High levels of chimerism in syngeneic BALB/c transplants were reported when hosts were exposed to 1 Gy (100 cGy) whole body irradiation (WBI) and infused with 40 × 106 marrow cells. The recovery of host stem cells and alterations of enhanced host engraftability at varying times after 1 Gy WBI have now been evaluated in this study. Male BALB/c marrow (40 × 106 cells) was infused into female BALB/c hosts immediately or at 6, 12, and 24 weeks after 1 Gy WBI of host female BALB/c mice; engraftment percentages 8 weeks after cell injection at week 0, 6, 12, or 24 were 68% ± 12%, 45% ± 15%, 51% ± 12%, or 20% ± 8%, respectively. Eight-week engraftment levels in nonirradiated hosts average 7.7%. Conversely, engraftable stem cells measured at 8 weeks postengraftment in 1 Gy– exposed hosts were reduced to 8.6% ± 3% of nonirradiated mice at time 0, 35% ± 12% 6 weeks later, 49% ± 10% at 3 months, and 21% ± 7% at 6 months. Engraftment was still increased and stem cell decreased 1 year after 1 Gy. Furthermore, the primary cells transplanted into 1 Gy hosts can be serially transplanted, and the predominant effect of 1 Gy is directly on engrafting stem cells and not through accessory cells. These data show that transplantation in 1 Gy mice may be delayed until recovery of hematopoiesis, suggesting strategies in allogeneic transplantation to avoid the adverse effects of cytokine storm. The incomplete recovery of engraftable stem cells out to 12 months indicates that stem cell expansion, especially in patients previously treated with radiomimetic drugs, may not be feasible.
Introduction
Marrow stem cells home to and engraft in normal nonmyeloablated hosts.1-6 The final percent donor chimerism is determined by the ratio of donor to host stem cells rather than the availability of open marrow space; thus, in essence, stem cell competition is the determinant of the percent donor chimerism in syngeneic marrow transplantation.
Engraftment into normal hosts was reported as early as the 1960s although its significance was not stressed.1 Later studies using various cell tracking techniques showed that variable, and at times significant, short-term chimerism could be obtained by simply infusing distinguishable donor cells into normal nonmyeloablated mice.2-5
Stewart et al6 extended these observations showing long-term (> 2 years) multilineage engraftment of male cells in female marrow, spleen, and thymus. Further studies showed that nonproliferating quiescent stem cells engrafted into normal hosts and that cell dose-response curves could be established.7-9Mathematical modeling of engraftment into normal hosts, assuming either stem cell replacement or stem cell augmentation, indicated that virtually all infused stem cells had engrafted; final host:donor marrow cell ratios appeared to be determined by stem cell competition.9 In a number of instances, engraftment levels were higher than theoretical maximums, suggesting that in some mice donor cells were favored over host cells.
If stem cell competition is the sole determinant of stem cell engraftment in normal nonmyeloablated hosts, then treatment that reduced host stem cells, even if only mildly myeloablative, would be expected to increase the donor-to-host stem cell ratio and thus the final percent chimerism. Low-dose irradiation is a treatment that appears to meet these criteria. A number of previous studies have reported D0 values for in vitro and in vivo murine stem progenitor cell assays ranging from 0.4 to 2.75 Gy (40 to 275 cGy), depending upon the assay and whether the marrow was stimulated with cytokines.10-14 We have confirmed that these levels of irradiation caused only temporary and mild depression of murine peripheral blood white cells and platelet levels, with little to no effect on marrow cellularity over 14 days after 1 Gy (100 cGy) whole body irradiation (WBI).15 We also established that 1 Gy was quite toxic to engraftable stem cells, reducing their numbers to less than 10% to 15% of normal. When 40 × 106 male BALB/c marrow cells were infused into 1 Gy–treated female hosts, very high levels of donor chimerism were seen at 2, 5, and 8 months postmarrow infusion. This result is predicted by the stem cell competition hypothesis: A reduction in host stem cells should increase donor chimerism. The use of 1 Gy WBI as a host conditioning regimen thus provides an effective approach to establishing high levels of syngeneic nontoxic chimerism.
