Thirty patients with chronic active Epstein-Barr virus (CAEBV) infection were analyzed. The study group included 18 male and 12 female patients, ranging in age from 5 to 31 years with a mean age of 14.2 years. Not all patients had high titers of EBV-specific antibodies, but all patients had high viral loads in their peripheral blood (more than 102.5 copies/μg DNA). Fifty percent of the patients displayed chromosomal aberrations, and 79% had monoclonality of EBV. Patients were divided into 2 clinically distinct groups, based on whether the predominantly infected cells in their peripheral blood were T cells or natural killer (NK) cells. Over a 68-month period of observation, 10 patients died from hepatic failure, malignant lymphoma, or other causes. Patients with T-cell CAEBV had a shorter survival time than those with NK-cell type of disease.
Introduction
Epstein-Barr virus (EBV) is ubiquitous in humans with most individuals being infected by early adulthood. Primary EBV infection is usually asymptomatic, but sometimes it results in infectious mononucleosis, which resolves spontaneously after the emergence of EBV-specific immunity.1,2 EBV causes chronic infections in apparently immunocompetent hosts. Chronic active EBV (CAEBV) infection was characterized by chronic or recurrent infectious mononucleosis–like symptoms persisting over a long time and by an unusual pattern of anti-EBV antibodies.3 Patients with this disease have no evidence of any prior immunologic abnormalities or of any other recent infection that might explain their condition.3,4 CAEBV is a disease with a high mortality and high morbidity with life-threatening complications, such as virus-associated hemophagocytic syndrome, interstitial pneumonia, lymphoma, coronary artery aneurysms, and central nervous system involvement.5-9
Straus4 defined the 3 main criteria of CAEBV infection as follows: (1) severe illness lasting more than 6 months that began as a primary EBV infection and that was associated with grossly abnormal EBV antibody titers, antiviral capsid antigens (VCA) immunoglobulin G (IgG) at least 5120, anti–early antigens (EA) IgG at least 640, or anti-EB nuclear antigens (EBNA) less than 2; (2) histologic evidence of major organ involvement such as interstitial pneumonia, hypoplasia of some bone marrow elements, uveitis, lymphadenitis, persistent hepatitis or splenomegaly; and (3) increased quantities of EBV in affected tissues. Subsequently, Okano and his colleagues8 proposed similar criteria to diagnose severe CAEBV infection. Importantly, many cases have been reported that do not satisfy the criteria described above. Some patients lack abnormal patterns of EBV-related antibodies, whereas other patients lack major organ involvement and have only skin symptoms, such as hypersensitivity to mosquito bites (HMB) or hydroa vacciniforme–like eruptions.10,11 On the other hand, we and others have reported patients with CAEBV infection had extremely high viral loads, as assessed by quantitative polymerase chain reaction (PCR).12,13 Moreover, accumulating evidence suggests that clonal expansion of EBV-infected T or natural killer (NK) cells could be associated with CAEBV infection.6,7,10 14-17 A review of the criteria for CAEBV infection appears to be necessary, based on these recent findings.
The purpose of this study was to elucidate the pathogenesis of CAEBV infection and to formulate more precisely the disease criteria to facilitate the development of more effective treatments. We analyzed 30 cases of CAEBV infection and found that about one third of the patients did not meet the proposed criteria of unusual patterns of EBV-related antibodies, but that all patients had high viral loads in their peripheral blood. Patients were divided into 2 groups, T-cell type and NK-cell type, based on the predominant infected cell population found in the peripheral blood. Distinct clinical features and prognoses were defined for each of these groups.
