Thrombosis is a life-threatening complication that occurs in a subset of patients with heparin-induced thrombocytopenia (HITT). The pathogenic mechanisms underlying the variable occurrence of thrombosis in HITT is poorly understood. It was hypothesized that monocyte activation leading to tissue factor expression may play a role in promoting a thrombogenic state in HITT. This study demonstrates that a human platelet factor 4 (PF4)/heparin-specific murine monoclonal antibody (KKO) binds to peripheral blood-derived human monocytes in a PF4-dependent manner. KKO and antibodies from patients with HITT induce monocytes to synthesize and secrete interleukin-8 and induce cell-surface procoagulant activity, which is abrogated following treatment with antihuman tissue factor antibody. The findings suggest a novel mechanism by which PF4/heparin antibodies may promote a hypercoagulable state in patients with HITT.
Introduction
Heparin-induced thrombocytopenia (HIT) is a common iatrogenic disease associated with a significant incidence of life-threatening thrombosis (HITT).1 We have recently demonstrated in a murine transgenic model that platelet factor 4 (PF4)/heparin-specific antibodies result in severe thrombocytopenia and thrombosis only when human PF4 and human platelet FcγRIIA are simultaneously coexpressed on murine platelets.2 Although it is clear that platelet activation by PF4/heparin antibodies is essential for thrombosis in HITT, clinical and laboratory data suggest that concurrent activation of the coagulation system may also be important to disease pathogenesis.
Indirect evidence for involvement of the clotting pathway in HITT is suggested by clinical findings that show an increased incidence of venous rather than arterial thromboses,3 occurrence of venous limb gangrene associated with increased thrombin generation,4 and therapeutic effectiveness of antithrombin agents.5 Additionally, laboratory findings of endothelial cell procoagulant activity by patient antibodies6 7suggest a more direct involvement of the coagulation system in HITT.
Like endothelial cells, monocytes are intravascular cells capable of significant tissue factor generation, and their role in HITT has not been addressed to date. Indeed, monocyte activation and tissue factor expression have been implicated in a variety of immune and nonimmune mediated thrombogenic disorders.8-11 We hypothesized that monocyte activation and tissue factor expression occurs in HITT and may contribute to the pathogenesis of thrombotic disease.
Study design
To examine the effects of HITT antibodies on monocyte activation, we employed a human PF4/heparin-specific murine monoclonal antibody, KKO, that mimics the in vivo and in vitro properties of antibodies from HITT patients,2,12 as well as plasma from 2 patients with HITT (both with clinical thrombosis). KKO, isotype control antibody (ISO, an immunoglobulin [Ig]G2bκ), antibodies from patients and one healthy volunteer, as well as antigen (PF4 and heparin) were obtained as previously described.12Approval from the institutional review board and informed consent was obtained for these studies as previously described.12Antigens were used at the following final concentrations: PF4 10μg/mL, heparin 1 U/mL, PF4 10μg/mL + heparin 1 U/mL, as previously determined.12 Monoclonal antibodies were diluted to a final concentration of 100 μg/mL, whereas human plasmas (HIT and control) were used at a final dilution of 1:50.
Peripheral blood monocytes (PBMOs) were derived from individual healthy donors (n = 6) and cultured as previously described.11 13 Flow cytometric studies were performed on Becton Dickinson FacsCaliber (San Jose, CA). Interleukin-8 (IL-8) levels were measured by using an antibody capture assay (IL-8 DuoSet; R&D Systems, Minneapolis, MN).
Endotoxin assay and removal
All reagents were tested for endotoxin contamination by using E-Toxate LAL Assay (Sigma, St Louis, MO). When detected, endotoxin was extracted from samples to reduce levels to less than 0.1 ng/mL, as previously described.14
Tissue factor assay
Reagents for determination of cell-surface tissue factor were kindly provided by Dr Walter Kisiel (Albuquerque, NM). PBMOs were incubated with antigen (PF4, heparin, or PF4 + heparin) and antibody (KKO, ISO, HITT, or control) for designated time intervals, and surface Factor Xa generation was measured by using chromogenic substrate S-2765 (Kabi Pharmacia Hepar, Franklin, OH) as previously described.15 Tissue factor concentration was estimated by using a standard curve constructed with relipidated tissue factor.
