was proportional to the dose of TBI or donor marrow cells. Pretransplantation fludarabine and posttransplantation Cy were both required for alloengraftment, but the drugs had additional effects. For example, fludarabine sensitized host stem cells to the toxicity of TBI, because animals conditioned with both agents had higher chimerism than animals conditioned with TBI alone (P < .05). Also, posttransplantation Cy attenuated lethal and nonlethal GVH reactions, because F1 recipients of host-reactive, parental spleen cells survived longer (P < .05) and had lower donor cell chimerism (P < .01) if they received posttransplantation Cy than if they did not. Finally, delayed infusions of donor lymphocytes into mixed chimeras prolonged survival after leukemia challenge (P < .0001) without causing lethal GVHD. These results indicate that stable engraftment of MHC-incompatible cells can be induced after fludarabine-based, nonmyeloablative conditioning and that it serves as a platform for adoptive immunotherapy with donor lymphocyte infusions.
Introduction
Many patients with disorders of hematopoiesis can be cured by myeloablative conditioning followed by HLA-identical, allogeneic blood or marrow transplantation (alloBMT).1Because of the existence of an expanding worldwide registry of HLA-typed volunteer donors, an HLA-identical donor can be identified for the majority of patients. For the 40% of patients for whom an HLA-identical donor cannot be found, one alternative is to receive a partially matched, or haploidentical, graft from a first-degree relative.2 However, partially HLA-mismatched marrow transplantations can be associated with severe toxicities, especially graft rejection and graft-versus-host disease (GVHD).3,4 T-cell depletion of the donor graft, administration of a high dose of stem cells,5 or ex vivo induction of tolerance in host-reactive donor T cells6 can reduce the risk of GVHD after haploidentical alloBMT. However, none of these strategies has diminished the need to intensively condition the recipient with cytotoxic and immunosuppressive agents to prevent graft rejection. Intensive treatment with cytotoxic agents, such as cyclophosphamide (Cy) or total body irradiation (TBI), reduces the risk of graft rejection but increases the risk of serious toxicities, including GVHD.7 8 In addition, graft rejection after myeloablative conditioning is inevitably fatal unless the patient can be rescued with reconditioning and a second stem cell graft. The outcome of haploidentical alloBMT may therefore be improved by the development of nonmyeloablative conditioning regimens that permit the induction of stable mixed hematopoietic chimerism. The use of nonmyeloablative conditioning would carry the additional safeguard of recovery of host hematopoiesis in the event of graft rejection.
In animal models of major histocompatibility complex (MHC)–mismatched alloBMT, immunosuppressive agents reduce but do not eliminate the requirement for myelosuppressive therapy to induce mixed chimerism using standard doses of donor marrow cells. Thus, mixed chimerism in MHC-disparate pairs of rodents has been achieved by combining dimethylmyleran, or low-dose TBI, with monoclonal antibodies to CD4 and CD8,9,10 CD3 and CD4,11 pretransplantation antilymphocyte serum and posttransplantation Cy,12 or combined blockade of B7-CD28 and CD40-CD154 interactions.13 Addition of these immunosuppressive agents lowers the dose of TBI required to obtain engraftment of MHC-incompatible cells from 700 cGy to approximately 300 cGy.12 In clinical trials in humans, engraftment of HLA-haploidentical cells has been achieved after conditioning with pretransplantation Cy and thymic irradiation, and peritransplantation antithymocyte globulin (ATG).14 Although ATG is effective at reducing the risks of graft rejection and GVHD, it kills T cells nonselectively, resulting in global immunodeficiency. Moreover, the mean elimination half-life of ATG in humans ranges from 5.7 days (equine source) to as long as 29.8 days (rabbit source),15and persisting activity of ATG may predispose the patient to opportunistic infections from viruses16 and fungi,17 even to virus-induced malignancy.18Thus, a nonmyeloablative conditioning regimen that eschews the use of T-cell–specific antibodies and produces a brief period of immunosuppression, sufficient for engraftment, is desirable.
Fludarabine is an immunosuppressive purine analogue that has been used mostly in the treatment of indolent lymphoid malignancies.19 Repeated cycles of fludarabine therapy induce a profound T-cell depletion, particularly of CD4+ T cells.20 Unlike other immunosuppressive cytotoxic drugs, such as Cy, fludarabine induces lymphocyte apoptosis in both dividing cells as well as cells in the Go-G1 phase of the cell cycle. This cell cycle independent activity may be attributed to the drug's inhibition of STAT1 signaling.21 Because of its potent immunosuppressive properties, fludarabine has been incorporated into nonmyeloablative conditioning for HLA-identical alloBMT.22-25 We were therefore interested to determine whether fludarabine-based nonmyeloablative conditioning could be used to achieve sustained engraftment of MHC-incompatible cells.
Materials and methods
Mice
AKR/NCr (H-2k), C57BL/6NCr (B6; H-2b), B6.SJL (H-2b), C57BL/10ScNCr (B10; H-2b), BALB/cAnNCr (H-2d), DBA/2NCr (H-2d), BALB/c × B6 (CB6) F1 (H-2b/d), B6 × C3H (B6C3) F1 (H-2b/k), and B6 × DBA/2 (B6D2) F1 (H-2b/d) mice were all obtained from the National Cancer Institute (Frederick, MD). B10.BR (B10.BR-H2k-H2-T18a/SgSnJ) mice were obtained from Jackson Laboratories (Bar Harbor, ME). Mice were maintained in microisolator cages and were fed ad libitum with autoclaved laboratory chow and acidified water. All mice were approximately 6 to 12 weeks of age at the time of transplantation.
Cell preparations
Donor spleens were removed aseptically and pressed through a nylon mesh to obtain single-cell suspensions. Bone marrow cell suspensions were prepared by flushing sterile media through the femoral and tibial marrow canals. In some experiments, mature T cells were depleted from the marrow cell suspension by using monoclonal antibodies against CD4, CD8, and Thy1.2, followed by treatment with guinea pig complement, as previously described.26 Cell suspensions were vigorously pipetted, counted, and washed into sterile phosphate-buffered saline (PBS) before injection.
Hematopoietic cell transplantation
The “Flu-XRT-Cy” regimen consists of fludarabine (Berlex Laboratories, Montville, NJ) 100 mg/kg/day (300 mg/m2/day) intraperitoneally (IP) on days −6 to −2, TBI (200 cGy) on day −1, and Cy (Bristol-Myers, Evansville, IN) 200 mg/kg IP on day +3. The Cy-XRT-Cy regimen substitutes Cy, 200 mg/kg IP on day −3, for fludarabine. Animals were irradiated by a dual source 137Cs irradiator (Gammacell 40; Atomic Energy of Canada, Ottawa, Ontario) at an exposure rate of approximately 82 cGy/minute. Donor marrow cells were injected on day 0 in a final volume of 0.5 mL PBS.