Patients and methods
Patients
Thirty patients, referred to the Nagoya University School of Medicine with suspected CAEBV infection, were enrolled in this study. All the patients fulfilled the following diagnostic criteria: (1) Illness of more than 6 months' duration: EBV-related illness or symptoms including fever, persistent hepatitis, extensive lymphadenopathy, hepatosplenomegaly, pancytopenia, uveitis, interstitial pneumonia, hydroa vacciniforme, or HMB. (2) Increased quantities of EBV in either affected tissues or peripheral blood. The amount of EBV was defined as increased when one or more of the following criteria were met: (a) EBV DNA was detected in tissues or peripheral blood by Southern blot hybridization, (b) EB-encoded small RNA 1 (EBER1) RNA+ cells were detected in tissues or peripheral blood, or (c) more than 102.5 copies/μg DNA were detected in peripheral blood mononuclear cells (PBMC).12 (3) No evidence of any prior immunologic abnormalities or of any other recent infection that might explain the condition. All the results for patients examined were negative for human immunodeficiency virus antibody.
Bone marrow aspiration was done in most cases, and various findings were obtained. Some patients showed normocellular marrow without any abnormal findings; others showed hypocellular marrow with mild or moderate hemophagocytosis. In all patients, however, bone marrow findings showed an absence of hematologic malignant disorders at the diagnosis of CAEBV infection.
Our disease criteria did not include antibody titers; therefore, patients with normal patterns of EBV antibodies were enrolled in this study. Patients who had 2 or more life-threatening complications were defined as having severe disease. Patients who had either one or no complications were defined as suffering mild disease.
Samples
Whole blood obtained from patients was centrifuged and separated into plasma and cell fractions. All the samples were obtained after informed consent from either patients or their parents. The cell fraction was separated into PBMC on Ficoll-Paque density gradients (Pharmacia Biotech, Piscataway, NJ). DNA was extracted from either 2 × 106 PBMC or 200 μL plasma using a QIAamp Blood Kit (Qiagen, Hilden, Germany) and resuspended in 50 μL distilled water.
Quantitation of EBV DNA
A real-time quantitative PCR assay with a fluorogenic probe was performed using the TaqMan PCR kit and a Model 7700 Sequence Detector (PE Applied Biosystems, Foster City, CA), as previously described.12 18 The amount of EBV DNA was calculated as the number of virus copies per microgram PBMC DNA or per milliliter plasma. The EBV detection limit in this assay was approximately 10 copies/μg DNA for PBMC and 50 copies/mL for plasma.
Determination of predominantly infected cells in peripheral blood
To determine which cells harbored EBV, the PBMC were fractionated into CD3+, CD4+, CD8+, CD16+, or CD19+ cells using an immunobeads method (DynaBeads; Dynal A/S, Oslo, Norway). The fractionated cells were analyzed by either quantitative PCR or an in situ hybridization assay. The in situ hybridization assay was performed using the EBER1 probe as described previously.12 Patients were defined as having the T-cell type of disease when mainly CD3+ cells gave a positive hybridization signal with EBER1 or had larger amounts of EBV DNA than other cells in the blood sample. Patients were defined as having NK-cell type when their CD16+ cells were the major group of cells harboring EBV.
Southern blot hybridization
The clonality of EBV was determined by Southern blotting using a terminal repeat probe as previously described.15 19Genomic DNA extracted from PBMC was digested with BamHI, subjected to gel electrophoresis, transferred to a nylon membrane, hybridized with a 32P-labeled Xho1 fragment from the terminal region of EBV, and visualized by autoradiography.
Detection of chromosomal abnormalities
The PBMC were cultured in the presence of 300 U/mL interleukin (IL) 2 for 3 to 5 days. The cells were subjected to standard cytogenetic procedures, without stimulation with phytohemagglutinin, and 20 cells in metaphase per sample were stained and counted.
Geneticanalysis of theSAP/SH2D1A gene
Analysis of the SAP/SH2D1A gene was performed as previously described.20 Briefly, DNA was extracted from PBMC, and 4 exons of the SAP/SH2D1A gene were amplified by PCR. The sequencing reaction was performed using the BigDye terminator cycle sequencing kit (PE Applied Biosystems) with an automated ABI PRISM 310 genetic analyzer (PE Applied Biosystems).