Results and discussion
Although immune-mediated platelet and endothelial cell injury have been long recognized as salient features of HITT, the role of other cellular effectors of thrombotic injury in HITT has not been investigated. In this study, we demonstrate that antibodies from patients with HITT or monoclonal PF4/heparin-specific antibodies bind to monocytes in the presence of PF4 and that they trigger cellular activation leading to expression of the proinflammatory cytokine, IL-8, as well as synthesis and expression of functional cell-surface tissue factor activity.
As revealed by flow cytometry, PF4/heparin-specific antibodies do not require exogenous heparin for binding to PBMOs but, rather, show optimal binding in the presence of PF4 alone (mean fluorescent intensity: KKO alone = 100, KKO + PF4 = 200, KKO + heparin = 138, KKO + PF4/heparin = 83 versus isotype control [ISO] alone = 91, ISO + PF4 = 84, ISO + heparin = 55, ISO + PF4/heparin = 73). These findings suggest that the antigenic target is likely to be PF4 in complex with cell-surface glycosaminoglycans, as previously shown for other cell lines.12
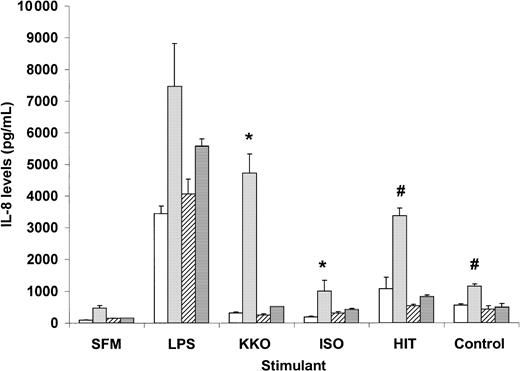
Binding of monocytes by PF4/heparin-specific antibodies is accompanied by markedly elevated IL-8 levels. As shown in Figure1, a statistically significant increase in IL-8 secretion is seen in the presence of PF4/heparin-specific antibodies (KKO or HITT) and PF4, as compared with control antibodies (ISO or control plasma) and PF4. IL-8 levels with KKO and PF4 were approximately 60% of maximal secretion induced by lipopolysaccharide, a potent monocyte activator, whereas binding of HITT plasma resulted in levels that were slightly lower (45%) than maximal secretion.
Interleukin-8 secretion by peripheral blood monocytes exposed to PF4/heparin or control antibodies.
Monocytes derived from individual healthy donors (see “Materials and methods”) were incubated in duplicate with antigen in serum-free media (SFM) alone (■) or SFM containing PF4 (░), heparin (▨), or PF4 + heparin (▤). The following stimulants were coincubated in the presence of antigen for 6 hours: SFM, lipopolysaccharide (LPS; 100 ng/mL), KKO, isotype control (ISO), HITT, or control plasma. IL-8 levels were measured in cell supernatants by enzyme-linked immunosorbent assay. Data shown for HIT patient are representative of 2 patients studied. P < .05 by a 2-tailed t test for KKO versus ISO (∗) or HITT versus control (#) in the presence of PF4.
Interleukin-8 secretion by peripheral blood monocytes exposed to PF4/heparin or control antibodies.
Monocytes derived from individual healthy donors (see “Materials and methods”) were incubated in duplicate with antigen in serum-free media (SFM) alone (■) or SFM containing PF4 (░), heparin (▨), or PF4 + heparin (▤). The following stimulants were coincubated in the presence of antigen for 6 hours: SFM, lipopolysaccharide (LPS; 100 ng/mL), KKO, isotype control (ISO), HITT, or control plasma. IL-8 levels were measured in cell supernatants by enzyme-linked immunosorbent assay. Data shown for HIT patient are representative of 2 patients studied. P < .05 by a 2-tailed t test for KKO versus ISO (∗) or HITT versus control (#) in the presence of PF4.
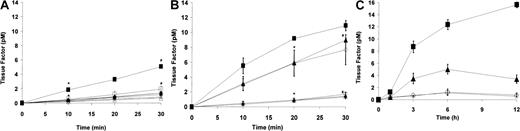
The inflammatory response by monocytes to PF4/heparin-specific antibodies is accompanied by the synthesis and cell-surface expression of tissue factor activity. As depicted in Figure2, cell-surface procoagulant activity, as measured by factor VIIa-dependent activation of factor X, was induced by HITT plasma (Figure 2A) in the presence of PF4 but not with heparin or with PF4/heparin. Similar results were seen with KKO in the presence of PF4 as compared with isotype control (Figure 2B). These findings are in agreement with preliminary observations by Pouplard et al,16 who demonstrated that tissue factor expression is increased by HITT sera or IgG in the presence of PF4.