Analysis of leukocyte chimerism
At designated times after transplantation, blood was obtained from the lateral tail vein, or animals were killed and suspensions of spleen and/or bone marrow were prepared. Erythrocytes from the peripheral blood were lysed by using ammonium chloride buffer before initiation of staining. For determination of lineage-specific chimerism in B10.BR→B10 or BALB/c→B10 chimeras, 1 million cells were stained with fluorescein (FITC)–conjugated antibody to H-2Kk or H-2Dd, respectively, and phycoerythrin (PE)–conjugated antibodies to CD4, CD8, or B220 (all from BD Pharmingen, San Diego, CA). For determination of lineage-specific chimerism in CD45.1-congenic pairs of donors and recipients, peripheral blood cells were stained with FITC-conjugated antibody against CD45.1 and either PE-conjugated antibodies against CD4 and CD8 (T-cell markers) or biotinylated antibody against CD11b (Mac1, a myeloid marker), followed by PE-avidin. In each experiment, samples of peripheral blood from at least 3 host strain mice not receiving transplants were stained for donor H-2 antigens. The mean + (3 × SEM) of the percentage of FITC+ cells in hosts not receiving transplants was calculated (and was < 0.5% for every experiment), and any transplant recipient containing a greater percentage of FITC+ cells than this value was considered to have donor cell engraftment.
Expression of selected Vβ gene products among host (H-2b) T cells of the B10.BR→B10 chimeras was determined by staining peripheral blood lymphocytes with cychrome-conjugated antibodies to CD4 or CD8, FITC-conjugated antibody against H-2Kb, and biotinylated antibodies to Vβ5, Vβ8, or Vβ11 (all from BD Pharmingen), followed by PE-conjugated avidin (Jackson Immunochemicals, West Grove, PA). After staining, a minimum of 1000 cychrome-positive (CD4+ or CD8+) lymphocytes per sample were acquired and analyzed by flow cytometry using a FACScan (Becton Dickinson).
Mixed lymphocyte cultures and immunologic cytotoxicity assays
Four million responder splenocytes and 2 million irradiated (3000 cGy) stimulator spleen cells were added to individual wells of a 24-well plate, each well containing 2 mL EHAA medium (Biofluids, Rockville, MD), 10% fetal calf serum (Gibco BRL, Gaithersburg, MD), 5 × 10−5 2-mercaptoethanol, glutamine, and antibiotics (complete medium; CM). After 5 days of culture in a 37°C, 5% CO2 incubator, responder cells were harvested, washed, and incubated in 96-well U-bottom plates, each well containing 0.2 mL CM and 104 labeled target cells (spleen cells cultured for 48 hours in CM containing 2 μg/mL Concanavalin A and pulsed for the last 16 hours with 3H-thymidine). Responder-to-target ratios indicated in the figure are based on the initial number of responder cells plated in mixed lymphocyte culture. Four to 6 hours later after coincubating responders with targets, cells were harvested onto glass fiber filters, and the incorporated radioactivity was counted on a β-scintillation counter.
Results
Induction of durable mixed hematopoietic chimerism across a full MHC barrier using pretransplantation fludarabine or Cy combined with pretransplantation TBI and posttransplantation Cy
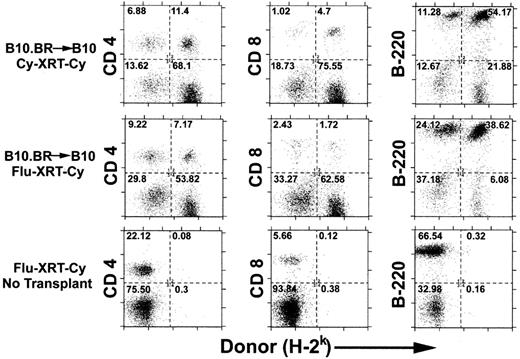
Previous studies have shown that the minimum dose of TBI that is required for the engraftment of MHC-incompatible cells can be reduced from 700 cGy to 500 cGy if Cy 200 mg/kg is given intraperitoneally 2 days after infusion of the allogeneic marrow.27 This irradiation dose could be further reduced to 300 cGy if antilymphocyte serum was administered before irradiation and posttransplantation Cy.12 We wished to determine whether pretransplantation Cy or fludarabine could substitute for antilymphocyte serum in the induction of tolerance to MHC-incompatible allografts. Thus, fludarabine 100 mg/kg/day IP for 5 days, equal to 25% of the LD10 of mice, or Cy 200 mg/kg IP on day −3 was added to pretransplantation TBI and posttransplantation Cy to create the Flu-XRT-Cy and Cy-XRT-Cy regimens. Either conditioning regimen was sufficient for the induction of mixed chimerism after the administration of 20 million B10.BR marrow cells to MHC-congenic C57BL/10 (B10) recipients (Figure 1, top and middle rows). Donor chimerism was detected in splenic CD4+ T cells (left), CD8+ T cells (middle), and B220+ B cells (right). Importantly, both regimens are nonmyeloablative, because mice that are conditioned with either regimen but reject or do not receive donor marrow remain healthy and recover autologous hematopoiesis (bottom row and data not shown).
Recipient conditioning with pretransplantation Cy or fludarabine in conjunction with low-dose TBI and posttransplantation Cy induces engraftment of MHC-incompatible cells.
C57BL/10 (B10; H-2b) mice were conditioned with Cy 200 mg/kg intraperitoneally on day −3 (top panel) or fludarabine 100 mg/kg intraperitoneally daily on days −6 to −2 (middle and bottom panels), followed by 200 cGy TBI on day −1 and Cy 200 mg/kg intraperitoneally on day +3 (all panels). On day 0, conditioned recipients received no marrow (bottom panel) or 2 × 107 marrow cells from MHC-congenic B10.BR (H-2k) donors (top and middle panels). Six weeks later, donor chimerism in splenic CD4+ and CD8+ T cells and B220+ B cells was analyzed by dual-color flow cytometry. The numbers in each of the quadrants represent the percentage of total cells analyzed.
Recipient conditioning with pretransplantation Cy or fludarabine in conjunction with low-dose TBI and posttransplantation Cy induces engraftment of MHC-incompatible cells.
C57BL/10 (B10; H-2b) mice were conditioned with Cy 200 mg/kg intraperitoneally on day −3 (top panel) or fludarabine 100 mg/kg intraperitoneally daily on days −6 to −2 (middle and bottom panels), followed by 200 cGy TBI on day −1 and Cy 200 mg/kg intraperitoneally on day +3 (all panels). On day 0, conditioned recipients received no marrow (bottom panel) or 2 × 107 marrow cells from MHC-congenic B10.BR (H-2k) donors (top and middle panels). Six weeks later, donor chimerism in splenic CD4+ and CD8+ T cells and B220+ B cells was analyzed by dual-color flow cytometry. The numbers in each of the quadrants represent the percentage of total cells analyzed.