Statistical analyses
The 2-tailed Student t test was used to compare the means of clinical and laboratory data for each group. The Fisher exact test was used for the comparison of clinical differences within groups. A regression analysis was used to compare EBV DNA copy numbers in PBMC and plasma. The probability of survival was estimated by the Kaplan-Meier method. The differences between 2 groups were studied by a log-rank test. P values less than .05 were considered statistically significant.
Results
Clinical manifestations of CAEBV infection
The clinical characteristics of each patient are summarized in Table 1. Detailed clinical features of some patients have been previously described elsewhere.19,21,22 The study group consisted of 18 male and 12 female patients, ranging in age from 5 to 31 years (mean, 14.2 years). The age at onset of CAEBV infection was 1 to 27 years (mean, 8.3 years), and at time of onset 14 of 30 patients had an infectious mononucleosis–like illness. Three of the latter patients developed viral-associated hemophagocytic syndrome at that time. The treatment for each patient is shown in the Table 1. Seven patients underwent allogeneic stem cell transplantation during the period of observation. The indications for transplantation were malignant lymphoma for 4 patients, intractable hemophagocytic syndrome for 2 patients, and acute leukemia for 1 patient. During the observation period ranging from 15 to 261 months (mean, 68 months), 10 patients died; causes of death were hepatic failure (3 patients), malignant lymphoma (2 patients), transplantation-related complications (2 patients), and sepsis, myocardial infarction, and hemophagocytic syndrome (1 patient each). The overall frequencies of individual symptoms and life-threatening complications are summarized in Table 2. HMB, which was recently shown to be one of the features in CAEBV infection,10 was observed in about 40% of the patients. Calcification in the basal ganglia21 was observed in approximately 20% of the patients. Four patients had hydroa vacciniforme–like eruptions.
EBV-related antibody titers at time of diagnosis
The EBV-related antibody titers of each patient are shown in Table1. Although most patients had high antibody titers against VCA IgG and EA IgG, only a limited number of patients fulfilled the criteria of Straus (ie, VCA IgG ≧ 5120, EA IgG ≧ 640, negative results for EBNA).4 Indeed, about one third of the patients (patients 7, 9, 14, 17, 18, 23, 24, 25, and 30) did not fulfill any of the above criteria.
Viral load in peripheral blood
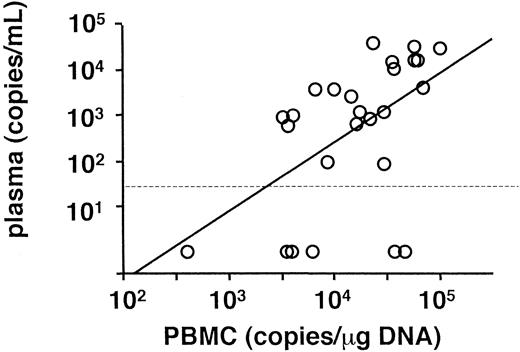
Peripheral blood was obtained from each patient on referral, and viral load was determined by real-time quantitative PCR (Table 1). The findings in all patients were positive for EBV DNA in PBMC and had EBV loads greater than 102.5 copies/μg DNA. On the other hand, findings in 6 patients were negative for plasma EBV DNA, although copy numbers of EBV DNA in PBMC and plasma generally correlated with each other (Figure 1). The mean copy numbers of EBV DNA were 104.2 copies/μg DNA in PBMC and 102.7 copies/mL in plasma.
Correlation of EBV DNA genome number between PBMC and plasma.
A regression analysis was used for the comparison (r = 0.52,P = .006). A dotted line shows the detection limit for plasma.
Correlation of EBV DNA genome number between PBMC and plasma.
A regression analysis was used for the comparison (r = 0.52,P = .006). A dotted line shows the detection limit for plasma.
We also studied whether viral loads correlated with disease severity. Patients were divided into 2 groups based on the number of life-threatening complications, listed in Table 2. Patients who had 2 or more life-threatening complications had higher viral loads (104.5 ± 0.4 copies/μg DNA in PBMC and 103.5 ± 1.0 in plasma; values are mean ± SD) compared with patients with mild disease severity (104.1 ± 0.6 copies/μg DNA in PBMC and 102.2 ± 1.7 copies/mL in plasma). The differences between the 2 groups were statistically significant in PBMC (P = .046 for PBMC values and P = .06 for plasma values).
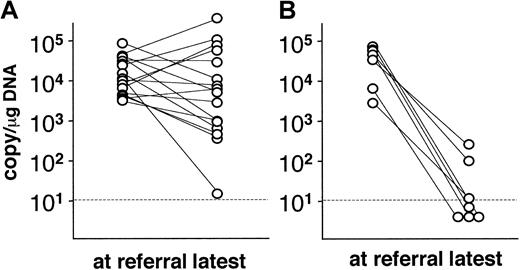
In 23 patients, the viral loads were measured serially over observation periods ranging from 4 to 72 months (mean, 20 months). Alterations in the viral load are shown in Figure 2. Viral loads in patients who had not undergone transplantation did not change appreciably during this period, except in the case of a single patient (Figure 2A). Significantly, 7 patients who had undergone stem cell transplantation showed significant decreases in viral load (< 102.5 copies/μg DNA) after transplantation. Although 4 of these patients died within 60 days of transplantation, the other 3 patients have maintained low viral loads and have been free of CAEBV-related symptoms.
Alterations in the viral load in PBMC.
Viral load was serially monitored in 23 patients and compared to the level at referral. (A) Patients without hematopoietic stem cell transplantation (n = 16). (B) Patients receiving transplants (n = 7). Dotted lines show the detection limit.
Alterations in the viral load in PBMC.
Viral load was serially monitored in 23 patients and compared to the level at referral. (A) Patients without hematopoietic stem cell transplantation (n = 16). (B) Patients receiving transplants (n = 7). Dotted lines show the detection limit.
SAP/SH2D1A gene analysis
X-linked lymphoproliferative syndrome (XLP), which is characterized by severe and often fatal infectious mononucleosis,23 has been shown to result from a defect in the SAP/SH2D1A gene.24-26 Because clinical characteristics of CAEBV infection might mimic those of XLP, we analyzed the SAP/SH2D1A gene of all 18 male patients in our study by direct sequencing. However, no mutations were found in the coding region of the gene, indicating that CAEBV infection might be differentiated from XLP.
Clonality and chromosomal examination
The clonality of EBV in 19 patients was analyzed by Southern blot hybridization using an EBV terminal repeat probe and 15 (79%) were defined as monoclonal (Table 1). We examined chromosomal material from isolated PBMC. Preliminary experiments showed that a standard chromosomal examination with phytohemagglutinin was normal. Because T cells and NK cells are reported to harbor EBV in patients with CAEBV infection, we stimulated PBMC in culture with IL-2 and then carried out the cytogenetic procedures. The PBMCs of 50% (10 of 20) of the patients examined in this way exhibited aberrant chromosomal patterns (Table 3). Some patients had multiple, different aberrations. There was no chromosomal specificity in patterns or accumulations. The clinical and virologic features were compared between patients with or without chromosomal aberrations. Although no statistically significant differences were observed between the 2 groups, patients who had chromosomal aberrations appeared to have higher rate of malignancy (40% versus 10%, P = .15). Other features were not different between the 2 groups.
T- and NK-cell types of CAEBV
To determine which cells were infected with EBV, PBMC were fractionated into CD3+, CD4+, CD8+, CD16+, or CD19+ cells and analyzed by either quantitative PCR or in situ hybridization. The results show that 16 of 30 patients were T-cell type (Table 1), because their CD3+cells were the predominant carriers of EBV. The T-cell type of patients were further classified as either CD4+ (3 patients) or CD8+ (2 patients). Twelve patients were classified as NK-cell type (Table 1), because their CD16+ cells, and not their CD3+ cells, were the main sources of EBV. None of patients showed predominant B-cell infection with EBV, and 2 patients could not be classified.
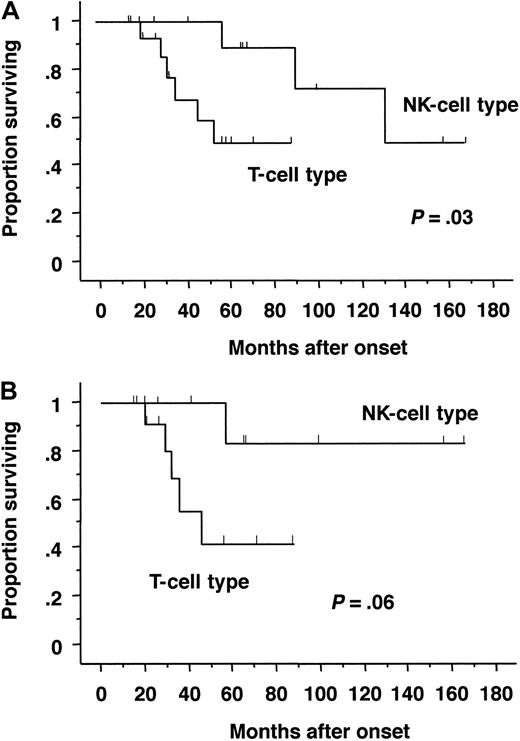
The symptoms and laboratory or virologic characteristics of the T-cell and NK-cell types were compared (Table4). The T-cell type of CAEBV infection was characterized by frequent high fever and high titers of VCA IgG and EA IgG. On the other hand, patients with the NK-cell type of infection were characterized by HMB, large granular lymphocytosis, and high IgE titers. The most striking difference observed between these 2 types was survival probability. Patients with the T-cell type of CAEBV infection had shorter survival times than those with NK-cell type of infection (Figure 3A). Because stem cell transplantation dramatically influences outcome, patients with transplantation were excluded and analyzed. Although statistical significance diminished because of the decrease of patient number, similar results were obtained (Figure 3B).
Probability of survival by Kaplan-Meier estimates.
Survival rates were compared between patients with the T-cell type and NK-cell type of CAEBV infection. The differences between groups were analyzed using a log-rank test. (A) All patients (T-cell type, 16 cases; NK-cell type, 12 cases). Probability of survival at 5 years was 0.55 ± 0.15 for T-cell type and 0.88 ± 0.11 for NK-cell type, respectively. (B) Patients without transplantation (T-cell type, 13 cases; NK-cell type, 9 cases). Probability of survival at 5 years was 0.41 ± 0.18 for T-cell type and 0.83 ± 0.15 for NK-cell type, respectively.
Probability of survival by Kaplan-Meier estimates.
Survival rates were compared between patients with the T-cell type and NK-cell type of CAEBV infection. The differences between groups were analyzed using a log-rank test. (A) All patients (T-cell type, 16 cases; NK-cell type, 12 cases). Probability of survival at 5 years was 0.55 ± 0.15 for T-cell type and 0.88 ± 0.11 for NK-cell type, respectively. (B) Patients without transplantation (T-cell type, 13 cases; NK-cell type, 9 cases). Probability of survival at 5 years was 0.41 ± 0.18 for T-cell type and 0.83 ± 0.15 for NK-cell type, respectively.