Monocyte tissue factor induction by PF4/heparin antibodies.
(A) Induction of tissue factor by HITT plasma. Monocytes derived from peripheral blood were incubated in duplicate for 6 hours with HITT (♦) or control plasma (⋄) alone, HITT plasma + PF4 (▪), control plasma + PF4 (■), HITT plasma + heparin (●), control plasma + heparin (○), HITT plasma + PF4/heparin (▴), or control plasma + PF4/heparin (▵). After incubation with antigen and antibody, cells were washed 3 times with Tris-buffered saline (TBS) containing 0.1% bovine serum albumin (BSA) and incubated in buffer (TBS/BSA) containing 10 mM calcium chloride and recombinant Factor VIIa (2 nM) for 30 minutes. Plasma-derived Factor X (200 nM) was added to wells, and activation to Factor Xa was measured at designated time intervals by using chromogenic substrate S-2765. Data shown are representative of at least 2 independent experiments, and results are depicted as means and SD. Data shown for HIT patient are representative of 2 patients studied. In the presence of PF4, P < .05 by one-tailed t test for HITT versus control plasma at 10 minutes (∗) and 30 minutes (#). (B) Specificity of tissue factor induction by KKO. Monocytes derived from peripheral blood were incubated in triplicate with PF4 in the presence of serum-free media (SFM; ○), KKO (▴), isotype control (▵), or lipopolysaccharide (LPS; 100 ng/mL; ▪). Additional wells containing KKO were incubated with polyclonal rabbit anti–tissue factor antibody (♦) or preimmune rabbit control IgG (⋄). Cell-surface procoagulant activity was determined as described in (A) legend. Data shown are representative of at least 3 independent experiments, and results are depicted as means and SD. P < .05 by a 2-tailedt test for KKO versus KKO + antitissue factor antibody at 20 minutes (∗) and 30 minutes (#). (C) Time course of tissue factor expression in the presence of antibody or antigen. To determine the time course of cell-surface tissue factor induction by PF4/heparin antibodies, monocytes derived from peripheral blood were incubated in duplicate with serum-free media containing (SFM; ○), or SFM and lipopolysaccharide (LPS; 100 ng/mL; ▪), SFM and KKO (▴), or SFM and isotype control (⋄) in the presence of PF4 for varying time intervals (0, 3, 6, and 12 hours). Data shown are representative of 2 independent experiments, and results are depicted as means and SD. Cell-surface procoagulant activity induced by KKO was maximal at approximately 6 hours, suggesting de novo synthesis of tissue factor. No significant time-dependent increase in tissue factor activity was seen in the presence of SFM or isotype control.
Monocyte tissue factor induction by PF4/heparin antibodies.
(A) Induction of tissue factor by HITT plasma. Monocytes derived from peripheral blood were incubated in duplicate for 6 hours with HITT (♦) or control plasma (⋄) alone, HITT plasma + PF4 (▪), control plasma + PF4 (■), HITT plasma + heparin (●), control plasma + heparin (○), HITT plasma + PF4/heparin (▴), or control plasma + PF4/heparin (▵). After incubation with antigen and antibody, cells were washed 3 times with Tris-buffered saline (TBS) containing 0.1% bovine serum albumin (BSA) and incubated in buffer (TBS/BSA) containing 10 mM calcium chloride and recombinant Factor VIIa (2 nM) for 30 minutes. Plasma-derived Factor X (200 nM) was added to wells, and activation to Factor Xa was measured at designated time intervals by using chromogenic substrate S-2765. Data shown are representative of at least 2 independent experiments, and results are depicted as means and SD. Data shown for HIT patient are representative of 2 patients studied. In the presence of PF4, P < .05 by one-tailed t test for HITT versus control plasma at 10 minutes (∗) and 30 minutes (#). (B) Specificity of tissue factor induction by KKO. Monocytes derived from peripheral blood were incubated in triplicate with PF4 in the presence of serum-free media (SFM; ○), KKO (▴), isotype control (▵), or lipopolysaccharide (LPS; 100 ng/mL; ▪). Additional wells containing KKO were incubated with polyclonal rabbit anti–tissue factor antibody (♦) or preimmune rabbit control IgG (⋄). Cell-surface procoagulant activity was determined as described in (A) legend. Data shown are representative of at least 3 independent experiments, and results are depicted as means and SD. P < .05 by a 2-tailedt test for KKO versus KKO + antitissue factor antibody at 20 minutes (∗) and 30 minutes (#). (C) Time course of tissue factor expression in the presence of antibody or antigen. To determine the time course of cell-surface tissue factor induction by PF4/heparin antibodies, monocytes derived from peripheral blood were incubated in duplicate with serum-free media containing (SFM; ○), or SFM and lipopolysaccharide (LPS; 100 ng/mL; ▪), SFM and KKO (▴), or SFM and isotype control (⋄) in the presence of PF4 for varying time intervals (0, 3, 6, and 12 hours). Data shown are representative of 2 independent experiments, and results are depicted as means and SD. Cell-surface procoagulant activity induced by KKO was maximal at approximately 6 hours, suggesting de novo synthesis of tissue factor. No significant time-dependent increase in tissue factor activity was seen in the presence of SFM or isotype control.