Table 1 summarizes the results of 3 experiments involving the transplantation of MHC ± minor H antigen-incompatible marrow after nonmyeloablative conditioning. Several points emerge from the data. First, in the B10.BR (H-2k)→B10 (H-2b) donor-recipient combination (Experiment 1), failure of engraftment after nonmyeloablative conditioning, presumably from graft rejection, can be overcome by increasing the dose of donor marrow. Donor cell engraftment, as determined by the presence of donor cells significantly above the background level seen in B10 mice not receiving transplants, occurred in 0 of 5 B10 recipients of 107 B10.BR marrow cells after Cy-XRT-Cy conditioning, as compared with 8 of 9 recipients of 2 × 107 B10.BR marrow cells after the same conditioning (compare group 2 with group 1, P = .003 by Fisher exact test). Second, engraftment of 107 MHC-incompatible marrow cells can be achieved in the majority of recipients by adding pretransplantation fludarabine to 200 cGy pretransplantation TBI and posttransplantation Cy (group 3). This result demonstrates that, at the doses of drugs that were used, pretransplantation fludarabine is more effective than pretransplantation Cy for promoting alloengraftment (compare group 3 with group 1). Third, in the BALB/c (H-2d)→B10 (H-2b) donor-recipient combination (Experiment 2), both pretransplantation fludarabine (compare group 7 with group 4) and posttransplantation Cy (compare group 7 with group 5) are required for the induction of long-term chimerism after the grafting of MHC-incompatible marrow. Indeed, the combination of pretransplantation fludarabine and posttransplantation Cy is sufficiently immunosuppressive to permit engraftment, defined as more than 0.5% donor cells detectable by flow cytometry (see “Materials and methods”), of fully histoincompatible marrow cells in 7 of 10 recipients even in the absence of TBI (group 6; see also Figure 3B). However, in the absence of TBI, donor chimerism is low, indicating that the combination of pretransplantation fludarabine and posttransplantation Cy exhibits minimal toxicity to hematopoietic stem cells. Finally, experiment 2 demonstrates that doubling the dose of fludarabine increases donor chimerism only marginally (compare group 7 with group 8). Similar results in the BALB/c→B10 donor-recipient combination were obtained in at least 2 independent experiments. In addition, stable mixed chimerism was induced by Cy-XRT-Cy in other fully MHC-mismatched or -haploidentical combinations, including B6.SJL (H-2b)→DBA/2 (H-2d), AKR (H-2k)→B6 × DBA/2 F1 (H-2b/d), and BALB/c × B6 F1 (H-2b/d)→B6 × C3H (H-2b/k) F1 (experiment 3).
Induction of stable, mixed chimerism with major histocompatibility complex-incompatible cells after nonmyeloablative conditioning
| Group . | Donor (H-2) . | Recipient (H-2) . | Fludarabine (mg/kg) IP d − 6 to − 2 . | Cy (mg/kg) IP d − 3 . | 200 cGy TBI d − 1 . | BM cells IV d 0 . | Cy 200 mg/kg IP d 3 . | Engrafted/ total . | Mean % donor chimerism (SEM)3-150 . |
|---|---|---|---|---|---|---|---|---|---|
| Experiment 13-151 | |||||||||
| 1 | B10.BR (H-2k) | B10 (H-2b) | — | 200 | + | 107 | + | 0/5 | None detected |
| 2 | B10.BR (H-2k) | B10 (H-2b) | — | 200 | + | 2 × 107 | + | 8/10 | 78.96 (8.96) |
| 3 | B10.BR (H-2k) | B10 (H-2b) | 100 | — | + | 107 | + | 8/9 | 52.39 (13.29) |
| Experiment 23-152 | |||||||||
| 4 | BALB/c (H-2d) | B10 (H-2b) | — | — | + | 2 × 107 | + | 0/5 | None detected |
| 5 | BALB/c (H-2d) | B10 (H-2b) | 100 | — | + | 2 × 107 | − | 0/5 | None detected |
| 6 | BALB/c (H-2d) | B10 (H-2b) | 100 | — | − | 2 × 107 | + | 7/10 | 2.31 (0.9) |
| 7 | BALB/c (H-2d) | B10 (H-2b) | 100 | — | + | 2 × 107 | + | 10/10 | 75.07 (4.04) |
| 8 | BALB/c (H-2d) | B10 (H-2b) | 200 | — | + | 2 × 107 | + | 5/5 | 81.77 (3.25) |
| Experiment 33-152 | |||||||||
| 9 | B6.SJL (H-2b) | DBA/2 (H-2d) | — | 200 | + | 2 × 107 | + | 4/4 | 88.15 (9.02) |
| 10 | CB6 F1 (H-2b/d) | B6C3 F1(H-2b/k) | — | 200 | + | 2 × 107 | + | 4/4 | 67.53 (4.8) |
| 11 | AKR (H-2k) | B6D2 F1(H-2b/d) | — | 200 | + | 2 × 107 | + | 4/4 | 78.32 (6.28) |
| Group . | Donor (H-2) . | Recipient (H-2) . | Fludarabine (mg/kg) IP d − 6 to − 2 . | Cy (mg/kg) IP d − 3 . | 200 cGy TBI d − 1 . | BM cells IV d 0 . | Cy 200 mg/kg IP d 3 . | Engrafted/ total . | Mean % donor chimerism (SEM)3-150 . |
|---|---|---|---|---|---|---|---|---|---|
| Experiment 13-151 | |||||||||
| 1 | B10.BR (H-2k) | B10 (H-2b) | — | 200 | + | 107 | + | 0/5 | None detected |
| 2 | B10.BR (H-2k) | B10 (H-2b) | — | 200 | + | 2 × 107 | + | 8/10 | 78.96 (8.96) |
| 3 | B10.BR (H-2k) | B10 (H-2b) | 100 | — | + | 107 | + | 8/9 | 52.39 (13.29) |
| Experiment 23-152 | |||||||||
| 4 | BALB/c (H-2d) | B10 (H-2b) | — | — | + | 2 × 107 | + | 0/5 | None detected |
| 5 | BALB/c (H-2d) | B10 (H-2b) | 100 | — | + | 2 × 107 | − | 0/5 | None detected |
| 6 | BALB/c (H-2d) | B10 (H-2b) | 100 | — | − | 2 × 107 | + | 7/10 | 2.31 (0.9) |
| 7 | BALB/c (H-2d) | B10 (H-2b) | 100 | — | + | 2 × 107 | + | 10/10 | 75.07 (4.04) |
| 8 | BALB/c (H-2d) | B10 (H-2b) | 200 | — | + | 2 × 107 | + | 5/5 | 81.77 (3.25) |
| Experiment 33-152 | |||||||||
| 9 | B6.SJL (H-2b) | DBA/2 (H-2d) | — | 200 | + | 2 × 107 | + | 4/4 | 88.15 (9.02) |
| 10 | CB6 F1 (H-2b/d) | B6C3 F1(H-2b/k) | — | 200 | + | 2 × 107 | + | 4/4 | 67.53 (4.8) |
| 11 | AKR (H-2k) | B6D2 F1(H-2b/d) | — | 200 | + | 2 × 107 | + | 4/4 | 78.32 (6.28) |
BMT indicates bone marrow transplantation; TBI, total body irradiation; IP, intraperitoneal; BM, bone marrow; IV, intravenous; Cy, cyclophosphamide; —, not applicable.
Chimerism was measured in peripheral blood 6 months after transplantation.
Donor and recipient differ in the expression of major histocompatibility antigens.
Donor and recipient differ in the expression of major + minor histocompatibility antigens.