The durability of the increased host engraftability was unknown, as was the recovery time for engraftable stem cells after 1 Gy WBI. The present studies evaluate the recovery of host stem cells and diminishment of the host's ability to accept and express a marrow graft at various times after 1 Gy WBI. In addition, the ability of marrow cells engrafted in 1 Gy hosts to serial transplantation was unknown, as was the possibility that accessory cell ablation played a role in the apparent stem cell effect of 1 Gy WBI. These issues are also addressed.
Materials and methods
Mice
Six- to 8-week-old BALB/c (H-2d) mice (Taconic Farms, Germantown, NY) were housed in a conventional clean facility for at least one week prior to experimental use. Mice received mouse chow and acidified water ad libitum.
Marrow cell suspension
BALB/c mice were killed by cervical dislocation, and bone marrow cells were collected from tibiae and femurs. Cells were flushed from femurs and tibiae with phosphate-buffered saline and filtered through a 40-μm cell strainer (Becton Dickinson, Franklin Lakes, NJ). The cells were washed once in phosphate-buffered saline, counted in crystal violet, and resuspended for injection in phosphate-buffered saline.
Transplantation
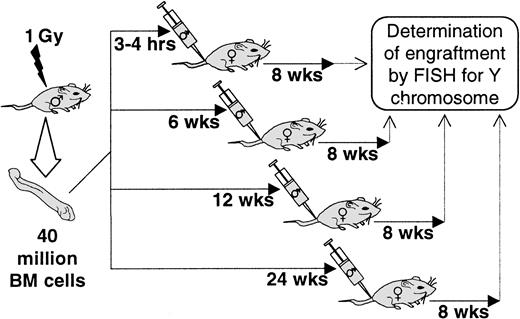
Female BALB/c mice were exposed to 1 Gy WBI (Gammacell, 0.89-1.02 Gy/min). These irradiated mice were then infused with 40 × 106 normal male BALB/c marrow cells 3 to 4 hours after host irradiation or, alternatively, as illustrated in Figure1, 6, 12, and 24 weeks after irradiation. These mice were then evaluated 8 weeks after cell infusion for engraftment by determining the percentage of male cells in female marrow using a fluorescent in situ hybridization (FISH) technique with a Y painting probe (see below) (Figure 1).
Schematic representation of host irradiation schedule prior to transplantation.
BALB/c female hosts were irradiated 0, 6, 12, and 24 weeks prior to intravenous infusion of 40 × 106 male BALB/c bone marrow cells. Percent engraftment was measured 8 weeks after transplantation by FISH for Y chromosomes.
Schematic representation of host irradiation schedule prior to transplantation.
BALB/c female hosts were irradiated 0, 6, 12, and 24 weeks prior to intravenous infusion of 40 × 106 male BALB/c bone marrow cells. Percent engraftment was measured 8 weeks after transplantation by FISH for Y chromosomes.
These studies were planned to establish host engraftability as a final percent of donor cells in host marrow at different times postirradiation.
We also evaluated host engraftable stem cell content at different time points after irradiation. In these experiements, male BALB/c mice were exposed to 1 Gy WBI, and then 3 to 4 hours or at 6, 12, and 24 weeks after irradiation marrow cells were harvested; the ability to engraft and proliferate in 1 Gy–treated female BALB/c mice was determined 8 weeks after cell infusion (Figure2).
Schematic representation of donor irradiation and harvest delay schedule.
BALB/c male donors were irradiated (1 Gy) 0, 6, 12, and 24 weeks prior to bone marrow harvest and intravenous infusion of 40 × 106 bone marrow cells into female BALB/c recipients treated with 1 Gy WBI. Percent engraftment was measured 8 weeks after transplantation by FISH for Y chromosomes.
Schematic representation of donor irradiation and harvest delay schedule.