To confirm that factor Xa generation was specifically due to the synthesis and the expression of cell-surface tissue factor, similar experiments were conducted with KKO and PF4 or HITT plasma and PF4 following incubation of PBMOs with either rabbit antihuman tissue factor IgG or rabbit preimmune IgG. Surface procoagulant activity induced by KKO (Figure 2B) or HITT plasma (data not shown) is completely eliminated after preincubation of stimulated monocytes with polyclonal antibodies to human tissue factor, excluding other potential mechanisms of monocyte procoagulant activity.17 Last, we found that cell-surface tissue factor activity by PF4/heparin antibodies requires approximately 6 to 12 hours for maximal induction (Figure 2C), suggesting de novo synthesis of tissue factor apoprotein, rather than de-encryption of preexisting tissue factor.
The clinical significance of monocyte activation and tissue factor expression in HITT remains to be determined. In monocytes and other cells, IL-8 expression occurs in response to a number of stimuli such as tumor necrosis factor α, IL-1α, IL-1β, IL-3, granulocyte-macrophage colony-stimulating factor, endotoxin, mitogens, and immune complexes.18 IL-8 induces neutrophil demargination, activation, and chemotaxis.18 IL-8 also binds to heparin, and auto-antibodies to IL-8 have been recognized in a subset of patients with HITT.19 Although IL-8 levels have not been measured in HITT patients, recent reports indirectly suggest that this cytokine may be up-regulated in some. In one study, significant tumor necrosis factor α levels were detected in 15% of patients with HITT,20 whereas another study reported findings of neutrophil activation and platelet-neutrophil aggregates by HITT sera.21
Our studies suggest that platelet activation, with release of PF4, is likely to be a prerequisite for monocyte activation and tissue factor induction. Clearly, the time dependence of monocyte activation and procoagulant expression in vitro (> 6 hours; Figure 2C) far exceeds the short incubation periods (≤ 1 hour) required for platelet activation by PF4/heparin-specific antibodies. It is tempting to speculate that the antibody-dependent hypercoagulable state induced in monocytes becomes clinically relevant after discontinuation of heparin, a period that is associated with a heightened risk of thrombosis.22 In this scenario, circulating PF4/heparin antibodies trigger platelet activation in the presence of heparin, releasing PF4 and providing the optimal conditions for monocyte activation on discontinuation of heparin. Alternatively, monocyte injury may be dictated by certain antibody characteristics (ie, epitope specificity, isotype, affinity, etc) that may be present in susceptible individuals receiving heparin therapy. Additional clinical and laboratory studies are under way to clarify the role of monocyte activation and tissue factor regulation in HITT.
We thank Drs Walter Kisiel, Douglas Cines, and Mortimer Poncz for their critical review of the manuscript.
Supported in part by Research Grants KO8 HL04009 (G.M.A.), a Beginning Grant-in-Aid from the American Heart Association Desert/Mountain Regional affiliate (G.M.A.), and an institutional award from the Cancer Research and Treatment Center at the University of New Mexico (G.M.A.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
G. M. Arepally, Department of Medicine, BRF 337G, 915 Camino de Salud NE, Albuquerque, NM 87131; e-mail:arepally@unm.edu.