Clonal deletion of donor-reactive host T cells in mixed chimeras prepared with nonmyeloablative conditioning
T-cell tolerance of donor or host antigens after allogeneic bone marrow transplantation may be maintained by clonal deletion,28 clonal anergy,29 or through active suppression.30 To determine the mechanism of tolerance in long-term (> 1 year) mixed chimeras prepared with fludarabine, 100 cGy TBI, and posttransplantation Cy, peripheral blood was analyzed for the presence of T cells reactive to donor antigens. In B10.BR but not in B10 mice, T cells expressing products of the Vβ5 or Vβ11 genes are preferentially deleted because of the presence of an endogenous, retrovirally encoded superantigen31 32 (Table 2). Residual host CD4+ and CD8+ T cells in the B10.BR→B10 chimeras contained a significantly lower percentage of cells expressing either Vβ5 or Vβ11 gene products than did T cells from B10 controls not receiving transplants. In contrast, expression of these gene products was not significantly different between chimeric host T cells and T cells from B10.BR donors. In 2 separate experiments, the percentage of CD4+, Vβ8+ T cells was higher in chimeric host T cells than in T cells from B10.BR mice not receiving transplants. Although the significance of this finding is unclear, the reduced numbers of Vβ5+ and Vβ11+ T cells of B10 origin in the B10.BR→B10 chimeras demonstrate that clonal deletion of host-reactive T cells is a major mechanism of tolerance of donor antigens after Flu-XRT-Cy conditioning. Although not statistically significant, the higher percentage of CD4+Vβ5+ and CD4+Vβ11+ T cells in the chimeras than in B10.BR mice leaves open the possibility that additional mechanisms of tolerance of donor cells, such as anergy or suppression, may be operative.
Clonal deletion of donor-reactive host T cells in B10.BR B10 chimeras
| . | % of CD4+ T cells (SEM) expressing . | % of CD8+ T cells (SEM) expressing . | ||||
|---|---|---|---|---|---|---|
| Vβ5 . | Vβ11 . | Vβ8 . | Vβ5 . | Vβ11 . | Vβ8 . | |
| Strain (n) | ||||||
| B10 (3) | 1.54 (.08) | 5.00 (.31) | 17.44 (1.08) | 11.06 (.67) | 6.84 (.61) | 16.79 (1.6) |
| B10.BR (3) | 0.17 (.17) | 0.17 (.03) | 15.36 (.52) | 1.59 (.63) | 1.54 (.42) | 19.82 (4.4) |
| B10.BR → B10* (4) | 0.43 (.16) | 0.30 (.05) | 18.69 (.69) | 0.94 (.23) | 1.58 (.12) | 20.78 (1.1) |
| Comparison, P | ||||||
| B10.BR → B10 vs B10 | .0026 | .00002 | .35 | .0005 | .0007 | .60 |
| B10.BR → B10 vs B10.BR | .31 | .09 | 0.02 | .38 | .94 | .86 |
| . | % of CD4+ T cells (SEM) expressing . | % of CD8+ T cells (SEM) expressing . | ||||
|---|---|---|---|---|---|---|
| Vβ5 . | Vβ11 . | Vβ8 . | Vβ5 . | Vβ11 . | Vβ8 . | |
| Strain (n) | ||||||
| B10 (3) | 1.54 (.08) | 5.00 (.31) | 17.44 (1.08) | 11.06 (.67) | 6.84 (.61) | 16.79 (1.6) |
| B10.BR (3) | 0.17 (.17) | 0.17 (.03) | 15.36 (.52) | 1.59 (.63) | 1.54 (.42) | 19.82 (4.4) |
| B10.BR → B10* (4) | 0.43 (.16) | 0.30 (.05) | 18.69 (.69) | 0.94 (.23) | 1.58 (.12) | 20.78 (1.1) |
| Comparison, P | ||||||
| B10.BR → B10 vs B10 | .0026 | .00002 | .35 | .0005 | .0007 | .60 |
| B10.BR → B10 vs B10.BR | .31 | .09 | 0.02 | .38 | .94 | .86 |
Numbers in this row indicate the percentage of host (B10)–derived T cells that express the indicated Vβ gene product. Mean (±SEM) peripheral blood donor chimerism in these animals was 14.46% ± 4.53%.
Donor-specific cytotoxic T-cell unresponsiveness in mixed chimeras prepared with nonlethal conditioning
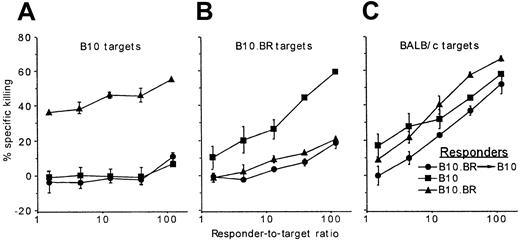
B10.BR→B10 mixed bone marrow chimeras were prepared after nonmyeloablative conditioning with fludarabine, 200 cGy TBI, and posttransplantation Cy. Six months after transplantation, spleen cells from the chimeras, as well as from untreated B10 and B10.BR mice, were tested for the generation of cytotoxic T lymphocytes (CTLs) against recipient strain B10 (Figure2A), donor strain B10.BR (Figure 2B), or third-party BALB/c stimulators (Figure 2C). Chimeric spleen cells generated effective CTLs against BALB/c stimulators but failed to respond to donor-type B10.BR or recipient-type B10 stimulators. Thus, long-term chimerism after nonmyeloablative alloBMT is associated with donor-specific CTL tolerance.
Donor chimerism induced after nonmyeloablative conditioning is associated with donor-specific CTL tolerance.
Spleen cells from B10 (▪) and B10.BR (▴) mice and from B10.BR→B10 chimeras generated after conditioning with Flu-XRT-Cy (●; as described in the legend to Figure 1) were cultured for 5 days with irradiated B10, B10.BR, or BALB/c stimulators and tested for killing of3H-labeled B10 (A), B10.BR (B), or BALB/c targets (C), respectively. Chimeras were tested 6 weeks after transplantation.
Donor chimerism induced after nonmyeloablative conditioning is associated with donor-specific CTL tolerance.
Spleen cells from B10 (▪) and B10.BR (▴) mice and from B10.BR→B10 chimeras generated after conditioning with Flu-XRT-Cy (●; as described in the legend to Figure 1) were cultured for 5 days with irradiated B10, B10.BR, or BALB/c stimulators and tested for killing of3H-labeled B10 (A), B10.BR (B), or BALB/c targets (C), respectively. Chimeras were tested 6 weeks after transplantation.