BALB/c male donors were irradiated (1 Gy) 0, 6, 12, and 24 weeks prior to bone marrow harvest and intravenous infusion of 40 × 106 bone marrow cells into female BALB/c recipients treated with 1 Gy WBI. Percent engraftment was measured 8 weeks after transplantation by FISH for Y chromosomes.
An experiment was designed to determine whether cells engrafted into a 1 Gy–treated host could be serially transplanted, indicating the stem cell nature of the original transplant (Figure3). Here we initially engrafted 40 × 106 normal male BALB/c marrow cells into 0- or 1 Gy–treated female BALB/c hosts. We also evaluated similar transplants with 1 Gy–exposed donor marrow. Eight weeks after the initial cell infusion, 20 × 106 chimeric (male plus female) marrow cells from the primarily engrafted group were reinfused into 10 Gy (1000 cGy)–exposed female mice. These groups were also followed for 8-week engraftment, evaluated, and 20 × 106 chimeric cells infused into 10 Gy–treated tertiary female hosts. Engraftability was determined 8 weeks after each infusion by FISH for percent male cells (see below). These experiments are outlined in Figure 3.
Schematic representation of sequential transplant experiments.
A total of 40 × 106 male BALB/c bone marrow cells were injected intravenously into 0- or 1 Gy–treated female BALB/c recipients. At 8 weeks, hosts were killed and engraftment evaluated by FISH for Y chromosomes. A secondary transplant was performed using 20 × 106 chimeric bone marrow cells into 10 Gy–treated female BALB/c. In the same way, a tertiary transplant was performed.
Schematic representation of sequential transplant experiments.
A total of 40 × 106 male BALB/c bone marrow cells were injected intravenously into 0- or 1 Gy–treated female BALB/c recipients. At 8 weeks, hosts were killed and engraftment evaluated by FISH for Y chromosomes. A secondary transplant was performed using 20 × 106 chimeric bone marrow cells into 10 Gy–treated female BALB/c. In the same way, a tertiary transplant was performed.
Accessory cell effects
We designed experiments to determine whether irradiation could be directly affecting the stem cells or indirectly affecting them through accessory or helper cells. We have approached this in 2 ways: (1) A total of 40 × 106 nonirradiated female marrow cells were admixed with 40 × 106 1 Gy–exposed male cells, and the mixture was compared with nonirradiated male cells for engraftment into both nonirradiated and 1 Gy–exposed hosts. (2) We purified Lin− Hoechst 33342lowRhodaminelow (see below) stem cells and then evaluated these cells after 0 or 1 Gy irradiation for 8-week engraftment into 1 Gy–exposed hosts.
FISH
The FISH technique identifies individual cells that contain the Y chromosome. Cells that are positive are male, and cells that are negative are female. Essentially 100% of male cells are labeled and 100% female cells are negative.
Cells are fixed in Carnoy fixative (75% methanol/25% acetic acid) once for 20 minutes and then transferred to Eppendorf tubes for long-term storage at −20°C. The cell preparation is washed in fresh Carnoy fixative once and then dropped on ethanol for 1 hour. The air-dried slides are then permeabilized with 0.1 N HCl/0.05% Triton X-100 at room temperature for 10 minutes; washed with 2 × SSC; denatured in 70% formamide in 2 × SSC at 70°C for 3 minutes; dehydrated in ice-cold 70%, 85%, and 100% ethanol, each for 2 minutes; and hybridized with a digoxigenin-labeled murine Y-chromosome painting probe at 37°C overnight. Unbound probe is removed by stringent washing in 50% formamide in 2 × SSC at 45°C for 5 minutes and then 2 × SSC at room temperature. The slides are blocked using a blocking buffer consisting of 5% fetal calf serum, 5% nonfat milk (Shaw's, Bridgewater, MA), and 0.05% Triton X-100 (Sigma, St Louis, MO) in 4 × SSC for 30 minutes. Detection of digoxigenin is done using antidigoxigenin fluorescein isothiocyanate–labeled Fab fragments (Boehringer Mannheim, Indianapolis, IN) incubated in the dark for 30 minutes. Nonbound antibody is removed through 3 light-protected washings in 4 × SSC containing 0.05% Triton X-100, each for 5 minutes. Finally, slides are mounted in the antifade media vectashield (Vector, Newcastle, United Kingdom) with 0.4 pM DAPI (4′6-diamidino-2-phenylindole · 2HCl) (Sigma), which counterstained the DNA. Specific positive signal is confirmed under the UV microscope by a visual check at excitation and emission wavelengths other than that of fluorescein isothiocyanate. Positive and negative controls consisted of male and female slide preparations, respectively. The percentage of positive male cells in female hosts is determined by male cell numbers divided by the total cells counted. For each sample, at least 100 cells were counted under the UV microscope.
