Abstract
In most human somatic cells telomeres progressively shorten with each cell division eventually leading to chromosomal instability and cell senescence. The loss of telomere repeats with cell divisions may also limit the replicative life span of antigen-specific T lymphocytes. Recent studies have shown that the replicative life span of various primary human cells can be prolonged by induced expression of the telomerase reverse transcriptase (hTERT) gene. To test whether introduction of hTERT can extend the life span of primary human T lymphocytes, naive CD8+ T lymphocytes were transfected with retroviral vectors containing the hTERTgene. Transduced T-cell clones expressed high levels of telomerase and either maintained or elongated their telomere lengths upon culture for extended periods of time. Two of the transduced subclones retained a normal cloning efficiency for more than 170 population doublings (PDs). In contrast, T-cell clones transfected with control vectors exhibited progressive telomere length shortening and stopped proliferation at around 108 PDs. Telomerase-positive T clones had a normal 46,XY karyotype, maintained their cytotoxic properties, and showed very little staining for the apoptotic marker annexin-V. These results indicate that ectopic hTERT gene expression is capable of extending the replicative life span of primary human CD8+cytotoxic T lymphocytes.
Introduction
After an antigenic challenge, naive T lymphocytes undergo differentiation and proliferation to become effector and memory T cells. T effector cells are the progeny of a limited number of antigen-specific precursor cells, and dramatic expansions of CD8+ T cells to achieve adequate immune responses have been described in several viral models.1,2 Moreover, memory responses are characterized by repetitive expansion of antigen-specific T-cell clones.3 Limitations in the proliferative life span of such clones could eventually lead to immune senescence4 and contribute to the increased incidence of infectious diseases observed in elderly individuals.
Telomeres are specialized structures at the end of eukaryotic chromosomes whose length decreases with cell divisions in vitro and in vivo.5,6 Recently, experiments have shown that artificial telomere elongation by transfection of the human telomerase reverse transcriptase (hTERT) gene results in extension of telomeres and increased proliferative life span of fibroblasts and of retinal pigment epithelial cells.7,8 Moreover, those cells appear indistinguishable from young normal cells and do not show signs of chromosomal aberrations or cellular transformations.9 10 Thus, these results have provided evidence for a direct linkage between telomere shortening and cellular senescence.
Despite transient telomerase activity upon mitogenic and antigen stimulation,11-13 cells of the immune system also show telomere shortening with age and with cellular replication in vitro.14-16 Indeed, lymphocytes maintained in culture have a finite life span and undergo replicative senescence after 30 to 50 population doublings (PDs; reviewed in reference 4). Moreover, the telomere length of memory CD4 and CD8 T lymphocytes is significantly shorter than that of naive T cells.16,17 Relative short telomere lengths have been found in lymphocytes from individuals with Down syndrome,15 in the memory CD4 and CD8 subsets of elderly individuals,18 and in the CD8+CD28− subset of normal individuals19 and those infected with human immunodeficiency virus.20 Taken together, most observations support the notion that telomere length reflects the replicative history of T cells and suggest that the proliferative life span of particular T-cell clones could be restricted by progressive telomere shortening.
Here we show that introduction of hTERT in human CD8+ T clones is able to maintain or elongate the telomere lengths during cell divisions and significantly extends their proliferative life span. Moreover, the hTERT-transduced T cells retained their cytotoxic properties and had a normal 46,XY karyotype, and 2 of the clones studied did not present any signs of apoptosis or senescence after more than 170 PDs.
Materials and methods
Isolation of naive and memory CD8+ T cells from peripheral blood
Peripheral blood mononuclear cells (PBMCs) were obtained following density centrifugation using Ficoll-Hypaque (Pharmacia, Uppsala, Sweden). The PBMCs (107) isolated from a male healthy individual were stained with anti-CD8 (allophycocyanin; Becton Dickinson, San Jose, CA), CD45RA-PE (phycoerythrin), and CD27-FITC (fluorescein isothiocyanate; Becton Dickinson). Naive CD8+CD45RA+CD27+ T lymphocytes were then sorted by fluorescence-activated cell sorting (FACStar Plus, Becton Dickinson) and subjected to phytohemagglutinin (PHA) T-cell cloning.