Effect of the dose of irradiation or donor bone marrow cells on engraftment and donor chimerism after conditioning with fludarabine, 200 cGy TBI, and posttransplantation Cy
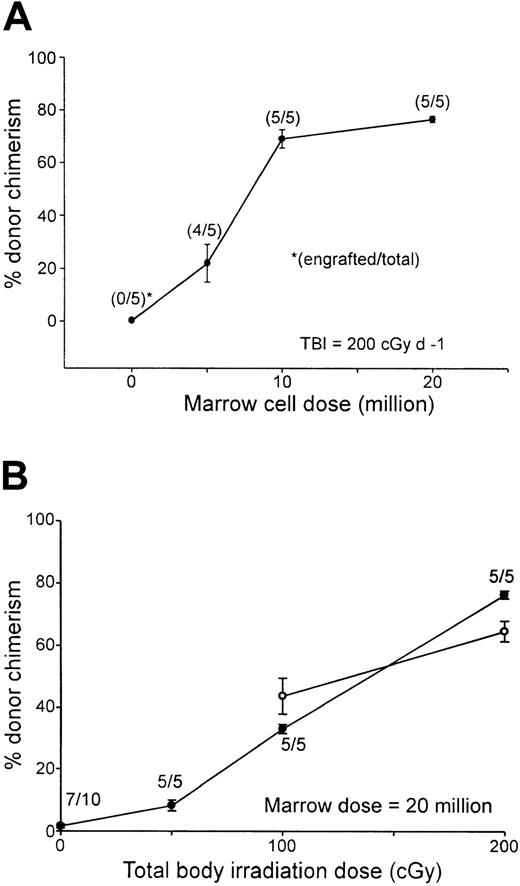
To facilitate comparisons to other nonmyeloablative conditioning regimens used to obtain engraftment of MHC-incompatible cells in mice, titrations of TBI or donor marrow cell dose were performed in the context of conditioning with fludarabine, 100 mg/kg IP on days −6 to −2 and Cy 200 mg/kg IP on day 3. Increasing the dose of TBI or donor bone marrow cells augmented the level of donor chimerism as measured 7 weeks after transplantation (Figure3). Donor chimerism in the peripheral blood increased from a mean of 22% in B10 recipients of 5 million BALB/c marrow cells to a mean of 68.9% in recipients of 10 million marrow cells, after which further increases in the marrow dose had a smaller effect (Figure 3A). Likewise, in B10 recipients of 20 million BALB/c marrow cells, donor chimerism increased from a mean of 8.2% in animals conditioned with 50 cGy TBI to a mean of 76.4% among recipients conditioned with 200 cGy (Figure 3B, closed circles). Significantly, donor chimerism was present in all animals receiving transplants of as few as 10 million marrow cells (Figure 3A) or conditioned with as little as 50 cGy TBI (Figure 3B). These results demonstrate that, in the context of fixed doses of pretransplantation fludarabine and posttransplantation Cy, the presence and level of donor chimerism are affected independently by the dose of TBI and donor marrow cells. Donor chimerism in the peripheral blood of animals conditioned with either 100 or 200 cGy TBI was measured again 1 year after transplantation (Figure 3B, open circles). Although donor chimerism in animals conditioned with 200 cGy TBI was significantly lower at 1 year than at 7 weeks after transplantation (P = .01), none of the animals experienced either graft rejection or conversion to full donor chimerism, indicating that the state of mixed hematopoietic chimerism was durable. Moreover, mixed T-cell chimerism was also present at 1 year (66.48% ± 2.86% in recipients of 100 cGy TBI and 63.98% ± 4.79% in recipients of 200 cGy TBI; n = 5/group).
Donor chimerism after Flu-XRT-Cy conditioning is proportional to the dose of transplanted marrow cells or TBI.
(A) B10 mice received fludarabine 100 mg/kg/day intraperitoneally on days −6 to −2, TBI (200 cGy) on day −1, graded doses of BALB/c marrow cells intravenously on day 0, and Cy 200 mg/kg intraperitoneally on day 3. (B) B10 mice received fludarabine 100 mg/kg/day intraperitoneally on days −6 to −2, graded doses of TBI on day −1, 20 million BALB/c marrow cells on day 0, and Cy 200 mg/kg intraperitoneally on day 3. (A,B) Seven weeks (closed symbols) or 1 year (open symbols) after transplantation, peripheral blood was stained with fluorescein-conjugated antibody to H-2Dd and evaluated for donor chimerism by flow cytometry.
Donor chimerism after Flu-XRT-Cy conditioning is proportional to the dose of transplanted marrow cells or TBI.
(A) B10 mice received fludarabine 100 mg/kg/day intraperitoneally on days −6 to −2, TBI (200 cGy) on day −1, graded doses of BALB/c marrow cells intravenously on day 0, and Cy 200 mg/kg intraperitoneally on day 3. (B) B10 mice received fludarabine 100 mg/kg/day intraperitoneally on days −6 to −2, graded doses of TBI on day −1, 20 million BALB/c marrow cells on day 0, and Cy 200 mg/kg intraperitoneally on day 3. (A,B) Seven weeks (closed symbols) or 1 year (open symbols) after transplantation, peripheral blood was stained with fluorescein-conjugated antibody to H-2Dd and evaluated for donor chimerism by flow cytometry.
In a separate experiment, the effect of depleting T cells from the donor marrow on the rate and level of donor engraftment was examined. Regardless of whether T-cell depletion was performed, donor cell engraftment at 2 months after transplantation was found in all B10 animals conditioned with Flu-XRT-Cy (200 cGy TBI) and receiving transplants of either 10 or 20 million BALB/c marrow cells (5 animals per group). The mean level of donor chimerism in the peripheral blood was actually higher in recipients of 10 million T-cell–depleted marrow cells (56.29% ± 3.57%) than in recipients of 10 million whole marrow cells (38.50% ± 6.16%; P = .04). Donor chimerism was also higher among recipients of 20 million T-cell–depleted marrow cells (76.42% ± 2.87%) than in recipients of 20 million whole marrow cells (68.30% ± 3.24%), although not significantly so (P = .1). This tendency toward a higher level of donor chimerism in recipients of T-cell–depleted marrow may be due to variations in experimental technique or to a higher stem cell content of a T-cell–depleted product. In either case, the results suggest that, in animals conditioned with Flu-XRT-Cy, mature T cells in the donor marrow do not significantly augment donor cell chimerism through a lymphohematopoietic GVH effect.
Effect of pretransplantation fludarabine on donor chimerism independent of immunosuppression
The presence and extent of donor chimerism after MHC-incompatible stem cell transplantation reflect the interaction of myeloablative and immunosuppressive effects of conditioning.9 Fludarabine is a potent immunosuppressive agent that induces significant reductions of host B and T lymphocytes.20 However, the effect of fludarabine on host stem cells, especially when the drug is given before nonmyeloablative doses of TBI, is less clearly defined. To address this issue, C57BL/6 mice (H-2b, CD45.1−, CD45.2+) were conditioned with fludarabine alone, 200 cGy TBI alone, or fludarabine and 200 cGy before receiving 20 million B6.SJL (H-2b, CD45.1+, CD45.2−) marrow cells. Because there is no immunologic barrier between these 2 congenic strains of mice, long-term donor myeloid chimerism in the recipients is a function of the effect of conditioning on host stem cells. Six months after transplantation, lineage-specific chimerism in the peripheral blood was evaluated by dual-color flow cytometry (Table 3). In mice conditioned with fludarabine alone, donor cells were detected at a low level in only one of 5 mice receiving transplants, indicating that the drug by itself exerts minimal toxicity to host stem cells. In contrast, mean total donor chimerism was 48.17% among B6 mice conditioned with 200 cGy TBI and receiving transplants of 20 million B6.SJL marrow cells. Interestingly, administration of fludarabine before 200 cGy TBI resulted in significantly higher donor chimerism 6 months after transplantation (68.66%) than was seen in animals conditioned with 200 cGy TBI alone (P < .05 by Studentt test), suggesting that fludarabine sensitizes host stem cells to the toxicity of low-dose TBI.