Isolation of Lin− Hoechst 33342lowRhodaminelow stem cells
Bone marrow was isolated from iliac bones, femur, and tibiae of BALB/c mice 6 to 8 weeks of age. A low-density fraction (< 1.077 g/cm2) was isolated on Nycoprep 1.077A (Accurate Chemical and Scientific, Westbury, NY). These cells were lineage-depleted with the following antibodies: Ter119, B220, Mac-1, GR-1, Lyt-2, L3t4, YW25.12.7, and Dynabeads MW450 antirat immunoglobulin G (Dynal, Lake Success, NY). The lineage-depleted cells were labeled with Rhodamine 123 at a concentration of 0.1 μg/mL and Hoechst 33342 at 10 μM. Cell were incubated in the dark for 30 minutes at 37°C, washed, and followed by an additional warm-buffer (37°C) incubation for 20 minutes at 37°C to efflux the Rhodamine. This last incubation was carried out twice before sorting using fluorescence activated cell sorting. The first through the third percentiles of Hoechst fluorescence and the first through the 13th percentiles of Rhodamine fluorescence were isolated.
Statistics
Results
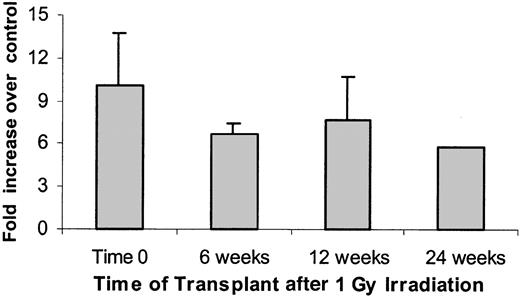
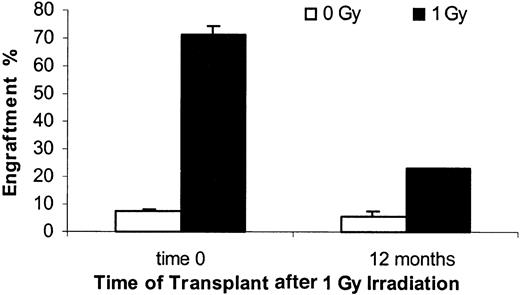
A total of 58 nonirradiated female BALB/c mice were infused with 40 ×106 male BALB/c marrow cells. The mean engraftment level at 8 weeks was 7.7%. Female mice exposed to 1 Gy WBI and then immediately infused with 40 ×106 male mouse cells showed mean 8-week engraftment levels of 67.7% ± 11.5% (SD) (Figure4). If marrow was infused 6 or 12 weeks after irradiation, male chimerism levels were 44.7% ± 15% and 50.5% ± 11.7%, respectively. A cohort of 5 mice was analyzed 6 months after irradiation and still showed elevated engraftment levels (19.5% ± 8.3%) compared with the nonirradiated controls (Figure4). Male engraftment in each individual experiment with their age-matched controls are shown in Table1. Expressed as a fold enhancement over engraftment seen in nonirradiated age-matched control hosts, engraftment at 0, 6, 12, and 24 weeks was enhanced 10.1 ± 3.6, 6.7 ± 0.8, 7.7 ± 3.0, and 5.75 ± 0.0, respectively (Figure5). These enhancements were all statistically significant at P ≤ .02. When female hosts were irradiated with 1 Gy immediately or 12 months before transplantation of 40 ×106 male marrow cells, the engraftment at 8 weeks was 71.1% ± 3.4% for time 0 and 23.2% ± 0.2% at 12 months, whereas the nonirradiated age matched controls had 7.2% ± 0.7% and 5.4% ± 2.1%, respectively (Figure 6). This represents a 9.9 ± 1.4-fold and 4.3 ± 1.7-fold enhancement of engraftment over controls, respectively.
