Abstract
In this study, the receptors and signals involved in collagen-induced platelet spreading were examined. It was found that platelet spreading on collagen (presenting a polygon shape with a number of filopodialike projections) was inhibited by the anti–integrin α2 antibody, suggesting the involvement of integrin α2β1 in this process. Studies with a glutathione-S-transferase fusion protein that binds specifically to activated Rac and in vitro p21-activated kinase (PAK) kinase assays revealed that Rac and PAK were activated during this collagen-activated process. Platelet spreading on collagen-coated surfaces was inhibited strongly by PP1 (a Src family kinase inhibitor) or weakly by wortmannin (a phosphatidylinositol 3-kinase [PI3-kinase] inhibitor) but not at all by Y-27632 (a Rho kinase inhibitor). The surfaces coated with anti–integrin α2β1antibodies also induced platelet spreading (presenting an almost complete round shape) and activation of Rac and PAK, although more slowly than collagen-coated surfaces. The antibody-induced responses were strongly inhibited by PP1 or wortmannin but not by Y-27632. The same concentration of Y-27632 inhibited collagen-induced shape change of platelets in suspension. These findings suggest that Rac and/or PAK activation, but not Rho, may play certain roles in platelet spreading via integrin α2β1 and that Src family kinases and PI3-kinase participate in these processes. Furthermore, the difference between spreading on collagen and the anti-integrin antibody suggests the involvement of other receptor(s) (in addition to the integrin α2β1) for collagen-induced spreading, the most likely candidate being glycoprotein VI.
Introduction
Platelet adhesion to collagen fibers through specific receptors is one of the initial processes in thrombus formation. Platelets adhere to, then spread on, collagen fibers, and finally form aggregates by recruiting other platelets through glycoprotein (GP)IIb/IIIa-fibrinogen binding. Platelet spreading and aggregation appear to involve different processes. The collagen receptors responsible for each process also seem to be different. A number of candidates for platelet collagen receptors have been proposed to date, including integrin α2β1, GPVI, GPIV, and p65.1 Among them, GPVI and integrin α2β1 are accepted as collagen receptors necessary for collagen-induced platelet aggregation, because human blood platelets, which lack the surface expression of integrin α2β12,3 or GPVI,4,5 fail to form aggregates on collagen stimulation. The GPVI-mediated activation signals that lead to platelet aggregation have been extensively investigated and several signaling molecules have been characterized.6-8 However, whether integrin α2β1 mediates activation signals during platelet aggregation remains controversial.
There have been several studies on the receptors responsible for platelet spreading on collagen-coated surfaces. Kehrel et al9 reported that only 9.9% of GPIa-deficient platelets spread on type I collagen, in contrast to 82% with control platelets. Nakamura et al10 showed that an anti–integrin α2β1 antibody markedly inhibited platelet spreading on collagen, whereas an anti-GPIV antibody had virtually no effect. They also reported that an anti-GPVI antibody had only slight inhibitory effects on platelet spreading, although Falet et al30 have recently shown that human platelets spread on collagen-related peptide (CRP)–coated surfaces, implying that signals from GPVI can also mediate platelet spreading. These findings suggest that integrin α2β1 is at least one of the major receptors responsible for platelet spreading and that it elicits certain signals necessary for this spreading. However, as described above for platelet aggregation, the presence of integrin α2β1-mediated activation signals has been challenged by others.1 11 Thus, we sought to characterize the integrin α2β1-mediated signals that lead to platelet spreading in this study.
Platelets adhere to collagen, then extend projections, and finally form curtainlike extensions between the projections during the process of spreading. These structures are reminiscent of filopodia and lamellipodia induced by the small guanosine triphosphate (GTP)–binding proteins, Rac/Cdc42. Cdc42 and Rac belong to the Rho family of small GTP-binding proteins, which contribute to the dynamic rearrangement of the actin cytoskeleton. In other cells, Cdc42 mediates the formation of long and thin actin-dependent extensions called filopodia, and Rac mediates the formation of curtainlike extensions called lamellipodia, although their functions cannot be clearly distinguished in many cases. Rho mediates the formation of actin stress fibers and focal adhesion, which appear to generate tension and stabilize adhesion. Its role in cell spreading has also been implied. Clark et al12reported that a significant reduction in cell spreading was observed in Rat-1 cells expressing dominant negative Rho. Rac and Cdc42 interact with a number of effector proteins. Among them, p21-activated kinases (PAKs) have been investigated most extensively in relation to Rac and Cdc42. Both Rac and Cdc42 in the GTP-bound state interact specifically with, and activate, PAK. PAKs are supposed to play an important role in cytoskeletal organization, including lamellipodia formation.13
Similarities between platelet spreading and lamellipodia/filopodia formation induced by Rac/Cdc42 prompted us to investigate the role of Rac/Cdc42 and their effector PAKs for platelet spreading. In this study, we present several lines of evidence to suggest that integrin α2β1 elicits the activation signals that involve Rac, Cdc42, and PAK and that PAK activation induced by Cdc42/Rac plays an important role in platelet spreading. Studies with inhibitors of Src family kinases, phosphatidylinositol 3-kinase (PI3-kinase), or Rho kinase, all of which are implicated for their roles in collagen-induced platelet activation1,6-8,14 or in cell spreading,12-16revealed that Src family kinases and PI3-kinase are important for platelet spreading, whereas Rho kinase is not involved in this process.
Materials and methods
Materials
Anti–integrin α2 monoclonal antibody (mAb), 7E10B, was generated as described previously.17 The hybridoma producing integrin β1 mAb (TS2/16) was from American Type Culture Collection (Manassas, VA). F(ab′)2fragments of 7E10B and TS2/16 were prepared by pepsin digestion as described elsewhere. Escherichia coli strain, BL21, transfected with pGEX-2T encoding glutathione-S-transferase (GST) fusion proteins containing p21-binding protein (PBD)18 was kindly donated by Dr Igarashi (Hokkaido University). Y-27632, a specific Rho kinase inhibitor,19was a gift from Welfide Corporation (Osaka, Japan).
The following materials were obtained from the indicated suppliers: anti-Rac1 mAb (Transduction Laboratories, Lexington, KY); anti-Cdc42 polyclonal antibody (pAb), anti-PAK pAb (C19), blocking peptides for anti-PAK pAb (sc-881 P), and peroxidase-conjugated goat anti–rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA); Type I collagen (Nycomed Pharma GMBH, Munich, Germany); Gly-Arg-Gly-Asp-Ser (Peptide Institute, Osaka, Japan); bovine serum albumin (BSA), prostaglandin I2 (PGI2), phenylmethylsulfonyl fluoride (PMSF), sodium orthovanadate, Triton X-100, tetramethyl rhodamine isothiocyanate (TRITC)–conjugated phalloidin and myelin basic protein (Sigma, St Louis, MO); protein A Sepharose and glutathione Sepharose 4B beads (Pharmacia Biotech, Uppsala, Sweden); peroxidase-conjugated goat anti–mouse IgG (Cappel Organon Teknika, Durham, NC); wortmannin (Sigma); PP1 (Biomol Research Laboratories, Plymouth Meeting, PA); and [γ-32P]ATP (NEN Life Science Products, Boston, MA).
Platelet preparation
Venous blood from healthy drug-free volunteers was collected into a tube containing acid-citrate-dextrose. Platelet-rich plasma (PRP) was obtained after centrifugation of whole blood at 160g for 10 minutes. PRP was incubated with 1 mM acetylsalicylic acid (ASA) for 30 minutes to exclude the secondary effects of thromboxane A2. The platelets were washed twice with a buffer containing 15% acid-citrate dextrose and 100 nmol/L PGI2 and then resuspended in a HEPES buffer containing 138 mM NaCl, 3.3 mM NaH2PO4, 2.9 mM KCl, 1.0 mM MgCl2, 1 mg/mL glucose, and 20 mM HEPES (pH 7.4) at a cell density of 2 to 4 × 108/mL, unless otherwise stated. The washed platelet suspensions were incubated for 30 minutes at 30°C before experiments.