Fludarabine augments the myelotoxicity of total body irradiation
| Group . | Flu 100 mg/kg IP d −6 to −2 . | 200 cGy TBI d −1 . | Cy 200 mg/kg IP d 3 . | Mean % total donor chimerism (SEM) . | Mean % T-cell chimerism (SEM) . | Mean % B-cell chimerism (SEM) . |
|---|---|---|---|---|---|---|
| 1* | + | − | − | <1 | Undetectable | Undetectable |
| 2 | − | + | − | 48.17 (4.82) | 46.38 (5.34) | 45.26 (5.92) |
| 3 | + | + | − | 68.66 (6.32)† | 67.56 (5.49)† | 70.43 (7.18)† |
| Group . | Flu 100 mg/kg IP d −6 to −2 . | 200 cGy TBI d −1 . | Cy 200 mg/kg IP d 3 . | Mean % total donor chimerism (SEM) . | Mean % T-cell chimerism (SEM) . | Mean % B-cell chimerism (SEM) . |
|---|---|---|---|---|---|---|
| 1* | + | − | − | <1 | Undetectable | Undetectable |
| 2 | − | + | − | 48.17 (4.82) | 46.38 (5.34) | 45.26 (5.92) |
| 3 | + | + | − | 68.66 (6.32)† | 67.56 (5.49)† | 70.43 (7.18)† |
Flu indicates fludarabine; IP, intraperitoneal; TBI, total body irradiation; Cy, cyclophosphamide;
n = 5 per group.
P < .05 compared with group 2 (unpaired Student t test).
Inhibition of GVH reactions by administration of posttransplantation Cy
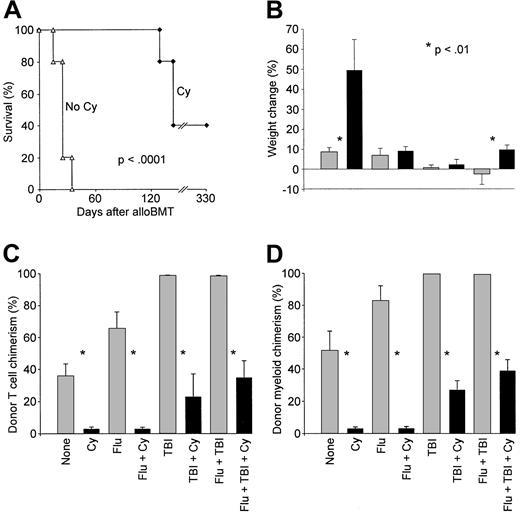
Cy, when administered 48 to 72 hours after MHC-identical alloBMT, prevents GVHD by selectively eliminating T cells that have been activated by recognition of host minor histocompatibility antigens.33 We wanted to determine whether posttransplantation Cy is also capable of preventing or ameliorating acute GVHD after MHC-mismatched BMT. For this purpose, we used a model in which acute GVHD is reliably induced.34 C57BL/6 × DBA/2 (B6D2) F1 mice were exposed to 850 cGy irradiation and received transplants the following day of 4 million bone marrow cells and 50 million spleen cells from B6.SJL donors. Three days after transplantation, the mice were left untreated or received Cy 200 mg/kg IP. Survival was prolonged in recipients of posttransplantation Cy compared with animals that received alloBMT without Cy (Figure4A; median survival 25 versus 145 days,P = .002 by log-rank test). All animals receiving transplants without Cy were dead by day 35 of acute GVHD, manifest as weight loss, hunched posture, and ruffled fur. In contrast, 2 of 5 recipients of Cy survived 330 days, whereas the other mice developed signs of chronic GVHD, including alopecia and dermatitis, before death. These results indicate that posttransplantation Cy inhibits acute GVHD mediated by T cells reactive to host MHC and minor H antigens.
Posttransplantation Cy ameliorates GVHD directed against major plus minor histocompatibility antigens.
(A) Lethally irradiated (850 cGy) C57BL/6 × DBA/2 (B6D2; H-2b/d) F1 mice received 4 × 106bone marrow cells and 5 × 107 spleen cells from C57BL/6 (H-2b) donors on day 0. On day +3, mice receiving transplants were left untreated (▵) or received Cy 200 mg/kg intraperitoneally (♦). Moribund mice were killed, and survival was plotted as a function of time after transplantation. (B-D) B6D2 F1 mice (CD45.1−) received transplants on day 0 of 107 bone marrow cells and 5 × 107spleen cells from B6.SJL (H-2b, CD45.1+) donors after conditioning with nothing, with fludarabine 100 mg/kg/day intraperitoneally on days −6 to −2, with 200 cGy TBI on day −1, or with both. On day 3, mice received nothing or Cy 200 mg/kg intraperitoneally. Each treatment group contained 10 animals, except for the group receiving posttransplantation Cy alone (n = 5). Each asterisk indicates that there is a significant difference (P < .01) between the values on either side of the asterisk. (B) Individual weights were recorded on the day of and 4 weeks after transplantation, and the mean (± SEM) percentage of weight change is plotted for each treatment group. (C) Donor T-cell chimerism 2 months after transplantation. (D) Donor myeloid chimerism 2 months after transplantation.
Posttransplantation Cy ameliorates GVHD directed against major plus minor histocompatibility antigens.
(A) Lethally irradiated (850 cGy) C57BL/6 × DBA/2 (B6D2; H-2b/d) F1 mice received 4 × 106bone marrow cells and 5 × 107 spleen cells from C57BL/6 (H-2b) donors on day 0. On day +3, mice receiving transplants were left untreated (▵) or received Cy 200 mg/kg intraperitoneally (♦). Moribund mice were killed, and survival was plotted as a function of time after transplantation. (B-D) B6D2 F1 mice (CD45.1−) received transplants on day 0 of 107 bone marrow cells and 5 × 107spleen cells from B6.SJL (H-2b, CD45.1+) donors after conditioning with nothing, with fludarabine 100 mg/kg/day intraperitoneally on days −6 to −2, with 200 cGy TBI on day −1, or with both. On day 3, mice received nothing or Cy 200 mg/kg intraperitoneally. Each treatment group contained 10 animals, except for the group receiving posttransplantation Cy alone (n = 5). Each asterisk indicates that there is a significant difference (P < .01) between the values on either side of the asterisk. (B) Individual weights were recorded on the day of and 4 weeks after transplantation, and the mean (± SEM) percentage of weight change is plotted for each treatment group. (C) Donor T-cell chimerism 2 months after transplantation. (D) Donor myeloid chimerism 2 months after transplantation.