Percent male phenotype at varying times after 1 Gy WBI.
A total of 40 × 106 male BALB/c marrow cells were transfused into female BALB/c mice immediately (time 0) or 6, 12, or 24 weeks after host exposure to 1 Gy WBI. Percentage male DNA in female marrow is presented as determined by FISH (see “Materials and methods”). There were 5 mice per experiment, except for 1 experiment at 12 weeks, which had 2 mice. There were 5, 4, 3, and 1 experiments at time 0, 6, 12, and 24 weeks, respectively. Nonirradiated mice showed an average of 7.7% engraftment with 40 × 106 cells (58 mice). Data are expressed as a mean ± 1 SD.
Percent male phenotype at varying times after 1 Gy WBI.
A total of 40 × 106 male BALB/c marrow cells were transfused into female BALB/c mice immediately (time 0) or 6, 12, or 24 weeks after host exposure to 1 Gy WBI. Percentage male DNA in female marrow is presented as determined by FISH (see “Materials and methods”). There were 5 mice per experiment, except for 1 experiment at 12 weeks, which had 2 mice. There were 5, 4, 3, and 1 experiments at time 0, 6, 12, and 24 weeks, respectively. Nonirradiated mice showed an average of 7.7% engraftment with 40 × 106 cells (58 mice). Data are expressed as a mean ± 1 SD.
Male BALB/c marrow engraftment into female BALB/c mice at different times after 1 Gy whole body irradiation
| Experiment no. . | Gy . | % Engraftment after 1 Gy female host irradiation . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 weeks . | 6 weeks . | 12 weeks . | 24 weeks . | ||||||
| No. . | Mean ± SD . | No. . | Mean ± SD . | No. . | Mean ± SD . | No. . | Mean ± SD . | ||
| 1 | 0 | 5 | 8.7 ± 3.4 | 4 | 6.3 ± 4.6 | — | — | — | — |
| 1 | 5 | 65.3 ± 8.4 | 5 | 42.7 ± 9.9 | 2 | 61.8 ± 9.0 | — | — | |
| 2 | 0 | 5 | 10.3 ± 4.7 | 5 | 10.7 ± 3.8 | 5 | 7.9 ± 1.6 | — | — |
| 1 | 5 | 77.9 ± 15.8 | 5 | 64.6 ± 10.0 | 5 | 46.3 ± 9.7 | — | — | |
| 3 | 0 | 4 | 4.2 ± 1.9 | 5 | 5.4 ± 1.2 | 4 | 6.0 ± 3.0 | — | — |
| 1 | 5 | 68.0 ± 6.6 | 5 | 39.3 ± 11.9 | 4 | 50.2 ± 13.7 | — | — | |
| 4 | 0 | 5 | 6.1 ± 1.8 | — | — | — | — | 5 | 3.3 ± 2.0 |
| 1 | 5 | 55.6 ± 6.0 | — | — | — | — | 5 | 19.5 ± 8.3 | |
| Experiment no. . | Gy . | % Engraftment after 1 Gy female host irradiation . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 weeks . | 6 weeks . | 12 weeks . | 24 weeks . | ||||||
| No. . | Mean ± SD . | No. . | Mean ± SD . | No. . | Mean ± SD . | No. . | Mean ± SD . | ||
| 1 | 0 | 5 | 8.7 ± 3.4 | 4 | 6.3 ± 4.6 | — | — | — | — |
| 1 | 5 | 65.3 ± 8.4 | 5 | 42.7 ± 9.9 | 2 | 61.8 ± 9.0 | — | — | |
| 2 | 0 | 5 | 10.3 ± 4.7 | 5 | 10.7 ± 3.8 | 5 | 7.9 ± 1.6 | — | — |
| 1 | 5 | 77.9 ± 15.8 | 5 | 64.6 ± 10.0 | 5 | 46.3 ± 9.7 | — | — | |
| 3 | 0 | 4 | 4.2 ± 1.9 | 5 | 5.4 ± 1.2 | 4 | 6.0 ± 3.0 | — | — |
| 1 | 5 | 68.0 ± 6.6 | 5 | 39.3 ± 11.9 | 4 | 50.2 ± 13.7 | — | — | |
| 4 | 0 | 5 | 6.1 ± 1.8 | — | — | — | — | 5 | 3.3 ± 2.0 |
| 1 | 5 | 55.6 ± 6.0 | — | — | — | — | 5 | 19.5 ± 8.3 | |
Engraftment determined by FISH (see “Materials and methods”).