T-cell cloning and culture
Monoclonal T cells were obtained by limiting dilution cultures (1 cell/well) in Terasaki 60-well microculture plates (Nunc, Glostrup, Denmark). Cells were cultured in 20 μL RPMI 1640 medium (Gibco, Grand Island, NY) containing 10% human serum (HS) supplemented with 1 μg/mL PHA (Murex Biotech, Dartford, United Kingdom), 100 U/mL recombinant interleukin 2 (rIL-2, Roche, Nutley, NJ) and 1 × 106/mL irradiated (3000 rad) allogeneic mononuclear feeder cells. After 8 to 10 days of culture, positive wells were transferred into 96 U-bottom plates in RPMI 1640 medium containing 10% HS and 100 U/mL rIL-2. After one additional round of stimulation with PHA (1 μg/mL), irradiated female feeder cells (1 × 106/mL), and rIL-2 (100 U/mL), enough cells (1-2 × 106) were obtained for telomere length analysis and further subcloning. PDs were calculated from the average cell count in the 8 T subclones chosen for telomere length measurements, using the following equation: PDs = log10 (average cell count after expansion) – log10 (1 cell seeded per well) / log10.
Retrovirus-mediated hTERT transduction in cytotoxic T clones
Gene transfer was achieved using retrovirus-mediated gene transduction. Murine stem cell virus (MSCV)–based retroviral vectors21 containing the gene for enhanced green fluorescent protein (GFP; Clontech, Palo Alto, CA) under the control of PGK promoter with or without the full-length hTERT complementary DNA (cDNA; kindly provided by Dr Robert Weinberg, Massachusetts Institute of Technology, Boston, MA) were constructed. Helper-free retrovirus pseudotyped with the Gibbon Ape leukemia virus envelope for efficient infection of human cells was generated using PG13 packaging cells.22 The progeny of one single naive CD8+T clone (N11) obtained by limiting-dilution was split and cultured for several days with either supernatant of virus containing the GFP control vector or with supernatant containing the hTERT vector. T cells expressing high GFP levels were sorted and recloned. The efficiency of transduction was estimated at around 1% to 3% from cells expressing GFP. Six subclones transduced either with the GFP control virus (G11.8 and G11.1) or with the hTERT/GFP virus (T11.9, T11.14, T11.7, and T11.10) were randomly selected and used for further subsequent cloning steps.
Telomerase repeat amplification protocol assay
Telomerase activity was measured by telomerase repeat amplification protocol (TRAP) assay using an end-labeled telomerase substrate (TS) primer as described.23 Cell extracts were obtained from a positive control cell line (HeLa) and from 1 × 104 quiescent T cells present in cultures 15 days after initial stimulation with 1.0 μg/mL PHA, 100 U/mL rIL-2, and 1 × 106/mL irradiated feeder cells. Extension of the TS primer by telomerase was performed for 30 minutes at 30°C, and the products generated were amplified by 27 cycles of polymerase chain reaction (PCR) at 94°C for 30 seconds and 60°C for 30 seconds using the ACX anchored return primer. One fourth of the amplified products were resolved on a 12% polyacrylamide gel, visualized by a phosphoimaging system and analyzed using Aida 1/D Evaluation software (Raytest USA, Wilmington, NC).
Telomere fluorescence in situ hybridization and flow cytometry
The average length of telomere repeats at chromosome ends in individual cells from T-lymphocyte clones and subclones was measured by fluorescence in situ hybridization (FISH) and flow cytometry (flow FISH) as previously described.16 FITC-labeled fluorescent calibration beads (Quantum TM-24 Premixed; Flow Cytometry Standards Corporation, San Juan, Puerto Rico) were used to convert telomere fluorescence data to molecules of equivalent soluble fluorescence (MESF) units. To estimate the telomere length in base pairs from telomere fluorescence in MESF units, the slope of the calibration curve described previously for lymphocyte subsets16 (y = 0.019x) was used in the following equation: (bp = MESF × 0.02604 × 0.019 × 103). Of note, GFP fluorescence of the T-cell clones did not interfere with telomere fluorescence measurements, because GFP fluorescence did not survive the denaturation step in the flow FISH protocol (10 minutes at 82°C in 70% formamide solution).
Quantitative FISH on metaphase chromosomes
The T-lymphocyte clones were cultured in the presence of 1.0 μg/mL PHA, 100 U/mL rIL-2, and 1 × 106/mL irradiated allogeneic feeder cells for 4 to 5 days before addition of colcemid for 1 hour. Cells were then treated with hypotonic KCl for 50 minutes at 37°C and fixed in methanol-acetic acid. Quantitative FISH on metaphase chromosomes (Q-FISH) was performed with Cy-3 labeled (CCCTAA)3 PNA probe and subsequent quantitative analysis of digital images as previously described.24 Briefly, slides were observed with an Axioplan (Zeiss, Thornwood, NY) microscope equipped with a CCD camera. Separate images were captured for DAPI and Cy-3, then subjected to telomere fluorescence measurements using the TFL-Telo software.25 Individual telomere length measurements were analyzed in 10 metaphases per sample. Data are expressed in telomere fluorescence units (TFU) with each unit corresponding to 1 kb of T2AG3 repeats as described.26
Annexin-V staining
The 105 T clones were incubated with annexin-V PE (Becton Dickinson) for 20 minutes, washed, resuspended in a phosphate-buffered saline (PBS) buffer containing propidium iodide at 10 μg/mL and analyzed on a FACSCalibur flow cytometer (Becton Dickinson).
Results
Transfer of hTERT gene into human CD8+T lymphocytes
Repetitive expansion of antigen-specific T-cell clones could be restricted by progressive telomere shortening. To test whether the replicative life span of human T lymphocytes could be prolonged by expression of hTERT, the progeny of one naive CD8+ T-cell clone (N11) was transduced with MCSV-based retroviral vectors containing the gene for GFP with or without full-length hTERT cDNA. GFP+ cells were sorted and recloned and 4 subclones containing the hTERT/GFP construct (T11.9, T11.14, T11.7, and T11.10) and 2 subclones containing the GFP control construct (G11.8 and G11.1) were randomly selected for further study and recloning. After each recloning step, the telomere fluorescence in 8 subclones was analyzed and a single subclone with average telomere length was chosen for subsequent recloning. This process was repeated until no further subclones could be obtained (GFP controls) or when this paper was submitted (hTERT/GFP clones).
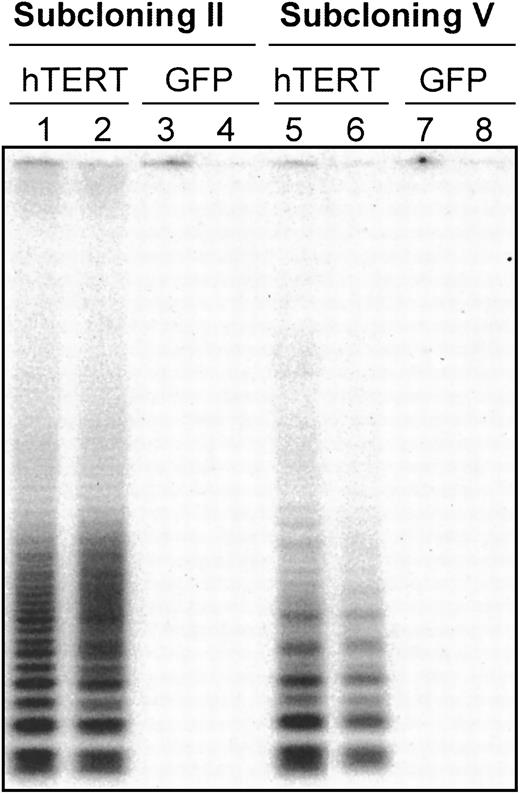
Telomerase expression was analyzed in 4 T-cell subclones (T11.9, T11.14, G11.8, and G11.1) by TRAP assay at a time point when essentially all the cells were quiescent (15 days after stimulation). After the second cloning step, high levels of telomerase activity were found in hTERT/GFP-transduced subclones T11.9 and T11.14, whereas subclones transduced with GFP only showed very low or undetectable levels of telomerase activity (Figure 1, lines 1-4). Moreover, the integration of the hTERT construct and its expression was relatively stable over time and after repeated rounds of cloning steps in these 2 hTERT-transduced T-cell clones. Thus, after the fifth generation of subclones, the cells transduced with hTERT/GFP still expressed at least 100-fold higher levels of telomerase activity than GFP-transduced control cells (Figure 1, lanes 5-8).
Telomerase activity in CD8
+ T clones transduced with a GFP control vector or with an hTERT/GFP vector. The 104 transduced cells after cloning step II (lanes 1-4) and V (lanes 5-8) were analyzed for telomerase expression by the TRAP assay.23 The 4 subclones are depicted as follows: T11.9 = lanes 1 and 5, T11.14 = lanes 2 and 6, G11.8 = lanes 3 and 7, G11.1 = lanes 4 and 8. T11.9 and T11.14 are clones transduced with hTERT construct, whereas G11.8 and G11.1 are clones transduced with the GFP control vector. Positive control extracts obtained from HeLa cell line and negative controls using RNAse-treated extracts were used in each experiment (data not shown).
Telomerase activity in CD8
+ T clones transduced with a GFP control vector or with an hTERT/GFP vector. The 104 transduced cells after cloning step II (lanes 1-4) and V (lanes 5-8) were analyzed for telomerase expression by the TRAP assay.23 The 4 subclones are depicted as follows: T11.9 = lanes 1 and 5, T11.14 = lanes 2 and 6, G11.8 = lanes 3 and 7, G11.1 = lanes 4 and 8. T11.9 and T11.14 are clones transduced with hTERT construct, whereas G11.8 and G11.1 are clones transduced with the GFP control vector. Positive control extracts obtained from HeLa cell line and negative controls using RNAse-treated extracts were used in each experiment (data not shown).
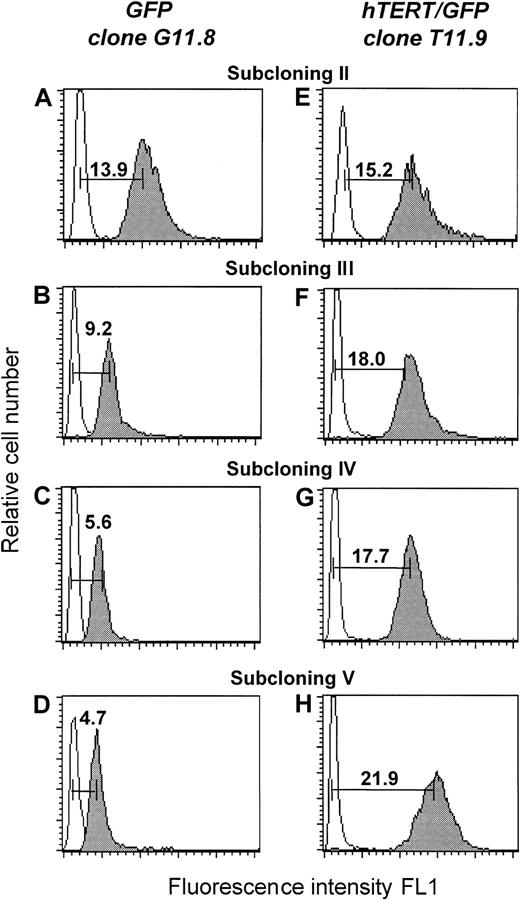
Telomere length elongation in serial passages of CD8+ T subclones after transduction with hTERT
The average length of telomere repeats at chromosome ends was measured in serial passages of transfected T-cell clones by flow FISH as previously described.16,18 As shown in Figure2, a progressive decline in telomere fluorescence was observed for clone G11.8 and its subclones (Figure 2A-D). As the number of PDs increased, the mean telomere fluorescence intensity of the 2 GFP-transduced subclones decreased from 15 kMESF to 5.5 kMESF (corresponding to about 2.7 kb; Figure3A). The telomere loss corresponded to a calculated telomere length shortening of approximately 60 bp/PD, comparable to the 50- to 100-bp/PD reported for in vitro cultured fibroblasts.6
Teleomere fluorescence.
Telomere fluorescence is shown from subclone G11.8 transduced with the GFP vector (A-D) and subclone T11.9 transduced with the hTERT/GFP construct (E-F). At each cloning step (II-V), the telomere fluorescence of a representative clone is depicted. The telomere fluorescence was calculated by subtracting the mean background fluorescence from the mean fluorescence obtained with the telomere probe and is expressed in kMESF units as described in “Material and methods” (1 kMESF unit corresponds to approximately 0.5 kb).
Teleomere fluorescence.
Telomere fluorescence is shown from subclone G11.8 transduced with the GFP vector (A-D) and subclone T11.9 transduced with the hTERT/GFP construct (E-F). At each cloning step (II-V), the telomere fluorescence of a representative clone is depicted. The telomere fluorescence was calculated by subtracting the mean background fluorescence from the mean fluorescence obtained with the telomere probe and is expressed in kMESF units as described in “Material and methods” (1 kMESF unit corresponds to approximately 0.5 kb).
Transduction with GFP and hTERT/GFP.
(A) Progressive loss of telomere fluorescence in subclones transduced with GFP vector (G11.8 and G11.1). (B) Elongation or maintenance of telomere fluorescence in subclones transduced with hTERT/GFP vector (T11.9, T11.14, T11.7 and T11.10). Eight clones were analyzed by flow FISH after each cloning (mean ± SD) and a single clone with average telomere length was picked for subsequent recloning; bp indicates base pair; PD, population doublings; n = 5, telomere fluorescence analysis of only 5 clones. Arrows represent the replicative senescence stage observed after the fifth round of cloning in subclones G11.1 and G11.8.
Transduction with GFP and hTERT/GFP.
(A) Progressive loss of telomere fluorescence in subclones transduced with GFP vector (G11.8 and G11.1). (B) Elongation or maintenance of telomere fluorescence in subclones transduced with hTERT/GFP vector (T11.9, T11.14, T11.7 and T11.10). Eight clones were analyzed by flow FISH after each cloning (mean ± SD) and a single clone with average telomere length was picked for subsequent recloning; bp indicates base pair; PD, population doublings; n = 5, telomere fluorescence analysis of only 5 clones. Arrows represent the replicative senescence stage observed after the fifth round of cloning in subclones G11.1 and G11.8.
In contrast, increased or stable telomere fluorescence was observed in the clones transduced with the hTERT/GFP construct (Figure 2E-H). The mean telomere fluorescence of clone T11.9 increased from 14.3 kMESF to 21.7 kMESF, corresponding to a gain of +33 bp/PD. Interestingly, although a significant increase in telomere fluorescence was also observed in subclones T11.7 and T11.10 (with a gain of around 25 bp/PD), the telomere fluorescence of clone T11.14 and its subclones was maintained at a mean telomere fluorescence of 15 kMESF (corresponding to 7.5 kb, Figure 3B). In summary, our results show that induced expression of hTERT into CD8+ T cells resulted in functional levels of telomerase activity that led in the majority of the clones (3 of 4) to significant telomere length elongation.
Long-term cultures of T lymphocytes often show multiple chromosomal abnormalities.27 To study chromosomes in the transfected T-cell clones, cytogenetic analysis was performed on clones (T11.9, T11.14, G11.8, and G11.1) from subcloning step III to VII using G-band preparations (data not shown). Early and late passage of GFP and hTERT/GFP transduced subclones all contained 46,XY normal chromosomes.
Induced expression of hTERT extends the life span of CD8+ T lymphocytes
To investigate the effect of increased hTERT expression on the life span of lymphocytes, the growth potential of hTERT/GFP and GFP subclones was compared. Both control GFP-transduced clones, G11.8 and G11.1, stopped dividing after the fifth round of cloning after a calculated total of 108 PDs (Table 1). Moreover, the cloning efficiency in those subclones dropped suddenly after the fifth cloning step and none or very few clones were produced beyond that point. In addition, the clones isolated from those late passages exhibited a reduced capacity to enter into the S phase of the cell cycle as determined by 3H-thymidine incorporation assays (data not shown). In contrast, hTERT-transduced subclones T11.9 and T11.7 had a growth potential that exceeded the life span of GFP-transduced clones by at least 3 further cloning steps or an estimated 67 additional PDs (Figure 3B; Table 1). Cloning efficiency for the hTERT-transduced clones T11.9 and T11.7 remained respectable (13% and 20%, respectively) after the eighth cloning step and no reduced proliferative responses were observed by3H-thymidine assays (Table 1 and data not shown). Similar results were observed for subclone T11.10 with a normal cloning efficiency at 23% after the sixth round of recloning. This subclone has now exceeded the maximum number of PDs observed with the GFP-control–transduced T-cell clones (135 PDs versus 108 PDs) and is still growing.
Subclone T11.14 shared most of the features found in senescent clones transduced with the GFP control vector. Indeed, although this subclone had a life span that exceeded that of the control cells by one additional cloning step, the cloning efficiency in T11.14 cells dropped from 40% to 2% after the seventh cloning step and a reduced proliferative capacity was already observed after the fifth cloning round (Table 1). However, most of the subclones generated after this sixth cloning step could be further maintained in culture for the following 4 months while cells divided with less than 2 PDs/wk (data not shown). Surprisingly, a higher growth rate was observed in bulk cultures of late passage T11.14 cells and telomere length analysis showed a significant increase in telomere fluorescence from 14.9 kMESF to 21.5 kMESF (corresponding to a gain of about 2.9 kb). In summary, ectopic expression of hTERT led to a dramatic extension of the life span of subclones T11.9, T11.7, and T11.10 when compared to the GFP control clones. Furthermore, whereas its expression was not sufficient to sustain continued rounds of cloning in T11.14 subclones, these hTERT-transduced cells still displayed a growth advantage over the control clones and subclones with extended telomeres emerged in mass culture.
Distribution of chromosome-specific telomere fluorescence in the hTERT/GFP- transduced subclones
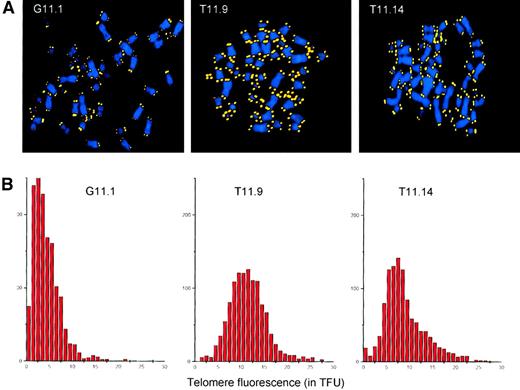
In normal human fibroblasts, short telomeres accumulate prior to senescence.28 A possible mechanism for the extended life span of the hTERT-transfected lymphocyte subclones could be the elongation of the shortest telomeres by telomerase. To test this hypothesis, we analyzed the telomere lengths at single chromosomes in metaphase spreads from the GFP-transduced subclone G11.1 and the hTERT/GFP-transduced subclones T11.9 and T11.14 by Q-FISH.26 As depicted in Figure4, the subclones transduced with the GFP control construct showed an overall decline in telomere fluorescence. Furthermore, these cells showed accumulation of short telomeres (Figure4A) and a skewed overall distribution of telomere fluorescence (Figure4B) similar to presenescent fibroblasts.28 In contrast, the average telomere fluorescence of the hTERT-transduced subclone T11.9 was equivalent to 11.5 kb with a normal overall distribution of the telomere fluorescence at chromosome ends. Thus, ectopic expression of hTERT had a direct effect on each chromosome end by preventing the accumulation of short telomeres and maintaining them at a stable length during culture for more than 170 PDs. Finally, subclone T11.14 that maintained its telomere lengths and showed signs of senescence after 125 PDs, presented a skewed distribution of telomere fluorescence. Although some chromosomes exhibited bright telomere fluorescence, an increased number of chromosomes with short telomeres was observed in T11.14 cells, with an average telomere fluorescence (corresponding to 8.9 kb) that was lower than measured in subclone T11.9.
Telomere analysis of transduced T-cell clones.
(A) Q-FISH analysis of metaphase chromosomes isolated from subclones transduced with the GFP vector (G11.1, second cloning step) and the TERT/GFP vector (T11.9 and T11.14, sixth cloning step). DAPI-stained chromosomes and Cy-3–labeled telomeres are shown in blue and yellow, respectively. (B) Distribution of TFUs in metaphase chromosomes from subclones transduced with GFP vector (G11.1) and TERT/GFP vector (T11.9 and T11.14). The average telomere fluorescence at individual chromosome ends was calculated to be 4.3 TFU for G11.1, 11.5 TFU for subclone T11.9, and 8.9 TFU for subclone T11.14. These values correspond closely to estimates of the average telomere length in these cells obtained by flow FISH (respectively 10.9, 21.7, and 14.9 kMESF units corresponding to an estimated18 5.4, 10.9, and 7.5 kb of telomere repeats for G11.1, T11.9, and T11.14 cells).
Telomere analysis of transduced T-cell clones.
(A) Q-FISH analysis of metaphase chromosomes isolated from subclones transduced with the GFP vector (G11.1, second cloning step) and the TERT/GFP vector (T11.9 and T11.14, sixth cloning step). DAPI-stained chromosomes and Cy-3–labeled telomeres are shown in blue and yellow, respectively. (B) Distribution of TFUs in metaphase chromosomes from subclones transduced with GFP vector (G11.1) and TERT/GFP vector (T11.9 and T11.14). The average telomere fluorescence at individual chromosome ends was calculated to be 4.3 TFU for G11.1, 11.5 TFU for subclone T11.9, and 8.9 TFU for subclone T11.14. These values correspond closely to estimates of the average telomere length in these cells obtained by flow FISH (respectively 10.9, 21.7, and 14.9 kMESF units corresponding to an estimated18 5.4, 10.9, and 7.5 kb of telomere repeats for G11.1, T11.9, and T11.14 cells).
Expression of CD28 on transfected T-cell clones
The costimulatory CD28 receptor molecule triggers CD28/B7 signal transduction allowing T-cell proliferation and expansion (reviewed in reference 29). Various reports have associated the progressive increase in T cells that lack expression of CD28 with aging in vivo as well as culture in vitro (reviewed in reference 30). Indeed, in bulk cultures of CD8+ T lymphocytes, more than 95% of the cells were CD28− at the time of senescence.31 The immortalized subclones generated in this study after the induction of hTERT expression represent a unique model to further analyze the correlation between CD28 and immunosenescence. The GFP and hTERT/GFP transduced subclones were stained with anti-CD28 monoclonal antibodies between the second and the eighth round of recloning. Although a low proportion of lymphocytes still expressed CD28 at cloning step II, all subclones had lost the CD28 cell surface marker by the time they had reached the fourth round of cloning (data not shown). Moreover, subclones from T11.9 that had a significant extension of life span further remained CD28− after the eighth cloning step. These results show that the lack of CD28 expression did not correlate with in vitro replicative senescence and that hTERT-transduced T-cell clones were capable of extensive rounds of cellular division without re-expression of CD28.
Ectopic expression of hTERT prevents cellular senescence in CD8+ T clones
To define phenotypic changes associated with cellular aging and replicative senescence, we stained the GFP-transduced T subclones and the hTERT/GFP-transduced subclones after the fifth cloning step with the apoptotic marker annexin-V and propidium iodide (PI). The percentage of annexin V+ and PI+ cells in GFP-transduced subclones (G11.8, 37% ± 11%; G11.1, 55% ± 12%) was significantly increased when compared to the hTERT-transduced clones T11.14 (18% ± 8%) and particularly T11.9 (6% ± 3%). These results suggest that the control T clones undergo increased cell death after about 100 PDs. On the contrary, the induced expression of hTERT in subclone T11.9 prevented cellular senescence and apoptosis. All subclones transduced either with the GFP or the hTERT/GFP construct retained the ability to kill the tumor cell line P815 (data not shown).
Discussion
The results described in this report allow several conclusions. The expansion potential of the control transfected CD8+ T clones exceeded more than 100 PDs, similar to the previously reported life span of the CD4+ T subset.16 Our data point to an enormous growth potential of CD8+ T-cell clones in culture. This extensive replicative capacity may be necessary to allow repeated rounds of proliferation of antigen-specific clones during successive immune responses.3 Although transient expression of telomerase could provide a partial compensation for the telomere loss in cultured T-cell clones, progressive telomere shortening and finally senescence was observed in all control (GFP-transfected) clones analyzed. Moreover, overexpression of telomerase activity in T-cell clones led to a significant extension of the life span in those cells. Two subclones, T11.9 and T11.7, displayed normal cloning efficiency after more than 170 PDs, while retaining a normal 46,XY karyotype and a phenotype similar to that of younger cells. Indeed, the average telomere lengths found in both clones and their subclones were similar to those of cord blood lymphocytes.18 Together, these data strongly support the hypothesis that telomere length in T-cell clones acts as a mitotic clock that monitors cell division and determines the eventual replicative capacity in T cells.
The regulation of telomerase activity seems to be more complex in peripheral blood T lymphocytes than in other somatic cells such as fibroblasts because T cells express low levels of endogenous hTERT.11,13 In this study, ectopic expression of hTERT allowed significant telomere length elongation in subclones T11.9, T11.7, and T11.10, whereas telomeres were maintained but not extended in subclone T11.14. In this clone, the replicative life span was extended by only one additional round of recloning. After that no more subclones were produced but the cells were further maintained in less constrained culture conditions without recloning. These observations could be explained by a low level of hTERT expression, for example, resulting from a particular integration site of the construct in genomic DNA or by silencing of ectopic gene expression. However, no difference in levels of telomerase expression was observed between subclones T11.9 and T11.14 by TRAP assay (Figure 1, data not shown). One possibility is that the TRAP assay is incapable of detecting small, but biologically relevant, differences in telomerase activity and telomerase levels in subclone T11.14 may have been insufficient to maintain the length of more than a limited number of short telomeres. Indeed, the decline of cellular growth may have been triggered in T11.14 cells as a consequence of the accumulation of short telomeres as it is most probably also the case for subclone G11.1 transduced with the GFP control construct (Figure 4). However, the analysis of telomere length at the level of single chromosomes did not reveal an increased number of short telomeres in subclone T11.14. Furthermore, the average telomere length after the sixth round of cloning in this clone was significantly longer than in subclone G11.1 (8.8 versus 4.3 TFU, respectively). These observations indicate that telomere length is only indirectly related to the induction of replicative senescence in T-cell clones as was previously shown for human32 and bovine fibroblasts.33 It is possible that senescence in clone T11.14 may have been triggered by telomere-independent mechanisms. Several studies have shown that induction of hTERT expression alone is not sufficient to immortalize all human cell types34,35and further studies are needed to dissect all the pathways involved in the induction of cellular senescence in T-cell clones.36
Recently, the effect of hTERT transfection on the life span of human CD8+ T lymphocytes was described in 2 independent reports.37,38 Although Migliaccio and colleagues37 found that ectopic expression of hTERT was not sufficient to immortalize bulk cultures of T lymphocytes, Hooijberg and coworkers showed an extended life span of 2 cytotoxic T lymphocyte (CTL) clones derived from a patient with melanoma.38 Our own data confirm that transfer ofhTERT gene into T-cell clones can result in an extension of their replicative potential and suggest that a very high level of sustained telomerase activity may be required to elongate both the telomere length and the replicative life span of primary T cells. Whereas Hooijberg and coworkers showed a relatively short follow up of 12 weeks,38 we furthermore show that 2 hTERT-transduced T-cell clones could be maintained for more than 170 PDs (equivalent to 10 months of sequential subcloning). Moreover, analysis of the telomere length at the level of single chromosome ends revealed that high levels of ectopic telomerase prevented the accumulation of short chromosomes and increased the telomere lengths to those found in cord blood T lymphocytes.
Although further studies are clearly needed to understand the precise role of telomere shortening and telomerase in lymphocyte biology, our results indicate that exogenous telomerase expression can result in telomere extension and elongation of the replicative life span of primary human T cells.
We thank Colette Grand, Solange Visher, and Elizabeth A. Chavez for excellent technical assistance. Dr Christine Cabrol and Jacqueline Tedesco are thanked for G-banded chromosome preparations and karyotyping analysis. We thank Dr Irène Garcia for helpful discussion. Dr Robert Weinberg and Dr Robert Hawley kindly provided the hTERT cDNA and MSCV vector, respectively.
Supported by a grant of the Swiss National Science Foundation (31-53774.98), a grant of the foundation Dr Henry Dubois-Ferrière-Dinu Lipatti, grant AI29524 from the National Institutes of Health, and a grant from the National Cancer Institute of Canada with funds from the Terry Fox Run.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Nathalie Rufer, Laboratory of AIDS Immunopathogenesis Hopital Beaumont, Ave Beaumont 29, CH-1011 Lausanne, Switzerland; e-mail: nathalie.rufer@chuv.hospvd.ch; or Peter M. Lansdorp, Terry Fox Laboratory, BC Cancer Agency, 601 W 10th Ave, Vancouver, BC, V5Z 1L3, Canada; e-mail: plansdor@bccancer.bc.ca.