The effect of posttransplantation Cy on GVH reactions after nonmyeloablative conditioning was also examined. Groups of 15 to 20 B6D2 F1 recipients were conditioned with nothing, with fludarabine on days −6 to −2, with 200 cGy TBI on day −1, or with both before the transplantation of 10 million marrow cells and 50 million spleen cells from B6.SJL donors. On day 3, at least 5 to 10 mice from each group also received Cy intraperitoneally, and all mice were then followed for survival and weight change. In addition, donor T cell and myeloid chimerism was measured in the peripheral blood 2 months after transplantation to evaluate for the presence and intensity of a lymphohematopoietic GVH reaction. In contrast to the frequent occurrence of fatal GVHD in animals given lethal conditioning, mortality from GVHD in sublethally conditioned mice was infrequent, occurring in only one mouse treated with 200 cGy TBI alone and one mouse conditioned with fludarabine and 200 cGy TBI. However, differences between groups that were or were not treated with posttransplantation Cy were readily apparent by other assays. For example, animals conditioned with nothing or with the combination of fludarabine and 200 cGy TBI gained significantly more weight by 4 weeks after transplantation if they were treated with posttransplantation Cy than if they were not (Figure 4B). In addition, all groups of animals that received posttransplantation Cy had significantly lower levels of donor T cell (Figure 4C) and donor myeloid chimerism (Figure 4D) than corresponding animals that received the identical conditioning without posttransplantation Cy. Because donor T cells augment donor chimerism through a lymphohematopoietic GVH reaction,35 36 the results demonstrate that posttransplantation Cy diminishes GVH reactions in sublethally conditioned recipients of major plus minor H antigen-incompatible T cells.
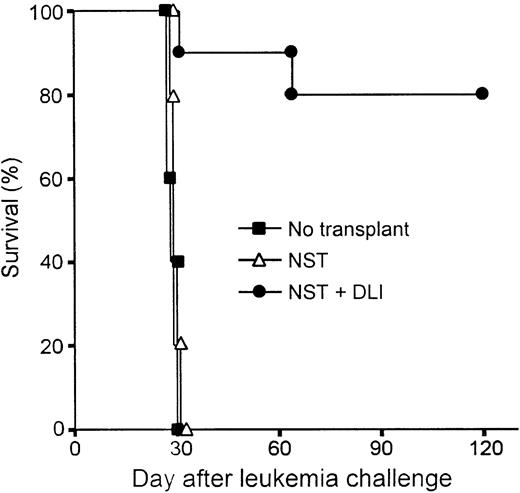
Graft-versus-leukemia effect after nonmyeloablative allogeneic BMT and donor lymphocyte infusion
The potency of donor lymphocyte infusion (DLI)–mediated GVH reactions, including the graft-versus-leukemia (GVL) effect, is significantly influenced by the immunogenetic disparity between donor and host, the intensity of transplantation conditioning,7and the interval between BMT and DLI.37 To assess the antitumor efficacy of DLI in mixed chimeras conditioned with Flu-XRT-Cy, an established model38 39 was used in which host leukemia cells and donor spleen cells are both administered after transplantation, thereby precluding any antitumor effects of the conditioning regimen. Groups of 10 C57BL/10 mice received B10.BR marrow cells in the context of Flu-XRT-Cy conditioning. On day 21, groups of animals received nothing or they received 20 million B10.BR spleen cells, and on day 30 all animals received an intravenous injection of 2 × 105 C1498 leukemia cells of C57BL/6 origin (Figure 5). The survival of mixed chimeras that received alloBMT without DLI was not significantly prolonged compared with those C57BL/10 recipients that did not receive alloBMT, but received only tumor cells. These results indicate that mature T cells contained in the donor marrow did not exert any antileukemic effects. In contrast, chimeric recipients of DLI survived significantly longer after leukemia challenge than animals that did not receive DLI (P < .0001 by log-rank test) and maintained their pre-DLI weight (data not shown). Flow cytometric analysis of the peripheral blood of DLI recipients confirmed the presence of full donor hematopoietic chimerism (data not shown). These results indicate that donor lymphocytes, administered to mixed chimeras conditioned with Flu-XRT-Cy, are capable of inducing strong lymphohematopoietic GVH reactions, including a GVL effect, without causing lethal GVHD.
Donor spleen cell infusions induce potent graft-versus-leukemia effects in mixed chimeras conditioned with Flu-XRT-Cy.
Nonmyeloablative allogeneic stem cell transplantation (NST) was performed by infusing 20 million B10.BR marrow cells on day 0 into B10 mice conditioned with fludarabine 100 mg/kg/day on days −6 to −2, with 200 cGy TBI on day −1, and with Cy 200 mg/kg intraperitoneally on day 3. On day 21, animals who had received transplants received nothing (▵; n = 5) or donor lymphocyte infusion (DLI) consisting of 20 million B10.BR spleen cells IV (●; n = 10). On day 30, all recipients of NST and a group of 5 B10 mice that hadn't received transplants (▪) received an intravenous injection of 2 × 105 C1498 leukemia cells, of B6 origin. Survival was monitored thrice weekly.
Donor spleen cell infusions induce potent graft-versus-leukemia effects in mixed chimeras conditioned with Flu-XRT-Cy.
Nonmyeloablative allogeneic stem cell transplantation (NST) was performed by infusing 20 million B10.BR marrow cells on day 0 into B10 mice conditioned with fludarabine 100 mg/kg/day on days −6 to −2, with 200 cGy TBI on day −1, and with Cy 200 mg/kg intraperitoneally on day 3. On day 21, animals who had received transplants received nothing (▵; n = 5) or donor lymphocyte infusion (DLI) consisting of 20 million B10.BR spleen cells IV (●; n = 10). On day 30, all recipients of NST and a group of 5 B10 mice that hadn't received transplants (▪) received an intravenous injection of 2 × 105 C1498 leukemia cells, of B6 origin. Survival was monitored thrice weekly.
Discussion
AlloBMT is a potentially curative treatment for various drug-resistant hematologic malignancies. However, only a small percentage of patients benefit from this therapeutic approach because of the limited availability of HLA-matched donors and the significant toxicity associated with treatment. HLA-haploidentical related donors are more readily available, but transplantation of partially HLA-mismatched marrow after lethal conditioning is associated with a high death rate from graft rejection, GVHD, and poor immunologic reconstitution with resulting susceptibility to infection. The results of haploidentical alloBMT are particularly poor in the adult population, because as many as 85% of patients whose donors are fully mismatched at one HLA haplotype (a 3-antigen mismatch) develop grade III to IV GVHD.40
The advent of low-toxicity, nonmyeloablative alloBMT offers multiple advantages for extending the use of alloBMT by using mismatched and haploidentical donors. We and others have been developing nonmyeloablative preparative regimens with the goal of separating alloBMT into 2 components: first, the induction of tolerance and mixed chimerism without GVHD, and, second, the achievement of full donor chimerism and GVL effects by donor lymphocyte infusions.41This concept of nonmyeloablative alloBMT as a 2-step procedure carries at least 3 potential advantages. First, patients with nonmalignant disorders of hematopoiesis, such as hemoglobinopathy, may forgo DLI and its associated risk of GVHD if they are cured by the induction of stable mixed hematopoietic chimerism. Second, animal studies have shown that mixed chimeras have a low incidence of GVHD42 and superior immunocompetence compared with full donor chimeras.43 The superior immunocompetence of mixed chimeras may be due to the persistence of host antigen-presenting cells, which are necessary for the activation of T cells that are restricted to recognition of antigen in the context of the MHC expressed on thymic epithelium. Third, in contrast to immediate posttransplantation administration of mature donor T cells, which is often associated with lethal GVHD,38,44 delayed infusion of donor lymphocytes can convert mixed to full donor hematopoietic chimerism35,36 and induce potent GVL effects38without significant GVHD.