Discussion
An accumulating body of evidence suggests that clonal expansion of EBV-infected T or NK cells is associated with CAEBV infection.6,7,10,14-17 In our study, most of the patients with CAEBV belonged to either the T- or NK-cell type. In healthy carriers, EBV exists latently in resting memory B cells.2It is unclear whether the invasion of blood cells other than B cells causes CAEBV infection or the invasion is an ordinary event, but the host's immunologic abnormalities allow the expansion of these cells. Recently, a defect in the SAP/SH2D1A gene was implicated in XLP, a fatal lymphoproliferative disorder.24-26 Patients with XLP are exclusively boys in whom primary infection with EBV causes lymphoproliferation and severe hepatitis, mimicking CAEBV infection.23,27 Therefore, we examined theSAP/SH2D1A gene in all the male subjects enrolled in our study, but did not find any abnormalities. However, it is possible that a defect in another gene essential for regulating lymphocyte activation and proliferation may be a cause of CAEBV infection. Such a defect or single nucleotide polymorphism might influence the function of virus-specific or nonspecific lymphocytes and thereby allow the expansion of EBV-infected T or NK cells. It has been reported that EBV-infected T and NK cells express a limited range of viral antigens.1,2 EBV-infected B cells express at least 9 antigens, some of which are antigenic enough to be presented to cytotoxic T lymphocytes. This is called latent infection type 1. EBV-infected T and NK cells express only EB nuclear antigen 1 and latent membrane protein 1; this is called latent infection type 2.1,2 The expression of viral antigens was examined in some of our patients, and they were type 2.15 19 Because these 2 proteins are less antigenic, infected cells can evade from immune surveillance by cytotoxic T lymphocytes. Therefore, they may proliferate and cause chronic infection.
There were several noteworthy differences between the T- and NK-cell types of CAEBV infection. T-cell type of disease was characterized by fever and high titers of EBV-related antibodies, whereas HMB and high IgE were observed in patients with the NK-cell type of CAEBV infection. Although not statistically significant, patients with T-cell type of CAEBV infection had higher IgG levels than those with NK-cell type. It remains unclear whether these 2 manifestations of disease represent different entities or simply appear different because of the nature of the infected cells. It is possible that EBV-infected T cells become activated and release inflammatory cytokines such as interferon- γ, IL-6, or tumor necrosis factor-α (TNF-α), resulting in severe inflammation and fever. In EBV-related hemophagocytic syndrome, it has been shown that viral-infected T cells release TNF-α and activate macrophages, resulting in massive hemophagocytosis.28 29These activated T cells might induce polyclonal B-cell activation through cytokine release and thereby induce high levels of IgG and EBV-related antibodies. On the other hand, it is unclear why HMB and high IgE were observed in patients with NK-cell type of CAEBV infection.
We should emphasize that determining the cell type is important in predicting disease prognosis, because the survival rates were different between the 2 groups. Differences in survival rates are particularly important in assessing treatment choices. To date, a treatment for CAEBV infection has not yet been established. Antiviral or immunomodulating agents, such as acyclovir, gancyclovir, vidarabine, interferon-α, and IL-2, have been tried.30-33 We reported the adoptive transfer of virus-specific cytotoxic T lymphocytes to a patient with CAEBV infection.34 However, most of these reports were anecdotal and there are few confirmatory reports. Immunochemotherapy consisting of etoposide, steroids, and cyclosporin A was proposed for use in patients with advanced CAEBV infection,35 but no evidence of its efficacy is available. Recently, successful treatment of CAEBV infection by allogeneic bone marrow transplantation has been reported.22 In our study, it is clear that viral loads decreased in all patients receiving transplants, some of whom appeared to be cured. However, hematopoietic stem cell transplantation constitutes a considerable risk, because 4 of 7 patients died after transplantation. Prospective studies analyzing a larger number of patients with CAEBV infection are necessary to confirm the efficacy of transplantation and to establish safer conditioning regimens.
Oshima and colleagues36 reported that chromosomal abnormality occurred in the lymph nodes of patients with CAEBV. In this study, 50% of the patients examined had chromosomal aberrations in their peripheral blood cells and 79% of patients showed monoclonality of EBV. Because there were no specific patterns of genetic aberration, and some patients displayed several different aberrations, the chromosomal abnormalities seen in patients with CAEBV infection might only reflect chromosomal fragility. However, clonality of the EBV genome and chromosomal aberrations generally indicate clonal expansion of EBV-infected cells. These results indicate that clonal expansion is a common feature of CAEBV infection, and this disease might therefore be considered lymphoproliferative rather than infectious.35 In fact, patients with CAEBV infection frequently develop neoplasms such as malignant lymphoma. It would therefore be better to call the cases presented here “chronic EBV–associated lymphoproliferative disorders.”
We analyzed patterns of EBV-related antibodies in 30 patients with CAEBV infection and found that approximately one third of them did not meet the previously accepted definition of CAEBV infection.4 8 These patients had symptoms typical for CAEBV infection and had extremely high EBV loads in their peripheral blood. We postulate that high titers of EBV-related antibody are not always necessary for the diagnosis of CAEBV infection. On the other hand, all the patients had more than 102.5 copies/μg EBV DNA in PBMC. Furthermore, it is of note that the copy number of EBV DNA in PBMC decreased below 102.5 copies/μg DNA in all 7 patients who underwent hematopoietic stem cell transplantation. These results indicate that viral loads greater than102.5copies/μg DNA can be used not only as diagnostic factors for CAEBV infection, but also as predictors of therapeutic efficacy.
Previous reports show that patients with CAEBV infection had cell-free EBV DNA in plasma, although the origin of the viral DNA is unclear.37,38 Usually, the plasma of healthy individuals does not contain EBV DNA.39 40 Therefore, the presence of EBV DNA in plasma may have significance for the diagnosis of CAEBV infection. However, it should be borne in mind that the plasma of patients with CAEBV infection did not always test positive for EBV DNA. Indeed, in this study, EBV DNA was not detected in plasma from 6 patients, whereas they had large amounts of EBV DNA in their PBMC. We recommend that PBMC, and not plasma, be used for diagnosing and monitoring patients with CAEBV infection. It is unclear why some patients did not have cell-free EBV DNA. We sometimes observed that patients with negative plasma results turned positive at a later visit. Cell-free viral DNA may fluctuate from day to day. The other possibility is that inhibitors in plasma might influence PCR reactions and cause false-negative results.
In conclusion, patients with CAEBV infection were divisible into 2 subgroups: T-cell and NK-cell CAEBV infection. Each group had different clinical features and prognosis. A high titer of EBV-related antibodies is not always a prerequisite for the diagnosis of CAEBV infection. Viral load, detected by quantitative PCR in PBMC, was useful for disease diagnosis and as an indicator of therapeutic efficacy. We propose that a viral load exceeding 102.5 copies/μg DNA be used as a diagnostic criterion for CAEBV infection.
We thank Dr Stephen E. Straus, National Institutes of Health, for critical reading of the manuscript. We thank the following people for contributions to the study: Mitsuaki Hosoya, Atushi Kikuta (Fukushima Medical University), Masahiro Sako (Osaka City General Hospital), Tsutomu Oh-ishi (Saitama Children Medical Center), Yasunobu Takeoka (Osaka City University), Shiro Oshima (Osaka University), Miho Maeda (Nippon Medical School), Yutaka Nishimura (Toyohashi City Hospital), Hiroko Kurozumi (Yokohama Minami Kyosai Hospital), Shinichi Toyabe (Niigata University), Yutaka Morita (Kariya General Hospital), Yuichi Hasegawa (Tsukuba University), Chikako Kanazawa (Yamagata University), Hiroyuki Moriuchi (Nagasaki University), Reiko Ito (Ogaki City Hospital), and Hitoshi Kiyoi and Tomoki Naoe (Nagoya University).
Supported by a grant from the Japan Society for the Promotion of Science (JSPS-RFTF97L00703). Presented in part at the 25th International Herpesvirus Workshop, Portland, OR, July 2000.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hiroshi Kimura, Dept of Pediatrics, Nagoya University Graduate School of Medicine, 65 Tsurumai-cho, Showa-ku, Nagoya 466-8550, Japan; e-mail: hkimura@med.nagoya-u.ac.jp.