Platelet adhesion study
For the preparation of microtiter plates coated with collagen or antibodies, 300 μL of 50 μg/mL collagen in an SKF buffer (Nycomed Pharma GMBH) or 10 μg/mL TS2/16 or 7E10B (intact molecules or F(ab′)2 fragments) was added to each well of a Falcon 24-well flat-bottomed plate (Becton Dickinson, Franklin Lakes, NJ), and the plates were left to stand overnight at room temperature. After 2 cycles of washing with phosphate-buffered saline (PBS), each well was blocked with 1% BSA in PBS for 30 minutes at room temperature. Prior to each experiment, 1% BSA-containing PBS was heated at 80°C for 10 minutes and sterilized by filtration. One hundred microliters of washed platelets (1 × 107 cells/mL) was then added to each well and incubated at 30°C for the indicated duration. After unbound platelets were removed by washing, wells were incubated with 3% paraformaldehyde for 30 minutes at room temperature. Platelet membranes were lysed with 0.2% Triton X-100, and the adhering platelets were stained by 0.1 μg/mL TRITC-conjugated phalloidin. Cells were viewed under a BH microscope (Olympus, Tokyo, Japan) and photographed on Fujicolor super G400 (Fujifilm, Tokyo, Japan). The number of adhering platelets was calculated on the photographs.
Precipitation with GST fusion proteins expressing PBD
Expression of GST fusion protein was induced by 0.5 mM isopropyl thiogalactopyranoside (IPTG). Bacteria were collected 2 hours after the addition of IPTG and suspended in a lysis buffer containing 50 mM Tris (pH 7.4), 150 mM NaCl, 10 mM MgCl2, 1 mM PMSF, 10 μg/mL leupeptin, and 1% Triton X-100. After standing on ice for 30 minutes, suspensions were sonicated and cleared by centrifugation at 10 000 rpm for 20 minutes. The supernatants were added to glutathione-Sepharose and incubated for 1 hour at 4°C. The beads were washed twice with a washing buffer and used for a binding assay. For the preparation of plates coated with collagen or TS2/16, 2 mL of 50 μg/mL collagen in an SKF buffer or 10 μg/mL TS2/16 was added to 10-cm diameter dishes for cell culture (Becton Dickinson). To each dish 1.2 mL washed platelets (4 × 108 cells/mL) were added, and the dishes were then incubated at 30°C for the indicated duration. Platelets were then lysed with 0.3 mL of 5 × ice-cold lysis buffer B (5% Triton X-100, 250 mM Tris/HCl, pH 7.4, 0.75 M NaCl, 50 mM MgCl2, 0.5% SDS, 5 mM PMSF, and 50 μg/mL leupeptin). After incubation for 30 minutes on ice, the platelet lysates were scraped by a cell scraper, sonicated 3 times for 5 seconds, then cleared by centrifugation at 16 000g for 5 minutes. The supernatants were incubated with 80 μL PBD-bound glutathione Sepharose 4B for 1 hour at 4°C. The beads were washed 4 times in a 1 × lysis buffer B. Proteins were eluted from the beads with 45 μL sodium dodecyl sulfate (SDS)–reducing buffer,20 boiled for 5 minutes, subjected to 15% SDS-polyacrylamide gel electrophoresis (PAGE) and electrophoretically transferred onto Clear Blot Membrane-P (Atto, Tokyo, Japan). The membranes were blocked with 1% BSA in PBS. After extensive washing with PBS containing 0.1% Tween 80, the immunoblots were incubated for 2 hours with the indicated antibody. Antibody binding was detected by using peroxidase-conjugated goat anti–mouse IgG or anti–rabbit IgG and visualized with electrogenerated chemiluminescence reaction reagents (Amersham, Buckinghamshire, United Kingdom) and Konica X-ray film (JX 8 × 10; Konica, Tokyo, Japan). Where indicated, levels of Rac/Cdc42 activation were quantified by using a PDI1400oe Scanner and Quantity One 2.5a software for Macintosh.
Immunoprecipitation kinase assay
The dishes coated with collagen or TS2/16 were prepared as described for Precipitation with GST fusion proteins expressing PBD. To each dish 1.5 mL washed platelets (2 × 108 cells/mL) pretreated with a vehicle solution or inhibitors was added, and the dishes were incubated at 30°C for the indicated duration. After removing unbound platelets, platelets were lysed with 0.6 mL of a 1 × ice-cold lysis buffer A (2% Triton X-100, 100 mM Tris/HCl pH 7.5, 5 mM EGTA, 2 mM vanadate, 1 mM PMSF, and 100 μg/mL leupeptin) and scraped by a cell scraper. The lysates were sonicated 3 times for 5 seconds and then cleared by centrifugation at 16 000g for 5 minutes. The protein concentrations of the resultant supernatants were determined by dye binding,21 and approximately 0.1 mg protein was used for immunoprecipitation. The samples were precleared with protein A-Sepharose 4B, and the resultant supernatants were immunoprecipitated with anti-PAK antibody. The immunoprecipitates were washed with 1 × lysis buffer A 3 times and then washed once with a low-salt buffer (10 mM Tris/HCl pH 7.2, 100 mM NaCl, 5 mmol/L MnCl2). The beads were incubated with 25 μL kinase reaction buffer (300 mM HEPES/NaOH, 15 mM MnCl2, 150 mM MgCl2, pH 8.0) with 2 μg myelin basic protein for 5 minutes, and reactions were initiated by the addition of 2 μM adenosine triphosphate (ATP; 10 μCi [37 Bq] [γ-32P]ATP). After 10 minutes at 20°C, reactions were stopped by the addition of a 3 × SDS sample buffer, then subjected to boiling for 5 minutes. The proteins were separated under reducing conditions by 14% SDS-PAGE and electrophoretically transferred onto Clear Blot Membrane-P. The membrane was dried and visualized with a BAS-2000 phosphor-image analyzer (Fuji Film, Japan).
Measurement of platelet shape change
Platelet shape change was measured by recording light transmission changes in an aggregometer as described previously22 at a cell density of 3 to 10 × 107/mL. Briefly, a vehicle solution or inhibitors were added to the platelet suspension at the indicated concentrations and incubated for 5 minutes. Collagen (20 μg/mL) was added to the platelet suspension, and light transmission changes were monitored for 5 minutes under stirring conditions. All the measurements were performed in the presence of 0.1 mM EGTA.
Results
Platelet spreading on collagen-coated surfaces and effects of the anti–integrin α2 antibody
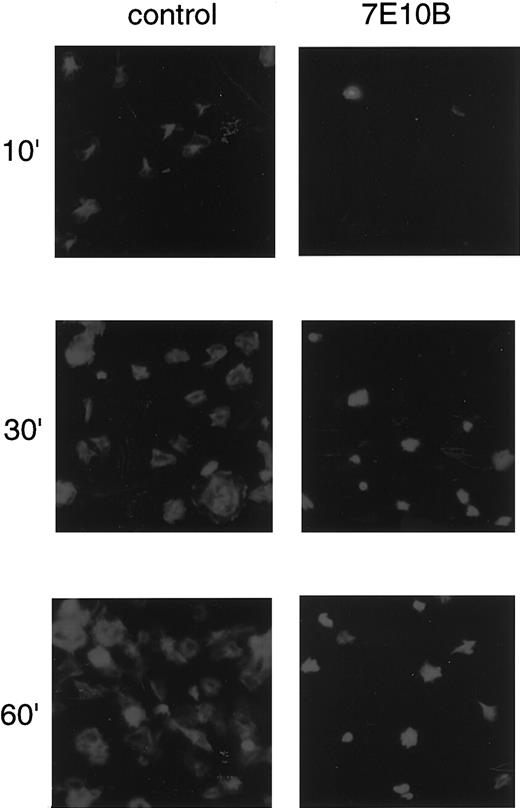
Washed platelets placed on collagen-coated surfaces adhered and extended filopodialike projections within 10 minutes and then spread over a period of approximately 1 hour, forming lamellipodialike structures (Figure 1, left panels). The number of adhered and spread platelets gradually increased in a time-dependent manner. To evaluate the contribution of integrin α2β1-mediated signals to platelet spreading, we pretreated platelets with anti–integrin α2antibody (7E10B), then seeded the platelets onto collagen-coated surfaces. The anti–integrin α2 antibody partially inhibited platelet adhesion, whereas it almost completely inhibited platelet spreading (Figure 1, right panels). Anti-GPIV antibody did not inhibit platelet spreading on collagen-coated surfaces at all (data not shown), which was consistent with a previous report.23Although we had no access to an anti-GPVI antibody, a previous study reported that an anti-GPVI antibody or RGDS peptide had only slight inhibitory effects.23 Our findings, combined with previous reports, suggest that integrin α2β1 plays a major role in platelet spreading on collagen-coated surfaces, whereas collagen receptors other than integrin α2β1, including GPVI, also contribute to platelet adhesion.
Anti–integrin α2 antibody inhibits platelet spreading on collagen-coated surfaces.
Washed platelets were seeded on collagen-coated surfaces with 10 μg/mL control mouse IgG or with 10 μg/mL anti–integrin α2 antibody (7E10B) for 10 minutes, 30 minutes, and 60 minutes at 30°C. After unbound platelets were removed, the platelets were fixed, then incubated with TRITC-conjugated phalloidin for actin fiber staining.
Anti–integrin α2 antibody inhibits platelet spreading on collagen-coated surfaces.
Washed platelets were seeded on collagen-coated surfaces with 10 μg/mL control mouse IgG or with 10 μg/mL anti–integrin α2 antibody (7E10B) for 10 minutes, 30 minutes, and 60 minutes at 30°C. After unbound platelets were removed, the platelets were fixed, then incubated with TRITC-conjugated phalloidin for actin fiber staining.
Platelets spread on the surfaces coated with F(ab)′2fragments of anti–integrin α2 or anti–integrin β1 antibody
We then investigated whether the signals mediated through integrin α2β1 alone could induce platelet spreading. Platelets placed on the surfaces coated with F(ab)′2fragments of TS2/16, an anti–integrin β1 antibody, or 7E10B, an anti–integrin α2 antibody, underwent spreading, forming lamellipodialike structures (Figure2A). Pretreatment of platelets with 10 μg/mL 7E10B almost completely inhibited platelet adhesion or spreading on 7E10B- or TS2/16-coated surfaces (data not shown), confirming that platelet spreading was specifically mediated by integrin α2β1. These findings suggest that cross-linking of integrin α2β1 alone can induce platelet spreading. Platelet spreading was observed 10 minutes after seeding on collage-coated surfaces, whereas it was observed 30 minutes after seeding on TS2/16-coated surfaces (Figure 2B).
Platelets spread on surfaces coated with collagen, anti–integrin β1 antibody, or integrin α2antibody.
(A) Washed platelets were seeded on surfaces coated with 1% fatty acid-free BSA, 50 μg/mL collagen, or 10 μg/mL anti–integrin β1 (TS2/16) or anti–integrin α2 antibody (7E10B) for 30 minutes at 30°C. After unbound platelets were removed, the platelets were fixed, then incubated with TRITC-conjugated phalloidin for actin fiber staining. (B) Washed platelets were seeded on surfaces coated with 50 μg/mL collagen or 10 μg/mL anti–integrin β1 (TS2/16) for the indicated time frames at 30°C. After unbound platelets were removed, the platelets were fixed, then incubated with TRITC-conjugated phalloidin for actin fiber staining. Platelets were photographed, and the extent of platelet spreading on collagen- (open circles) or TS2/16-coated (solid circles) surfaces was tentatively evaluated by the following scoring: a fully spread cell (2 points), a mildly spread cell (1 point), and a nonspread cell (0 point). After evaluating more than 25 platelets, the total points for 10 platelets were calculated (for the platelet spreading index); the data represent the mean of the total points. The extent of platelet adhesion on collagen- (open bars) or TS2/16-coated (hatched bars) surfaces was evaluated by counting the number of adhered platelets on 2 photographs (randomly taken) at a high-power field. The data represent the mean ± SD of the total numbers.
Platelets spread on surfaces coated with collagen, anti–integrin β1 antibody, or integrin α2antibody.
(A) Washed platelets were seeded on surfaces coated with 1% fatty acid-free BSA, 50 μg/mL collagen, or 10 μg/mL anti–integrin β1 (TS2/16) or anti–integrin α2 antibody (7E10B) for 30 minutes at 30°C. After unbound platelets were removed, the platelets were fixed, then incubated with TRITC-conjugated phalloidin for actin fiber staining. (B) Washed platelets were seeded on surfaces coated with 50 μg/mL collagen or 10 μg/mL anti–integrin β1 (TS2/16) for the indicated time frames at 30°C. After unbound platelets were removed, the platelets were fixed, then incubated with TRITC-conjugated phalloidin for actin fiber staining. Platelets were photographed, and the extent of platelet spreading on collagen- (open circles) or TS2/16-coated (solid circles) surfaces was tentatively evaluated by the following scoring: a fully spread cell (2 points), a mildly spread cell (1 point), and a nonspread cell (0 point). After evaluating more than 25 platelets, the total points for 10 platelets were calculated (for the platelet spreading index); the data represent the mean of the total points. The extent of platelet adhesion on collagen- (open bars) or TS2/16-coated (hatched bars) surfaces was evaluated by counting the number of adhered platelets on 2 photographs (randomly taken) at a high-power field. The data represent the mean ± SD of the total numbers.
The shape of platelet spreading on antibody-coated surfaces was slightly different from that on collagen-coated surfaces (Figure 2A). Platelet spreading on collagen presented a polygon shape with a number of filopodialike projections, whereas platelet spreading on TS2/16 or 7E10B-coated surfaces presented an almost complete round shape. Signals mediated through receptors other than integrin α2β1 may be responsible for the slightly different shape.
Effects of inhibitors on platelet spreading on the surfaces coated with collagen or TS2/16
To investigate the signals that lead to platelet spreading, we examined the effects of various inhibitors that are known to inhibit platelet aggregation induced by collagen. Because platelets showed more extensive spreading on the TS2/16-coated surfaces than that on the 7E10B-coated ones under visual evaluation, TS2/16 was subsequently used to induce platelet spreading. As shown in Figure3, 10 μM PP1, a specific inhibitor of Src family kinases, strongly inhibited platelet spreading on collagen-coated surfaces as well as that on TS2/16-coated ones, suggesting that Src family kinases play a crucial role in platelet spreading. Wortmannin (100 nM), a PI3-kinase inhibitor, inhibited platelet spreading on the surfaces coated with collagen or TS2/16, although the inhibitory effect on collagen-coated surfaces was relatively weak. From these findings, we suggest that PI3-kinase plays a vital role in TS2/16-induced platelet spreading, whereas its contribution is only partial in platelet spreading on collagen-coated surfaces.
PP1 or wortmannin, but not Y-27632, inhibits platelet spreading on surfaces coated with collagen or anti–integrin β1 antibody.
Washed platelets were incubated without (control [C]) or with 20 μM PP1 (P), 20 μM Y-27632 (Y), and 100 nM wortmannin (W) for 10 minutes at 37°C. Then, they were seeded on surfaces coated with 50 μg/mL collagen (A) or 10 μg/mL anti–integrin β1 (TS2/16) (B) for 30 minutes at 30°C. After unbound platelets were removed, the platelets were fixed, then incubated with TRITC-conjugated phalloidin for actin fiber staining. (C) The extent of platelet spreading was tentatively evaluated as described in the legend for Figure 2. After evaluating more than 200 platelets, the total points for 100 platelets were used as the platelet spreading index. The data represent the mean ± SD of the total points.
PP1 or wortmannin, but not Y-27632, inhibits platelet spreading on surfaces coated with collagen or anti–integrin β1 antibody.
Washed platelets were incubated without (control [C]) or with 20 μM PP1 (P), 20 μM Y-27632 (Y), and 100 nM wortmannin (W) for 10 minutes at 37°C. Then, they were seeded on surfaces coated with 50 μg/mL collagen (A) or 10 μg/mL anti–integrin β1 (TS2/16) (B) for 30 minutes at 30°C. After unbound platelets were removed, the platelets were fixed, then incubated with TRITC-conjugated phalloidin for actin fiber staining. (C) The extent of platelet spreading was tentatively evaluated as described in the legend for Figure 2. After evaluating more than 200 platelets, the total points for 100 platelets were used as the platelet spreading index. The data represent the mean ± SD of the total points.
Y-27632 has been used widely as a specific inhibitor of Rho kinase, which is a Rho effector. Since Clark et al24 suggested the role of Rho in cell spreading of Rat-1 cells, the effects of Y-27632 on platelet spreading were examined. We found that 20 μM Y-27632 inhibited neither platelet spreading on collagen-coated surfaces nor that of TS2/16. However, the same concentration of Y-27632 reduced collagen-induced platelet shape change in suspension to approximately 30% ± 19% of the control (n = 5). Recently, it has been reported that both Rho/Rho kinase activation and Ca++ mobilization are involved in adenosine diphosphate (ADP)–induced platelet shape change.25 26 Because ADP is released on platelet activation, we next sought to exclude the possibility that this inhibitory effect of Y-27632 is due to the inhibition of ADP-induced Rho/Rho kinase activation. Even in the presence of apyrase, an ADP scavenger, the inhibitory effects of Y-27632 were essentially the same as those in the absence of apyrase (data not shown). These findings suggest that Rho/Rho kinase activation through collagen receptors is not required for platelet spreading on collagen, although it plays an important role in collagen-induced platelet shape change in suspension.
Rho family small GTPases, Rac and Cdc42, are activated during platelet spreading on collagen- or TS2/16-coated surfaces
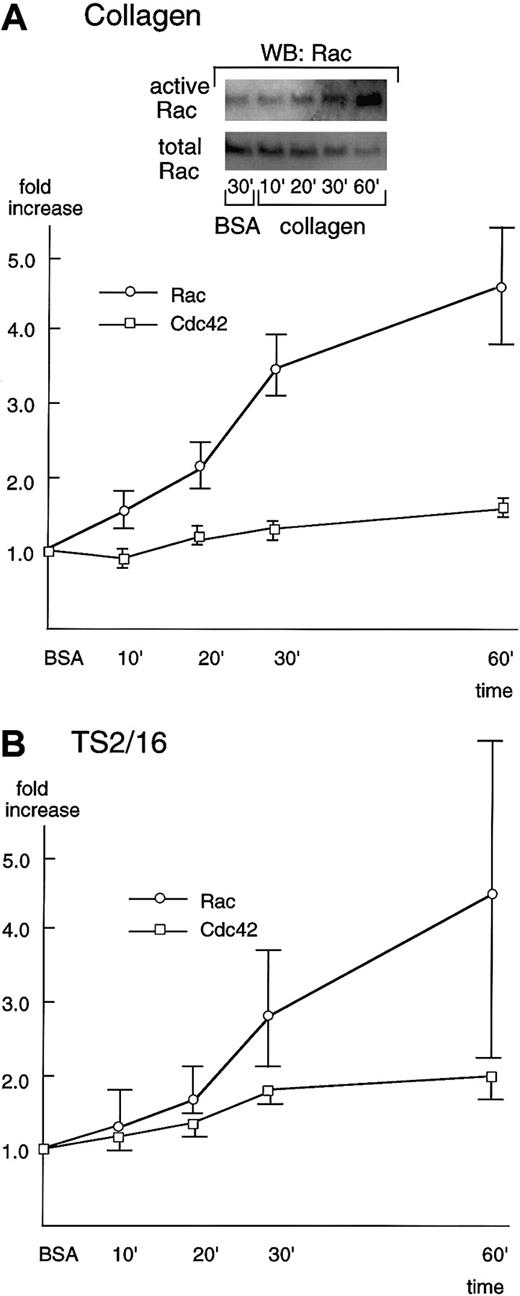
When platelets adhere to collagen, they spread rapidly by extending projections, which is followed by the formation of curtainlike extensions. These structures are reminiscent of filopodia and lamellipodia mediated by the small GTP-binding proteins, Cdc42 and Rac, respectively. Thus, we investigated the activation of Rac/Cdc42 during platelet spreading.
To detect the activation of Rac/Cdc42, we used a recombinant Rac/Cdc42 binding domain of PAK (PBD) that specifically binds to the activated Rac/Cdc42. Washed platelets were placed on collagen-, TS2/16-, or BSA-coated dishes, incubated for the indicated periods of time, then lysed with a lysis buffer. Activated Rac/Cdc42 proteins were precipitated by GST fusion proteins of PBD, followed by Western blotting with an anti-Rac or Cdc42 antibody.
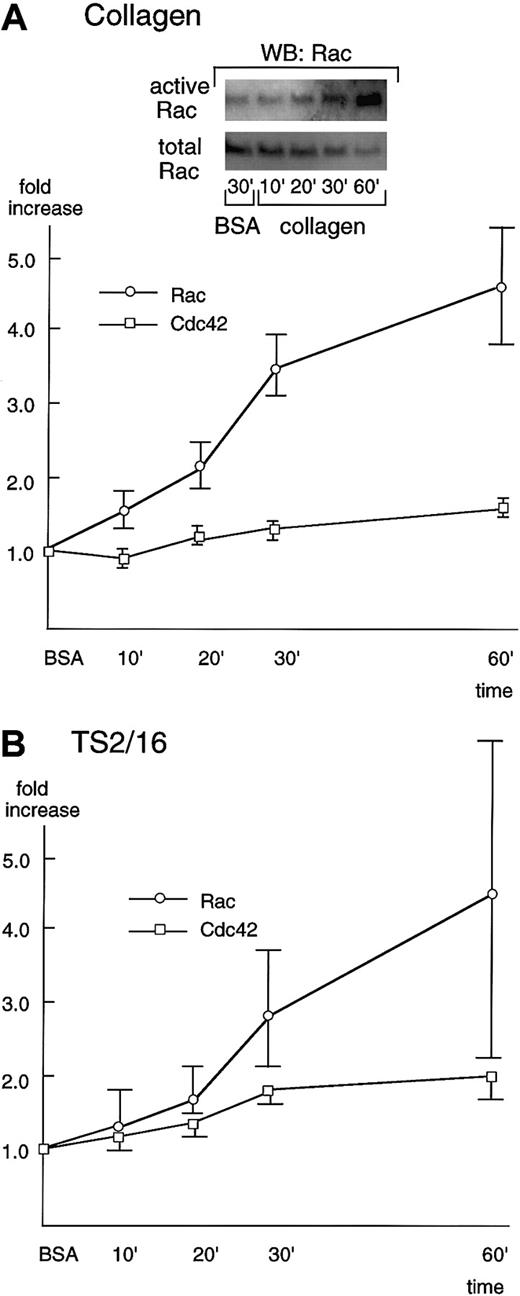
Rac showed only minimal activation even 30 minutes after platelets were placed on BSA-coated surfaces (Figure4A, inset). However, in the case of collagen-coated dishes, Rac was activated 10 minutes after platelet seeding, and its activation increased in a time-dependent manner (Figure 4A). The Rac activity reached the maximum level (about a 5-fold increase) at 60 minutes, the longest time point we examined in this study. At 60 minutes, the recovery of Rac usually decreased, which may be due to its degradation or its translocation to the cytoskeletal fraction. As was the case with collagen-coated surfaces, platelet spreading on TS2/16-coated surfaces resulted in activation of Rac 10 to 20 minutes after stimulation and the activity reached its peak at 60 minutes (Figure 4B), although the extent of activation was lower than that of collagen. Cdc42 was also activated in platelet spreading both on collagen- and TS2/16-coated surfaces, but the extent of activation was lower than that of Rac activation (Figure 4A,B). The slight activation of Cdc42 may be due to the limited sensitivity of our assay method. Alternatively, this finding may imply that the role of Cdc42 in platelet spreading on collagen is minimal.
Rac and Cdc42 are activated during platelet spreading on surfaces coated with collagen or TS2/16.
Washed platelets were added to collagen- (A) or TS2/16-coated (B) dishes and incubated at 30°C for the indicated time frame. Reactions were terminated with lysis buffer B, and a small aliquot of each supernatant was used to check the equal loading of samples (see total Rac in A, inset). The rest of the lysate was used to precipitate active forms of Rac (see active Rac in A, inset) or Cdc42 with PBD-bound glutathione Sepharose 4B. The sample was then Western blotted with anti-Rac or Cdc42 antibody. Levels of the active Rac (open circles) and Cdc42 (open squares) were quantified and adjusted by the total Rac and Cdc42, respectively. The data are expressed as the fold increases relative to those on BSA-coated surfaces (the mean ± SD of the total points).
Rac and Cdc42 are activated during platelet spreading on surfaces coated with collagen or TS2/16.
Washed platelets were added to collagen- (A) or TS2/16-coated (B) dishes and incubated at 30°C for the indicated time frame. Reactions were terminated with lysis buffer B, and a small aliquot of each supernatant was used to check the equal loading of samples (see total Rac in A, inset). The rest of the lysate was used to precipitate active forms of Rac (see active Rac in A, inset) or Cdc42 with PBD-bound glutathione Sepharose 4B. The sample was then Western blotted with anti-Rac or Cdc42 antibody. Levels of the active Rac (open circles) and Cdc42 (open squares) were quantified and adjusted by the total Rac and Cdc42, respectively. The data are expressed as the fold increases relative to those on BSA-coated surfaces (the mean ± SD of the total points).
Effects of inhibitors on Rac activation during platelet spreading on collagen- or TS2/16-coated surfaces
The effects of the inhibitors that had been assessed on platelet spreading were also evaluated with Rac activation. We chose to evaluate the effects of inhibitors on Rac, rather than Cdc42, because Cdc42 activation was of a lower degree compared with that of Rac. We evaluated the Rac activities 30 minutes after platelet seeding on collagen- or BSA-coated surfaces, because the recovery of Rac was constant within this time frame. Similar to the effects on platelet spreading, 20 μM PP1 or 100 nM wortmannin inhibited Rac activation in platelets seeded on collagen- or TS2/16-coated surfaces (Figure5). These findings suggest that Src family kinases and PI3-kinase play a crucial role in Rac activation. However, we cannot totally exclude the possibility that these inhibitory effects were overestimated, because we found that PP1 and wortmannin also inhibited the basal activity of Rac (data not shown). In contrast, 20 μM Y-27632 failed to inhibit Rac activity during platelet spreading both on collagen- and TS2/16-coated surfaces, which is in agreement with the absence of inhibitory effects on platelet spreading. These findings taken together imply that Rho/Rho kinase activation is not necessary in the process of platelet spreading.
PP1 or wortmannin, but not Y-27632, inhibits Rac activation during platelet spreading on collagen- or TS2/16-coated surfaces.
(A) Washed platelets were incubated without (control [C]) or with 20 μM PP1 (P), 100 nM wortmannin (W), or 20 μM Y-27632 (Y) for 10 minutes at 37°C. Then, they were seeded to BSA-coated or collagen-coated dishes and incubated at 30°C for 30 minutes. Reactions were terminated with lysis buffer B, and a small aliquot of each supernatant was used to check the equal loading of samples (lower panels). The rest of the lysate was used to precipitate active forms of Rac with PBD-bound glutathione Sepharose 4B (upper panels). The sample was then Western blotted with anti-Rac antibody. The data are representative of at least 3 experiments. (B) Washed platelets incubated without (control [C]) or with 20 μM PP1 (P), 100 nM wortmannin (W), or 20 μM Y-27632 (Y) for 10 min at 37°C. Then, they were added to BSA-coated or TS2/16-coated dishes and incubated at 30°C for 30 minutes. The procedure thereafter was the same as described for Figure 5A.
PP1 or wortmannin, but not Y-27632, inhibits Rac activation during platelet spreading on collagen- or TS2/16-coated surfaces.
(A) Washed platelets were incubated without (control [C]) or with 20 μM PP1 (P), 100 nM wortmannin (W), or 20 μM Y-27632 (Y) for 10 minutes at 37°C. Then, they were seeded to BSA-coated or collagen-coated dishes and incubated at 30°C for 30 minutes. Reactions were terminated with lysis buffer B, and a small aliquot of each supernatant was used to check the equal loading of samples (lower panels). The rest of the lysate was used to precipitate active forms of Rac with PBD-bound glutathione Sepharose 4B (upper panels). The sample was then Western blotted with anti-Rac antibody. The data are representative of at least 3 experiments. (B) Washed platelets incubated without (control [C]) or with 20 μM PP1 (P), 100 nM wortmannin (W), or 20 μM Y-27632 (Y) for 10 min at 37°C. Then, they were added to BSA-coated or TS2/16-coated dishes and incubated at 30°C for 30 minutes. The procedure thereafter was the same as described for Figure 5A.
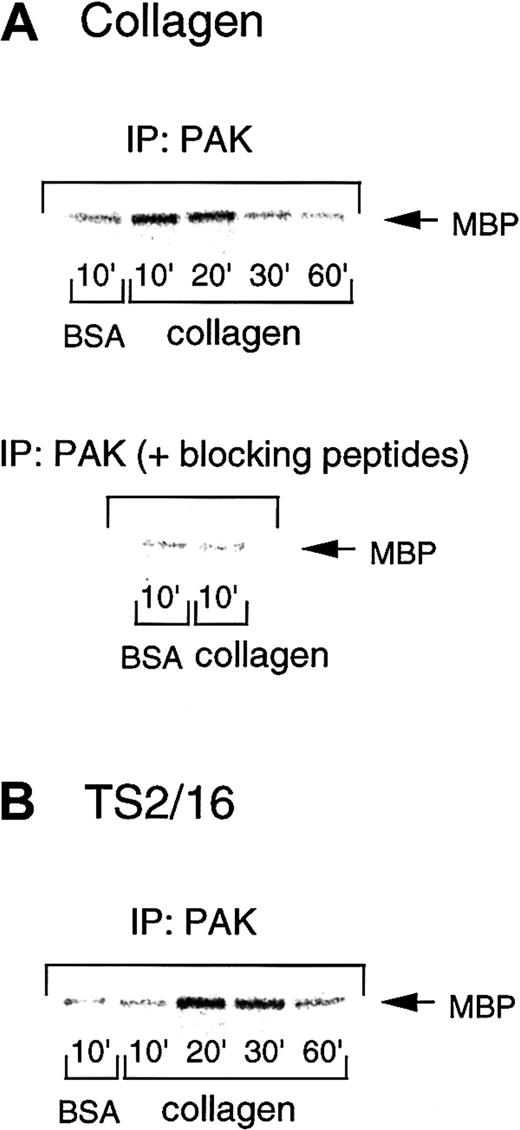
PAK is activated during platelet spreading on collagenor TS2/16-coated surfaces
Because PAK is one of the most precisely characterized effectors of Rac/Cdc42, we investigated PAK activation during platelet spreading on collagen- or TS2/16-coated plates. To address this issue, we performed in vitro kinase assays of PAK immunoprecipitates by using myelin basic protein as an exogenous substrate. The PAK activity increased 10 minutes after platelet seeding on collagen-coated surfaces and decreased after 30 minutes (Figure6A, upper panel). However, platelets had only marginal PAK activity when placed on BSA-coated plates. In some cases, PAK activity gradually increased up to 30 minutes, but it always returned to the control level by 60 minutes. With TS2/16-coated surfaces, PAK was activated at a time point (20 minutes) later than collagen-coated surfaces, then decreased after 60 minutes (Figure 6B). This is consistent with the findings that platelets on TS2/16-coated surfaces showed apparent spreading 30 minutes after seeding, which occurred later than the spreading on collagen-coated surfaces (10 minutes after seeding). In both cases, PAK activity showed about a 2- to 9-fold increase at the maximum levels, as assessed by densitometry. These findings suggest that the activation of this pathway is an early response initiated by cell adhesion that precedes cell spreading.
PAK is activated during platelet spreading on surfaces coated with collagen or TS2/16.
Washed platelets were added to collagen- (A) or TS2/16-coated (B) dishes and incubated at 30°C for the indicated time frame. After removing unbound platelets, reactions were terminated with a lysis buffer, and PAK was isolated by immunoprecipitation with anti-PAK antibody. In some experiments, an anti-PAK antibody was incubated with blocking peptides before immunoprecipitation (A, lower panel). The immunoprecipitates were then subjected to in vitro kinase assays with myelin basic protein as an exogenous substrate. The data are representative of at least 3 experiments.
PAK is activated during platelet spreading on surfaces coated with collagen or TS2/16.
Washed platelets were added to collagen- (A) or TS2/16-coated (B) dishes and incubated at 30°C for the indicated time frame. After removing unbound platelets, reactions were terminated with a lysis buffer, and PAK was isolated by immunoprecipitation with anti-PAK antibody. In some experiments, an anti-PAK antibody was incubated with blocking peptides before immunoprecipitation (A, lower panel). The immunoprecipitates were then subjected to in vitro kinase assays with myelin basic protein as an exogenous substrate. The data are representative of at least 3 experiments.
To confirm that this kinase activity was specific to PAK, we incubated the blocking peptides of this antibody (commercially available antigen peptides for the anti-PAK antibody) before the immunoprecipitation. PAK immunoprecipitates showed negligible kinase activity after blocking peptide treatment (Figure 6A, lower panel), as compared with those without the peptides (Figure 6A, upper panel). This finding confirms that the kinase activity we measured in this study represents that of PAK.
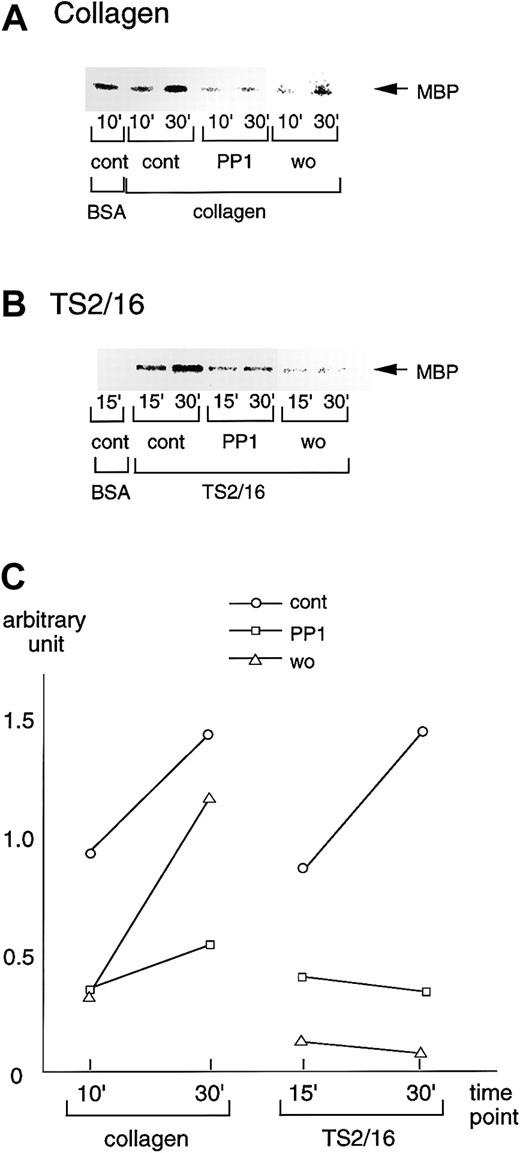
Effects of inhibitors on PAK activation during platelet spreading on collagen- or TS2/16-coated surfaces
We also sought to evaluate the effects of the inhibitors that suppressed platelet spreading on PAK activation. Consistent with the effects on platelet spreading and Rac activation, 20 μM PP1 potently inhibited PAK activation in platelets seeded on collagen-coated surfaces, whereas the inhibition by 100 nM wortmannin was marginal (Figure 7A). These findings suggest that PI3-kinase and Src family kinases contribute to PAK activation, as well as to Rac activation and platelet spreading. On TS2/16-coated surfaces, both PP1 and wortmannin, which showed inhibitory effects on platelet spreading and Rac activation induced by TS2/16, also inhibited PAK activation (Figure 7B). The inhibitory effects on PAK activation of collagen- or TS2/16-coated surfaces were quantified as shown in Figure7C. We also found that Y-27632 failed to inhibit PAK activation during platelet spreading on collagen- or TS2/16-coated surfaces (data not shown).
PP1 or wortmannin inhibits PAK activation during platelet spreading on surfaces coated with collagen or TS2/16.
(A) Washed platelets were incubated without (cont) or with 20 μM PP1 (PP1), 100 nM wortmannin (wo) for the indicated durations at 37°C. Then, they were added to BSA- or collagen-coated dishes and incubated at 30°C for 30 minutes. After removing unbound platelets, reactions were terminated with a lysis buffer. After the protein concentrations were adjusted, PAK proteins were isolated by immunoprecipitation with anti-PAK antibody. The immunoprecipitates were used for in vitro kinase assays with myelin basic protein as the exogenous substrate. The data are representative of at least 3 experiments. (B) Washed platelets were incubated without (cont) or with 20 μM PP1 (PP1), 100 nM wortmannin (wo) for 10 minutes at 37°C. The platelets were then added to BSA- or F(ab)′2 fragments of TS2/16-coated dishes and incubated at 30°C for 30 minutes. The procedures thereafter were the same as described for A. (C) Activation of PAK was quantified with Quantity One image analyzing software.
PP1 or wortmannin inhibits PAK activation during platelet spreading on surfaces coated with collagen or TS2/16.
(A) Washed platelets were incubated without (cont) or with 20 μM PP1 (PP1), 100 nM wortmannin (wo) for the indicated durations at 37°C. Then, they were added to BSA- or collagen-coated dishes and incubated at 30°C for 30 minutes. After removing unbound platelets, reactions were terminated with a lysis buffer. After the protein concentrations were adjusted, PAK proteins were isolated by immunoprecipitation with anti-PAK antibody. The immunoprecipitates were used for in vitro kinase assays with myelin basic protein as the exogenous substrate. The data are representative of at least 3 experiments. (B) Washed platelets were incubated without (cont) or with 20 μM PP1 (PP1), 100 nM wortmannin (wo) for 10 minutes at 37°C. The platelets were then added to BSA- or F(ab)′2 fragments of TS2/16-coated dishes and incubated at 30°C for 30 minutes. The procedures thereafter were the same as described for A. (C) Activation of PAK was quantified with Quantity One image analyzing software.
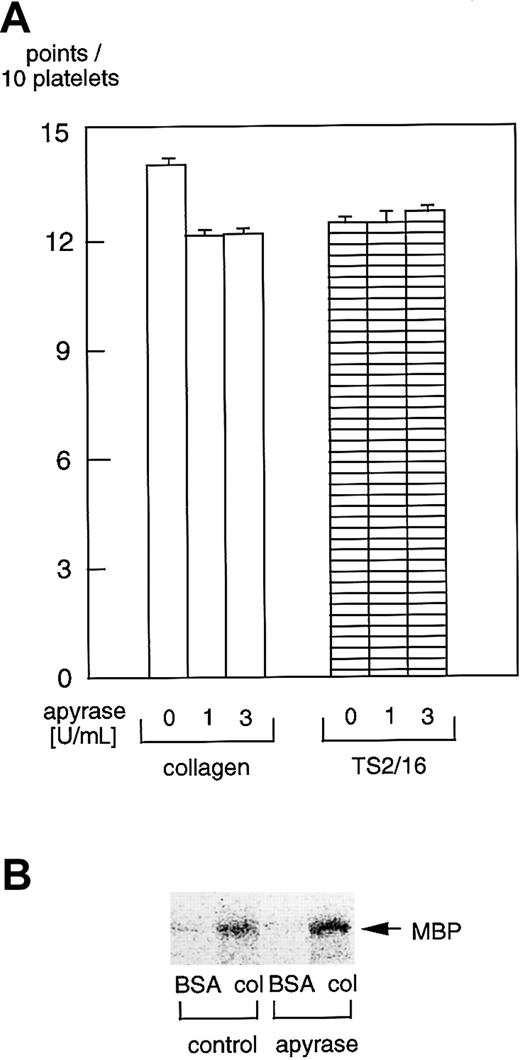
Effects of apyrase on platelet spreading and PAK activation
It was reported that ADP scavengers inhibit platelet spreading on fibrinogen-coated surfaces.27-29 Hence, we sought to evaluate the effects of apyrase, an ADP scavenger, on platelet spreading on collagen- or TS2/16-coated surfaces. There were virtually no inhibitory effects of 1 U/mL or 3 U/mL (Figure8A) apyrase on platelet spreading on collagen- or TS2/16-coated surfaces. Furthermore, 3 U/mL apyrase did not inhibit PAK activation in platelets on collagen-coated surfaces (Figure 8B) or TS2/16-coated surfaces (data not shown), which is consistent with the effects on platelet spreading. These findings suggest that ADP has little effect on platelet spreading on collagen, although it may play an important role in platelet spreading on fibrinogen.
Apyrase does not inhibit platelet spreading or PAK activation in platelets on collagen- or TS2/16-coated surfaces.
(A) Washed platelets were incubated without or with 1 U/mL or 3 U/mL apyrase for 10 minutes at 37°C. Then, they were seeded on surfaces coated with 50 μg/mL collagen or 10 μg/mL anti–integrin β1 (TS2/16) for 30 minutes at 30°C. After unbound platelets were removed, the platelets were fixed, then incubated with TRITC-conjugated phalloidin for actin fiber staining. The extent of platelet spreading on collagen-coated surfaces was tentatively evaluated as described in the legend for Figure 2. After evaluating at least more than 25 platelets, the total points for 10 platelets were calculated as the platelet spreading index. The data represent the mean ± SD of the total points. (B) Washed platelets were incubated without or with 3 U/mL apyrase for 10 minutes at 37°C. Then they were added to BSA- or collagen-coated dishes and incubated at 30°C for 10 minutes. After removing unbound platelets, reactions were terminated with a lysis buffer. After the protein concentrations were adjusted, PAK proteins were isolated by immunoprecipitation with anti-PAK antibody. The immunoprecipitates were used for in vitro kinase assays with myelin basic protein as the exogenous substrate.
Apyrase does not inhibit platelet spreading or PAK activation in platelets on collagen- or TS2/16-coated surfaces.
(A) Washed platelets were incubated without or with 1 U/mL or 3 U/mL apyrase for 10 minutes at 37°C. Then, they were seeded on surfaces coated with 50 μg/mL collagen or 10 μg/mL anti–integrin β1 (TS2/16) for 30 minutes at 30°C. After unbound platelets were removed, the platelets were fixed, then incubated with TRITC-conjugated phalloidin for actin fiber staining. The extent of platelet spreading on collagen-coated surfaces was tentatively evaluated as described in the legend for Figure 2. After evaluating at least more than 25 platelets, the total points for 10 platelets were calculated as the platelet spreading index. The data represent the mean ± SD of the total points. (B) Washed platelets were incubated without or with 3 U/mL apyrase for 10 minutes at 37°C. Then they were added to BSA- or collagen-coated dishes and incubated at 30°C for 10 minutes. After removing unbound platelets, reactions were terminated with a lysis buffer. After the protein concentrations were adjusted, PAK proteins were isolated by immunoprecipitation with anti-PAK antibody. The immunoprecipitates were used for in vitro kinase assays with myelin basic protein as the exogenous substrate.
Discussion
This study aimed to identify and characterize the signaling pathways that lead to platelet spreading mediated by collagen receptors. We showed that an anti–integrin α2 blocking antibody, but not an anti-GPIV antibody, potently inhibited platelet adhesion and spreading on collagen-coated surfaces. This finding is consistent with previous reports, which showed that platelet adhesion23 and spreading10 on collagen-coated surfaces were markedly reduced by an anti–integrin α2antibody (6F1). Our finding is also in agreement with the report of Kehrel et al,9 which showed that integrin α2-deficient platelets showed marginal spreading on collagen. These findings taken together suggest that integrin α2β1 is involved in collagen-induced platelet spreading. However, it should be noted that spreading on antibodies to the integrin α2β1 is different from that on collagen in that (1) the former occurs slower than the latter; (2) the PI3-kinase pathway plays a greater role in spreading induced by the antibodies than that induced by collagen; and (3) the pattern of spreading is distinct between the 2 stimuli, ie, platelet spreading on collagen presented a polygon shape with a number of filopodialike projections, and platelet spreading on the antibody-coated surfaces presented an almost complete round shape. These results suggest the involvement of other receptor(s) (in addition to the integrin α2β1) for collagen-induced spreading, the most likely candidate being GPVI.30 31
Similarities between platelet spreading and lamellipodia/filopodia formation induced by Rac/Cdc42 prompted us to investigate the role of Rac/Cdc42 and PAKs. We found that Rac/Cdc42 and PAK were activated during platelet spreading on collagen- or TS2/16-coated surfaces, although the precise roles of Rac/Cdc42 and PAK activation in platelet spreading remain to be determined. To the best of our knowledge, this is the first report to show Rac/Cdc42 or PAK activation during platelet spreading induced by collagen or integrin α2β1 stimulation. Rac was activated 10 minutes after platelet seeding on collagen- or TS2/16-coated surfaces, reaching its peak at 60 minutes (the longest time frame investigated). The Rac activation during platelet spreading seems to last longer than that induced by soluble ligands; Rac/Cdc42 activation increased over 30 seconds and decreased 6 minutes after TRAP (thrombin receptor–activating peptide) stimulation in suspended platelets.32 In integrin αIIbβ3-transfected Chinese hamster ovary cells, plating on fibrinogen-coated dishes induced tyrosine phosphorylation of Vav1, the guanine nucleotide exchange factor for Rac, which lasts more than 45 minutes.33 Although direct measurement of Rac activation was not performed, this finding implies the sustained activation of Rac. These findings suggest that immobilized ligands appear to induce sustained activation of Rac, whereas soluble ligands appear to induce its transient activation. PAK activation, most likely stimulated by Rac/Cdc42, occurred 10 minutes after platelet seeding and diminished after 60 minutes, when Rac was still activated. The time course of PAK activation during platelet spreading is virtually similar to that of fibronectin-stimulated PAK activation in NIH3T3 fibroblasts.15 These findings suggest that PAK is inactivated by certain mechanisms after activation by Rac/Cdc42 during platelet seeding. Modifiers of PAK were also reported in other cells, such as p85Cool-1/βPix (stimulator) or p50Cool-1 (inhibitor).34 These molecules may also participate in regulation of PAK activity in platelets.
The signaling pathway downstream of PAK activation also requires investigation. Recent studies showed that PAK activates LIM-kinase that inhibits the activity of cofilin, an actin-depolymerization factor.34-36 Yang et al37 reported that the expression of an inactive form of LIM-kinase suppressed lamellipodia formation induced by Rac or insulin. This expression may be because rapid phosphorylation/dephosphorylation cycles of cofilin (hence increased phosphatase activity as well as LIM-kinase activity) are associated with Rac stimulation.35 Cofilin is expressed in platelets38 and may serve as a PAK downstream signal to regulate platelet spreading.
We then sought to define the signal transduction pathways that lead to Rac and PAK activation and, thus, platelet spreading. To address this issue, we investigated the effects of various inhibitors on platelet spreading and activation of Rac and PAK. We found that PP1 strongly inhibited the platelet spreading on collagen- or TS2/16-coated plates. Consistent with these findings, both Rac and PAK activation were inhibited by PP1. We previously found that Src constitutively associates with integrin α2β1, and that it is activated after rhodocytin stimulation, a putative integrin α2β1 agonist.17 These findings suggest that Src, which probably lies at the most proximal step of the integrin α2β1-induced signaling pathway, seems to be crucial for activating Rac and PAK and for subsequent platelet spreading on collagen-coated surfaces.
We found that wortmannin, a PI3-kinase inhibitor, inhibited platelet spreading on collagen- or TS2/16-coated surfaces and the resultant activation of Rac and PAK. This inhibition was especially the case with TS2/16-coated surfaces. Miranti et al33 reported that a PI3-kinase inhibitor inhibited integrin αIIbβ3-mediated lamellipodia formation, but not tyrosine phosphorylation of Syk and Vav, which locate upstream of Rac activation. Missy et al39 reported that lipid products of PI3-kinase interact with Rac1 GTPase and stimulate guanosine diphosphate dissociation. Our findings are consistent with these reports and suggest that PI3-kinase also stimulates Rac activation in platelets and facilitates lamellipodia formation during platelet spreading mediated by integrin α2β1. In contrast to integrin α2β1-mediated platelet spreading, inhibition of PI3-kinase resulted in weak suppression of platelet spreading on collagen-coated surfaces and the related Rac or PAK activation. These findings suggest that there may be pathways distinct from PI3-kinase that participate in platelet spreading and Rac and PAK activation in the case of collagen stimulation. Signals from other collagen receptors, including GPVI, may be involved in the processes.
An ADP scavenger, apyrase, failed to inhibit platelet spreading and PAK activation on collagen- or TS2/16-coated surfaces. There is a considerable body of evidence that ADP plays an important role in platelet spreading on fibrinogen mediated through integrin αIIbβ3.27-29 However, our findings suggest that this is not true with platelet spreading on collagen, which is mediated at least partly through integrin α2β1. Our findings are consistent with the report by Haimovich et al27 showing that apyrase failed to inhibit protein tyrosine phosphorylation in platelets on collagen-coated surfaces, whereas it inhibited that of fibrinogen-coated surfaces.27 Although it has been reported that ADP removal reduces platelet deposition onto collagen40 or platelet adhesion on the subendothelium of rabbit aorta,41 these studies were conducted in a flow chamber system using whole blood, completely different from our study, ie, static conditions using washed platelets.
Y-27632 failed to inhibit platelet spreading on collagen-coated surfaces, whereas this Rho kinase inhibitor potently inhibited platelet shape change induced by collagen. Our findings suggest that the Rho/Rho kinase pathway plays a pivotal role in shape change induced by collagen and that it may not be required for platelet spreading on collagen-coated surfaces. We next examined the possibility that ADP released on platelet activation is responsible for collagen-induced platelet shape change through the Rho/Rho kinase pathway. ADP is reported to activate the Rho/Rho kinase pathway,25,26although there are several lines of evidence to show that ADP-induced platelet shape change is mainly dependent on Ca++, and that the Rho/Rho kinase pathway plays a minimal role.42 45 In this study, apyrase did not modify the inhibitory effect of Y-27632, suggesting that activation of Rho/Rho kinase, which leads to platelet shape change, was mediated through collagen receptors.
It has been reported that myosin light chain (MLC) phosphorylation is a crucial event in triggering shape change43 and that Rho/Rho kinase and Ca++/calmodulin-regulated MLC kinase are involved in MLC phosphorylation independently of each other.44 Rho/Rho kinase inhibits MLC phosphatase activity, which results in an increase of phosphorylated MLC.44 Bauer et al45reported that CRP-induced platelet shape change is largely dependent on Ca++/calmodulin-regulated MLC kinase, but not on Rho/Rho kinase-regulated MLC phosphatase. In that study, 20 μg/mL collagen induced only a mild increase in intracellular Ca++ in aspirin-treated platelets (data not shown) in contrast to CRP. Their findings and ours taken together suggest that Ca++/calmodulin is the main factor for platelet shape change induced by CRP, whereas Rho/Rho kinase is the main factor for platelet shape change induced by collagen. We speculate that a collagen receptor other than GPVI is responsible for Rho/Rho kinase activation during collagen-induced shape change.
In conclusion, we suggest that platelet spreading on collagen involves Rac and PAK activation, which is regulated by Src and PI3-kinase.
We thank Dr Atsushi Wada and Dr Yasuyuki Igarashi for donating BL21 transfected with pGEX-2T encoding GST fusion proteins containing PBD.
Supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Yukio Ozaki, Dept of Clinical and Laboratory Medicine, Yamanashi Medical University, Shimokato 1110, Tamaho, Nakakoma, Yamanashi 409-3898, Japan; e-mail:yozaki@res.yamanashi-med.ac.jp.



![Fig. 3. PP1 or wortmannin, but not Y-27632, inhibits platelet spreading on surfaces coated with collagen or anti–integrin β1 antibody. / Washed platelets were incubated without (control [C]) or with 20 μM PP1 (P), 20 μM Y-27632 (Y), and 100 nM wortmannin (W) for 10 minutes at 37°C. Then, they were seeded on surfaces coated with 50 μg/mL collagen (A) or 10 μg/mL anti–integrin β1 (TS2/16) (B) for 30 minutes at 30°C. After unbound platelets were removed, the platelets were fixed, then incubated with TRITC-conjugated phalloidin for actin fiber staining. (C) The extent of platelet spreading was tentatively evaluated as described in the legend for Figure 2. After evaluating more than 200 platelets, the total points for 100 platelets were used as the platelet spreading index. The data represent the mean ± SD of the total points.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/13/10.1182_blood.v98.13.3708/6/m_h82411889003.jpeg?Expires=1766067244&Signature=m5yPn4pJpC~3cJKLjSs~J-Ad1GyRWFpNrTMYTQycXYE-iMvzAxTMZyoHlqE6eTxbzWz3ddoyayCqyR55hGtPLZchdXwcFYZHiGvtVfp2Tk4EO~~FaNXLwUkpxIwQh-qyI9HHD8DJGwnQLrnosUquaPe0LPes02PYxo29RrgaoFSDdo9Xes25Myc095NYnvDI97A42e5sVCqvnp-qCgINypXH2btkFcOtAGwN2lxd-6UHhLmloHsO2sgsRmCn3ayDd3WzCbrfIePgy5Lzjw86mCtj6KBG0vNaZq6AKnTcCBMK7DydMpuVeqDuzbjzZzeBfCfjNh1ev7QYEj29czmSgA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. PP1 or wortmannin, but not Y-27632, inhibits Rac activation during platelet spreading on collagen- or TS2/16-coated surfaces. / (A) Washed platelets were incubated without (control [C]) or with 20 μM PP1 (P), 100 nM wortmannin (W), or 20 μM Y-27632 (Y) for 10 minutes at 37°C. Then, they were seeded to BSA-coated or collagen-coated dishes and incubated at 30°C for 30 minutes. Reactions were terminated with lysis buffer B, and a small aliquot of each supernatant was used to check the equal loading of samples (lower panels). The rest of the lysate was used to precipitate active forms of Rac with PBD-bound glutathione Sepharose 4B (upper panels). The sample was then Western blotted with anti-Rac antibody. The data are representative of at least 3 experiments. (B) Washed platelets incubated without (control [C]) or with 20 μM PP1 (P), 100 nM wortmannin (W), or 20 μM Y-27632 (Y) for 10 min at 37°C. Then, they were added to BSA-coated or TS2/16-coated dishes and incubated at 30°C for 30 minutes. The procedure thereafter was the same as described for Figure 5A.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/13/10.1182_blood.v98.13.3708/6/m_h82411889005.jpeg?Expires=1766067244&Signature=d68TQ2lDI8jxEQMoT44gMCpu3qiSrxmRC6XjLrOnZba5jt1FwOz98kaE0SJr1~z6n0pWwrDblVpeS1H32oVeBtR1wRHAZMm8iibwfqldjtua1NEX4nqb3~xKf7Bz8zFfSnsOMWwrzLji-F8VES7C6x~L-mStvVnQtrttwaezMomTupMyEChqMd2JC0Dhsp0dxX-FAZVXbkMnanTizdxYQZGU2KVudho~WXDj7naId8AHE7VTElDU3vKYNoWFSeaZZV6bls180~ffuZs7sHbKOgH11qOw3lZNiKYfo-7Cfe4ZtRdev0G2v9QZAf9JtntFuZJ3NsiHRmQqHLPMHRxzVA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)




![Fig. 3. PP1 or wortmannin, but not Y-27632, inhibits platelet spreading on surfaces coated with collagen or anti–integrin β1 antibody. / Washed platelets were incubated without (control [C]) or with 20 μM PP1 (P), 20 μM Y-27632 (Y), and 100 nM wortmannin (W) for 10 minutes at 37°C. Then, they were seeded on surfaces coated with 50 μg/mL collagen (A) or 10 μg/mL anti–integrin β1 (TS2/16) (B) for 30 minutes at 30°C. After unbound platelets were removed, the platelets were fixed, then incubated with TRITC-conjugated phalloidin for actin fiber staining. (C) The extent of platelet spreading was tentatively evaluated as described in the legend for Figure 2. After evaluating more than 200 platelets, the total points for 100 platelets were used as the platelet spreading index. The data represent the mean ± SD of the total points.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/13/10.1182_blood.v98.13.3708/6/m_h82411889003.jpeg?Expires=1766081931&Signature=I6QJeGIz1TOhLlypMatElmGI1Sg2McxBzZ44hxAPv8kTPEHHJnKtTcosCN8o6ERNahgAjtTlsLWuAg66EZhMfqCpFXUAnhUJ~WFF63YqiIg6Mwfw6y5scTtgFKVqyUgNgwaDx8i0v9FtFPz-Kzob6QCFZYAN4dvjD2gKodUP2HFbhA77DapKxJmbEW~S7bCjQQbIABsz5DPFZ24FiM42PBTkRpF9ai5q-g6342oki0xyCSfrXjS6O0qI1UmdNe6QVYq79YUWRyra9XJvMdowbORk8s0SfZ6kqaAwJRGOFR2p4MQaiP6nXkdvgsuVg5K-F3fQz2NobPkMCMpMUwxivA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. PP1 or wortmannin, but not Y-27632, inhibits Rac activation during platelet spreading on collagen- or TS2/16-coated surfaces. / (A) Washed platelets were incubated without (control [C]) or with 20 μM PP1 (P), 100 nM wortmannin (W), or 20 μM Y-27632 (Y) for 10 minutes at 37°C. Then, they were seeded to BSA-coated or collagen-coated dishes and incubated at 30°C for 30 minutes. Reactions were terminated with lysis buffer B, and a small aliquot of each supernatant was used to check the equal loading of samples (lower panels). The rest of the lysate was used to precipitate active forms of Rac with PBD-bound glutathione Sepharose 4B (upper panels). The sample was then Western blotted with anti-Rac antibody. The data are representative of at least 3 experiments. (B) Washed platelets incubated without (control [C]) or with 20 μM PP1 (P), 100 nM wortmannin (W), or 20 μM Y-27632 (Y) for 10 min at 37°C. Then, they were added to BSA-coated or TS2/16-coated dishes and incubated at 30°C for 30 minutes. The procedure thereafter was the same as described for Figure 5A.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/13/10.1182_blood.v98.13.3708/6/m_h82411889005.jpeg?Expires=1766081931&Signature=aEHMsRJ-bqnitY20apZNKwwysCoXhUxeRdeatRAdXTUjD-HlIspi1h4ogujrI3i2K82WPASlxlYKIfte36B3c4L2noJFagSbP9BQaDtgEDQjVa513OKI8nAKB810HL-vMggageS4zVOyqYV8fOoZUFCqJo4eErWCVyfmQ493HRCoQ2yZmdruMHsPp9SxSHZTD5CKZ7VWULPB8cULpMh6L6RYBamNGcppsul91iq8mnbdc-joe~UE3HpmYstcECzB9DNu4zooDWEzXlGrr4KocZWeqUw~UPobZZIUD19E0LVBBiZlz4t-h53Pcrgv5JHpCsNNtGgr152wXtIlCL8SOg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)