In the present study, mixed chimerism was achieved by transplanting MHC+/− minor H antigen-incompatible marrow after conditioning with pretransplantation fludarabine or Cy, 200 cGy TBI, and posttransplantation Cy. Significant donor chimerism was achieved across a number of immunologic boundaries, including MHC only, MHC + minor H antigens, and haploidentical donor-recipient pairs, the latter analogous to parent→child or child→parent transplantations. In particular, the combination of fludarabine and posttransplantation Cy facilitated the induction of stable mixed chimerism in all recipients conditioned with as little as 50 cGy TBI or as few as 107donor marrow cells, equivalent to 5 × 108 mononuclear cells/kg. Deletion of donor-reactive host T cells in mixed chimeras was substantial; however, we cannot formally rule out the existence of a small population of donor-reactive cells whose unresponsiveness is maintained by anergy or suppression. Thus, although chimerism was stable in pathogen-free conditions, it remains possible that, after infection or other immune stimulation, tolerance of donor cells could be broken and donor chimerism, especially in low-level chimeras, could be lost.
Because adoptive immunotherapy with allogeneic T cells may be most effective in the treatment of minimal residual cancer, the use of conditioning agents that have antitumor activity is desirable. Fludarabine and Cy have antitumor as well as immunosuppressive activities and are therefore attractive alternatives to T-cell–specific antibodies or T-cell costimulation inhibitors in conditioning regimens for nonmyeloablative alloBMT in the treatment of hematologic malignancies. Tomita et al10 have reported the induction of tolerance and stable engraftment of MHC-incompatible cells by administering 108 donor spleen cells intravenously on day 0, Cy 200 mg/kg intraperitoneally and busulfan 25 mg/kg intraperitoneally on day 2, and 107 T-cell–depleted donor marrow cells on day 3.45 As in our approach, this method combines Cy-induced tolerance with the administration of donor stem cells after partial myeloablation to achieve significant donor chimerism.
The metabolism and pharmacokinetics of fludarabine phosphate differ between humans and animals.46 The activity of deoxycytidine kinase, which converts the prodrug F-ara-a into F-ara-AMP (adenosine monophosphate), is 10 times greater in humans than in dogs or mice. Accordingly, the maximum tolerated dose of fludarabine is 10 to 30 times lower in humans than in these species. Fludarabine, given to mice for 5 consecutive days at a dose of 100 mg/kg per day (25% of the LD10), decreases the absolute number of splenic CD4+ and CD8+ T cells by 25% and 15%, respectively (data not shown). A similar level of T-cell depletion, using the same dose of fludarabine in the mouse, has been reported recently by Petrus et al.47 In addition, these investigators have shown that Cy is very potent in reducing lymphocytes in the mouse and that the combination of fludarabine and Cy has a synergistic effect in depleting host T cells. However, because the metabolism and toxicity of these drugs differ significantly in humans and mice, caution must be exercised when translating the results of studies in mice to the clinic. Our data from the syngeneic BMT model (Table 2) also demonstrate the existence of an additive effect of fludarabine and 200 cGy TBI on donor engraftment. This result may be explained by the ability of fludarabine to inhibit the repair of DNA damaged by TBI or Cy, or by the redistribution of cells into a phase of the cell cycle that is more sensitive to apoptosis induced by genotoxic damage.48 49
The percentage of engrafting animals and the level of donor chimerism obtained in mice conditioned with the Flu-XRT-Cy regimen compare favorably with the results achieved with other regimens incorporating low-dose TBI or dimethylmyleran combined with antibodies against T-cell surface molecules.9-12,50 Efforts have focused on reducing the level of cytotoxic conditioning of the host14 and on substituting agents that induce alloantigen-specific tolerance rather than global immunosuppression.6 Tolerance and multilineage donor chimerism across MHC barriers in mice can be achieved without myelosuppressive conditioning by infusing more than 100 million cells in conjunction with monoclonal antibodies against T cells.51,52 In contrast, the Flu-XRT-Cy regimen described here is sufficient conditioning to obtain engraftment with 10 million MHC-incompatible marrow cells and substitutes fludarabine for T-cell–specific antibodies. Two properties of the Flu-XRT-Cy regimen may also mitigate the severity of GVHD after MHC-mismatched alloBMT. First, as shown in Figure 4, posttransplantation Cy decreases the incidence and severity of acute GVHD after transplantation of MHC-incompatible marrow. The ability of Cy to prevent GVHD without causing global immunosuppression is consistent with the drug's selective toxicity to T cells recently activated by antigen recognition.53 Second, the incidence and severity of GVHD after alloBMT is proportional to the irradiation dose; thus, by reducing the dose of irradiation to 200 cGy, the risk of GVHD is further minimized.
Earlier studies in rodents indicate that donor cell dose is critical for the engraftment of allogeneic bone marrow. Uharek et al54 have studied the relationship of donor cell dose to the intensity of conditioning required for engraftment of MHC-incompatible marrow in rats. These investigators have shown that engraftment is most dependent on the donor marrow dose when immunosuppression of the recipient is suboptimal. In the present study, small changes in the dose of TBI or the dose of marrow cells profoundly influenced the rate of graft acceptance and the level of donor chimerism after nonmyeloablative conditioning. Nonetheless, the relationship of the level of donor chimerism to immunologic competence after BMT and to the efficacy of DLI remains to be determined.
Donor lymphocyte infusions are a clinically validated therapy of drug-resistant hematologic malignancies in relapse after alloBMT.55,56 Here we show, as has been documented for DLI after MHC-identical alloBMT in mice,57 that infusion of MHC-mismatched donor lymphocytes on day 21 after nonmyeloablative alloBMT results in potent GVL effects and conversion to full donor chimerism without GVHD. Although the precise mechanisms are unclear, the decreased incidence of lethal GVHD seen when lymphocyte infusions are delayed after BMT may be due to resolution of the peritransplantation “cytokine storm,”58 which augments GVH reactions, or to the presence of donor-derived immunoregulatory cells that suppress GVHD.30 59 Regardless of the cellular mechanisms involved, our studies suggest that potent GVL effects may be induced without lethal GVHD, even across MHC barriers, by infusing donor lymphocytes into mixed chimeras generated after nonmyeloablative conditioning. A more direct comparison of DLI-mediated GVHD and GVL effects after nonmyeloablative versus myeloablative alloBMT and the cellular requirements for optimal antitumor immunity in these distinct settings are the focus of future studies.
Supported by a grant from Berlex Pharmaceuticals, a Clinical Investigator Award from the Cancer Research Institute (E.J.F.), and grant P01-CA15396-24 from the National Cancer Institute. L.L. is a Fellow of the Cure for Lymphoma Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ephraim J. Fuchs, Cancer Research Building, Room 488, 1650 Orleans St, Baltimore, MD 21231; e-mail: ejf@jhmi.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal