Abstract
Development of T and natural killer (NK) cells is critically dependent on cytokine signaling, and defects in cytokine receptor complex subunits have been shown to result in severe combined immunodeficiency (SCID) syndromes in humans and in murine models. An infant boy had typical clinical features of SCID and was found to lack NK cells in his peripheral circulation. Molecular analysis did not reveal abnormalities in his γc or JAK-3 genes, and he was investigated for defects in the interleukin-15 (IL-15) receptor complex because functional IL-15 signaling is essential for NK cell development. Expression of the IL-2R/IL-15Rβ chain was significantly reduced in the patient's peripheral blood mononuclear cells (PBMCs) by immunoblot, flow cytometry, and Northern blot analysis. Furthermore, IL-2 stimulation of PBMCs showed only minimal tyrosine phosphorylation of JAK-3. These data demonstrate that defects in IL-2R/1L-15Rβ expression can lead to a unique NK-deficient SCID immunophenotype.
Introduction
Severe combined immunodeficiency (SCID) syndromes are a heterogeneous group of conditions arising from defects in the development and function of T-, B-, and natural killer (NK)–cell populations. The molecular basis of many of the immunologic phenotypes has now been identified1 but remains unclear in a significant number of patients. Cytokine receptor signaling pathways are essential in the early stages of lymphocyte development, and defects in these pathways have been shown to result in SCID phenotypes in murine models and in humans. The X-linked form of SCID is caused by mutations in the common γ-chain (γc) subunit, which is a component of the high-affinity interleukin-2 (IL-2), -4, -7, -9, and -15 receptors.2,3 This abnormality results in the absence of T-cell and NK-cell development and an as yet uncharacterized defect of B cells (denoted T−B+NK− SCID). Other components of this signaling pathway are also affected in certain forms of SCID. The JAK-3 tyrosine kinase binds directly to and is activated by γc after receptor stimulation and is defective in the autosomal recessive form of T−B+NK− SCID.4IL-7 receptor α mutations result in a more lineage-restricted T−B+NK+ form of SCID, thus illustrating the essential role of IL-7 signaling in T-lymphocyte development.5
Evidence suggests that the development and survival of NK cells is dependent on a functional IL-15/IL-15 receptor-signaling pathway. The IL-15 receptor consists of a unique IL-15Rα chain that combines with a β chain (which is shared with the IL-2R complex and is termed IL-2R/IL-15Rβ) and γc subunits to create a high-affinity receptor complex. Mice with homozygous mutations in any of these 3 receptor subunits,6-8 in the IL-15 cytokine itself,9or in interferon regulatory factor-110 (IRF-1, a transcription factor necessary for IL-15 expression) show profound defects of NK cell development and survival. In contrast, mice deficient in IL-2, IL-2Rα, or IL-7R have normal numbers of NK cells, suggesting a specific requirement for IL-15 signaling in NK cell development.11-13 In this article, we describe a child in whom abnormalities in the IL-15R complex resulted in a unique NK-deficient form of SCID.
Study design
Staining and fluorescence-activated cell-sorting analysis
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque density centrifugation, washed, and resuspended in 1 mL RPMI 1640 (Life Technologies, Gaithersburg, MD). Then 5 μL of the indicated antibodies were added to 100 μL cell suspension and analyzed according to standardized protocols (Becton Dickinson, Oxford, United Kingdom).
JAK-3 activation
PBMCs from 5 mL EDTA blood were resuspended in 1 mL RPMI. One hundred units IL-2 (Chiron, Harefield, United Kingdom) was added, and a JAK-3 tyrosine phosphorylation assay was performed as previously described.14
Immunoblots
PBMCs were isolated and lysed in NP40 lysis buffer. Immunoblotting was performed according to previously described protocols.15 The primary antibodies (anti–IL-2R/IL-15Rβ, anti–IL-15Rα, anti–IL-15, and JAK-3) (Santa Cruz Biotechnology, Santa Cruz, CA) were used at a concentration of 10μL in 1 mL milk–PBS-T (phosphate-buffered saline with Tween). For all protein expression and functional assays, age-matched control samples were analyzed.
Northern blot analysis
Northern blot analysis of IL-2R β messenger RNA (mRNA) was performed as described previously.16
Analysis of genomic DNA for exon-intron boundary defects
The exon-intron boundaries were investigated in genomic DNA by single-stranded conformational polymorphism analysis (SSCP) or sequencing of the coding exons.2-10 Primers were designed in-house from the genomic sequence (GenBank GI 4090209/AL022314) (primer sequences and polymerase chain reaction conditions available on application). SSCP conditions have been previously decribed,17 and sequencing used an ABI377 automated sequencer and Big Dye Chemistry (PE Applied Biosystems, Warrington, United Kingdom).
Results and discussion
An infant boy, P1, the second child of nonconsanguineous parents, had severe viral and fungal infections. In the first year of life, he had recurrent episodes of respiratory syncytial virus (RSV) bronchiolitis, Candida enteritis with significant failure to thrive, hepatomegaly, and an episode of meningo-encephalitis for which no causative organism was found. Investigation of his immune system, detailed in Table 1, showed a T↓B+ NK− immunophenotype with poor specific antibody production. His clinical condition stabilized after courses of antibacterial, antifungal, and antiviral therapy, and he underwent successful allogeneic unrelated donor bone marrow transplantation at 17 months of age with good immune reconstitution and resolution of clinical symptoms.
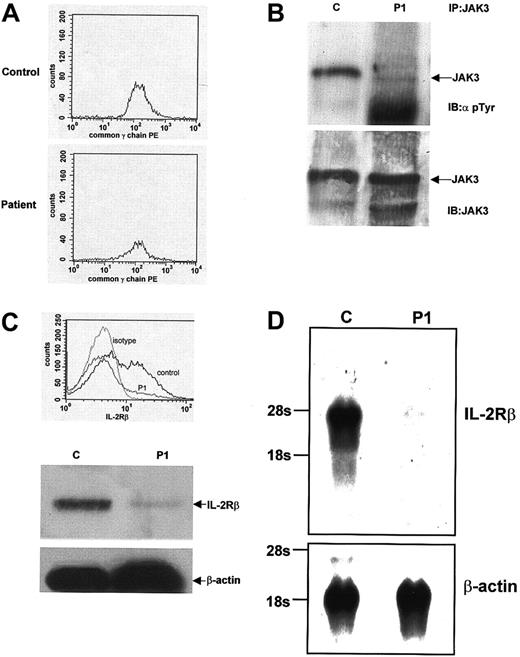
In view of the low T-cell numbers and lack of NK cell development, the patient was suspected to have an atypical form of γc- orJAK-3–deficient SCID that resulted in a small amount of T- cell development, consistent with previous reports.18 19Flow cytometric analysis of PBMCs showed normal expression of γc (Figure 1A). Screening of the gene by (SSCP) did not reveal any abnormalities, thus making a diagnosis of X-SCID highly unlikely. To investigate JAK-3 activation and expression, we examined tyrosine phosphorylation in response to IL-2 stimulation of PBMCs. A control sample showed normal JAK-3 tyrosine phosphorylation, but only minimal JAK-3 phosphorylation was detectable in PBMCs from P1 (Figure 1B, top panel). Immunoblotting with anti–JAK-3 antibody demonstrated that P1 expressed normal amounts of JAK-3 (Figure 1B, bottom panel). The JAK-3 gene was further screened by SSCP analysis and did not show any abnormalities. From these initial studies, we concluded that although there was no abnormality in either the γc or the JAK-3 molecules, the lack of JAK-3 tyrosine phosphorylation indicated a significant abnormality in signaling through the IL-2R complex.
IL-2 signaling and IL-2R/IL-15Rβ expression abnormalities in T↓B+NK− SCID.
(A) Flow cytometric analysis of PBMCs for cell surface expression of γc. Equivalent amounts of γc expression are seen in both a control sample and sample from P1. (B) Tyrosine phosphorylation of JAK-3 after IL-2 stimulation. PBMCs from a control and P1 were stimulated with IL-2, lysed, and immunoprecipitated using an anti–JAK-3 antibody. JAK-3 immunoprecipitates were then immunoblotted using an antiphosphotyrosine antibody. Normal JAK-3 tyrosine phosphorylation is seen in the control sample, but only minimal tyrosine phosphorylation is seen in P1 (top panel). The filter was stripped and reblotted with an anti–JAK-3 antibody, and the blot shows equivalent amounts of JAK-3 expression in both samples (bottom panel). (C) Flow cytometric and Western blot analysis of IL-2R/IL-15Rβ expression. PBMCs from a control and P1 were analyzed by flow cytometry for cell surface expression of IL-2R/IL-15Rβ. Normal expression is seen in a control sample, but significantly decreased expression is seen in the sample from P1. Western blot analyses of whole cell lysates from a control and P1 also show decreased expression of IL-2R/IL-15Rβ. Stripping the filter and reblotting with an anti–β-actin antibody shows equivalent protein loading in each lane. (D) Northern blot analysis ofIL-2R/IL-15Rβ expression. mRNA prepared from PBMCs from a control subject and from P1. Northern blot analysis using anIL-2R/IL-15Rβ cDNA probe shows 7% of control IL-2R/IL-15Rβ expression in the sample from P1. Equivalent mRNA loading was demonstrated after stripping and reblotting the filter with a probe for β-actin.
IL-2 signaling and IL-2R/IL-15Rβ expression abnormalities in T↓B+NK− SCID.
(A) Flow cytometric analysis of PBMCs for cell surface expression of γc. Equivalent amounts of γc expression are seen in both a control sample and sample from P1. (B) Tyrosine phosphorylation of JAK-3 after IL-2 stimulation. PBMCs from a control and P1 were stimulated with IL-2, lysed, and immunoprecipitated using an anti–JAK-3 antibody. JAK-3 immunoprecipitates were then immunoblotted using an antiphosphotyrosine antibody. Normal JAK-3 tyrosine phosphorylation is seen in the control sample, but only minimal tyrosine phosphorylation is seen in P1 (top panel). The filter was stripped and reblotted with an anti–JAK-3 antibody, and the blot shows equivalent amounts of JAK-3 expression in both samples (bottom panel). (C) Flow cytometric and Western blot analysis of IL-2R/IL-15Rβ expression. PBMCs from a control and P1 were analyzed by flow cytometry for cell surface expression of IL-2R/IL-15Rβ. Normal expression is seen in a control sample, but significantly decreased expression is seen in the sample from P1. Western blot analyses of whole cell lysates from a control and P1 also show decreased expression of IL-2R/IL-15Rβ. Stripping the filter and reblotting with an anti–β-actin antibody shows equivalent protein loading in each lane. (D) Northern blot analysis ofIL-2R/IL-15Rβ expression. mRNA prepared from PBMCs from a control subject and from P1. Northern blot analysis using anIL-2R/IL-15Rβ cDNA probe shows 7% of control IL-2R/IL-15Rβ expression in the sample from P1. Equivalent mRNA loading was demonstrated after stripping and reblotting the filter with a probe for β-actin.
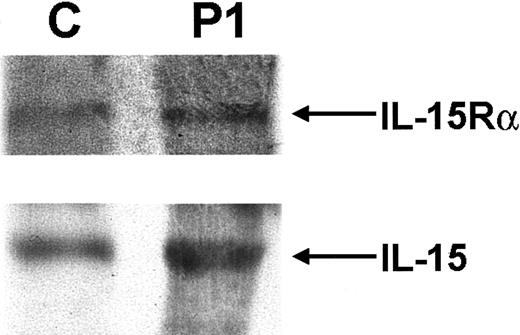
The most notable feature of the patient's immunophenotype was the complete absence of NK cells. Because IL-15 signaling is essential for NK-cell development, components of the IL-15 cytokine and receptor complex were analyzed. Normal expression of both IL-15 and the IL-15Rα chain was detected in P1, as demonstrated by immunoblot analysis of PBMCs (Figure 2). Having previously shown normal γc expression, the third component of the IL-15 receptor complex, IL-2R/IL-15Rβ, was analyzed. Flow cytometric analysis showed that less than 7% of PBMCs from P1 were positive for surface IL-2R/IL-15Rβ expression. This was markedly low in comparison with a control sample (Figure 1C, top panel). Immunoblot analysis also showed a significant decrease in IL-2R/IL-15Rβ expression in P1 compared with control samples, whereas expression of β-actin was equivalent in both (Figure 1C, bottom panels). Similar results were found when mRNA was isolated from PBMCs and analyzed forIL-2Rβ expression by Northern blot analysis. In comparison with a control sample, P1 expressed only 7% of theIL-2R/IL-15Rβ message as quantitated by Phosphorimager (Molecular Dynamics, Sunnyvale, CA; Figure 1D). These results all suggested a significant abnormality in IL-2R/IL-15Rβ expression—a protein that is normally constitutively expressed.20Numerous genetic analyses of the IL-2R/IL-15Rβ gene were undertaken. IL-2R/IL-15Rβ gene complementary DNA (cDNA) was initially amplified and sequenced (the 2 alleles could be distinguished by the polymorphism c750c-> t (G250G)). In addition, genomic sequence containing 1.1 kbp promoter region of the human IL-2R/IL-15Rβ gene was also amplified and sequenced. To exclude splice-site mutations, 8 of 9 coding exons including exon/intron boundaries were directly sequenced, and exon 5, which could not be sequenced for technical reasons, was analyzed by SSCP analysis. No abnormalities were detected in any of these assays. Furthermore, DNA from P1 was analyzed by Southern blot analysis, but there was no evidence of duplications or gross deletions (data not shown). After bone marrow transplantation, when the NK-cell lineage was fully reconstituted, expression of IL-2R/IL-15Rβ on PBMCs by flow cytometry and immunoblot analysis was normal (data not shown).
IL-15Rα and IL-15 expression.
Western blot analysis of IL-15Rα and IL-15 expression in PBMCs of P1 shows expression equivalent to that seen in a normal control.
IL-15Rα and IL-15 expression.
Western blot analysis of IL-15Rα and IL-15 expression in PBMCs of P1 shows expression equivalent to that seen in a normal control.
Many different SCID immunophenotypes have now been described, but this is the first report of SCID in which the major developmental abnormality is in the NK-cell lineage. The experiments described suggest that the defect occurred as a result of abnormal IL-15 and IL-2 signaling caused by a marked decrease in IL-2R/IL-15Rβ expression. Although no evidence of an abnormality in theIL-2R/IL-15Rβ gene was identified, some explanations are possible. The sensitivity of the techniques used was not 100%, and it is possible that a mutation was not detected. It is also possible that the defect lay in an uncharacterized regulatory region of the gene. Transcription factors such as Egr-1 (early growth response protein 1) and the Sp family proteins have been shown to bind to the −170 to −139 IL-2R/IL-15Rβ enhancer region,21 and it is conceivable that a defect in one of these molecules or another transcription factor was responsible for the lack of IL-2R/IL-15Rβ expression.
Although the most complete defect was in IL-15–mediated NK- cell development, it is important to note that the patient had abnormalities in T- and B-cell function (Table 1). Impairment of IL-15 signaling, which is known to have important effects on T cell function,6 9 might have contributed to the T cell defects, and IL-2 receptor mediated abnormalities would be predicted to affect both T and B lymphocyte function. This case study again highlights the critical nature of cytokine receptor signaling pathways to lymphocyte and NK-cell development and function.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hubert B. Gaspar, Molecular Immunology Unit, Institute of Child Health, 30, Guilford St, London WC1N 1EH, United Kingdom; e-mail: h.gaspar@ich.ucl.ac.uk.