Percent male phenotype expressed as a fold increase over age-matched nonirradiated controls in each experiment.
The data presented in Figure 4 are presented here as a fold increase engraftment in postirradiation female mice given as 40 × 106 male BALB/c cells compared with engraftment seen when age-matched nonirradiated mice are infused with 40 × 106 male BALB/c cells. There were statistically significant differences between 0 and 1 Gy in each experiment and at each week (Wilcoxon rank sum test, P ≤ .02). The time effect was assessed in matched experiments using the ANOVA method, adjusting in the model for experimental effect and possible interactions and stratifying by radiation level (ie, separately for 0 and 1 Gy). At 0 Gy, no significant differences in engraftment at the different time points were observed. At 1 Gy, engraftment data at 6 and 12 weeks were significantly different from that of 0 weeks (P < .0002). No difference between 6 and 12 weeks was observed. Data are expressed as a mean ± 1 SD.
Percent male phenotype expressed as a fold increase over age-matched nonirradiated controls in each experiment.
The data presented in Figure 4 are presented here as a fold increase engraftment in postirradiation female mice given as 40 × 106 male BALB/c cells compared with engraftment seen when age-matched nonirradiated mice are infused with 40 × 106 male BALB/c cells. There were statistically significant differences between 0 and 1 Gy in each experiment and at each week (Wilcoxon rank sum test, P ≤ .02). The time effect was assessed in matched experiments using the ANOVA method, adjusting in the model for experimental effect and possible interactions and stratifying by radiation level (ie, separately for 0 and 1 Gy). At 0 Gy, no significant differences in engraftment at the different time points were observed. At 1 Gy, engraftment data at 6 and 12 weeks were significantly different from that of 0 weeks (P < .0002). No difference between 6 and 12 weeks was observed. Data are expressed as a mean ± 1 SD.
Engraftment of normal male bone marrow cells into hosts irradiated 12 months earlier (1 Gy).
Female BALB/c recipient mice were given 0 or 1 Gy WBI prior to immediate or 12-month delayed infusion of 40 × 106 male bone marrow cells intravenously. Engraftment was evaluated by FISH at 8 weeks after transplantation. Significant differences were observed between time 0 and 12 months for 1 Gy groups and with age-matched controls within each time group. Mean ± SE is shown.
Engraftment of normal male bone marrow cells into hosts irradiated 12 months earlier (1 Gy).
Female BALB/c recipient mice were given 0 or 1 Gy WBI prior to immediate or 12-month delayed infusion of 40 × 106 male bone marrow cells intravenously. Engraftment was evaluated by FISH at 8 weeks after transplantation. Significant differences were observed between time 0 and 12 months for 1 Gy groups and with age-matched controls within each time group. Mean ± SE is shown.
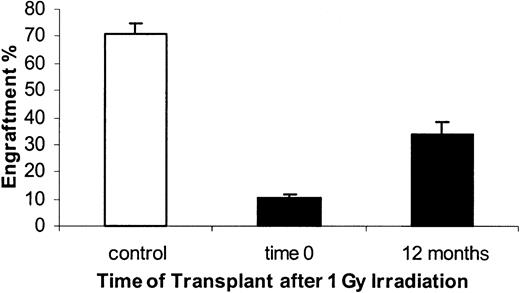
The residual host engraftable stem cells, as measured at 8 weeks after cell infusion, were analyzed immediately and at 6, 12, and 24 weeks after 1 Gy exposure. As previously reported, engraftable stem cells were markedly suppressed when determined immediately after 1 Gy WBI. Engraftment capacity of marrow from 1 Gy–exposed mice was only 8.62% ± 2.7% of the engraftment capacity of marrow from nonirradiated BALB/c mice (Figure 7). This represents a 7.9-fold decrease in stem cell engraftment capacity (Figure 8). When marrow stem cells were assayed 6 weeks after 1 Gy engraftment, capacity was 34.5% ± 12% of age-matched nonirradiated marrow. This represented a 2.1-fold decrease in engraftment capacity. At 12 weeks postirradiation, engraftment capacity had recovered to 48.5% ± 10% of nonirradiated controls. In a small number of mice evaluated at 6 months after 1 Gy engraftment, capacity was still suppressed (20.9% ± 6.8% of nonirradiated control), with a 1.5-fold decrease compared with age-matched control. Engraftment of irradiated marrow at 6 and 12 weeks was significantly higher than that at 0 weeks (Wilcoxon rank sum test, P < .001) and showed an increasing time trend (Cuzick nonparametric trend test, P < .0l). When male donors were irradiated with 1 Gy immediately or 12 months before bone marrow harvest and transplantation of 40 × 106 cells into 1 Gy–treated female recipients, the engraftment at 8 weeks was 10.5% ± 1.2% for time 0 and 33.6% ± 4.6% at 12 months, whereas the nonirradiated bone marrow control group had 71.1% ± 3.4% at time 0. There was no age-matched control for this experiment (Figure 9).
Engrafting marrow stem cells at varying times after 1 Gy WBI.
Male BALB/c mice were exposed to 1 Gy WBI and marrow was harvested immediately (time 0) or 6, 12, or 24 weeks later. This marrow was then assayed for engraftment potential in 1 Gy–irradiated female hosts 8 weeks after cell infusion. Data are expressed as a percentage of male cells as determined by FISH. There were 4 to 5 mice per experiment and 5, 4, 2, and 1 experiments at 0, 6, 12, and 24 weeks, respectively. Nonirradiated marrow (40 × 106 cells) infused into 1 Gy–treated hosts gave a mean of 68% ± 8% (5 experiments, 25 mice). Data are expressed as a mean ± 1 SD.
Engrafting marrow stem cells at varying times after 1 Gy WBI.
Male BALB/c mice were exposed to 1 Gy WBI and marrow was harvested immediately (time 0) or 6, 12, or 24 weeks later. This marrow was then assayed for engraftment potential in 1 Gy–irradiated female hosts 8 weeks after cell infusion. Data are expressed as a percentage of male cells as determined by FISH. There were 4 to 5 mice per experiment and 5, 4, 2, and 1 experiments at 0, 6, 12, and 24 weeks, respectively. Nonirradiated marrow (40 × 106 cells) infused into 1 Gy–treated hosts gave a mean of 68% ± 8% (5 experiments, 25 mice). Data are expressed as a mean ± 1 SD.
Engraftable stem cells expressed as fold decreases compared with nonirradiated controls.
The data presented in Figure 6 are presented here as a fold decrease in engraftment of post–1 Gy marrow at different times after 1 Gy compared with engraftment seen with nonirradiated marrow. Engraftments at 6 and 12 weeks are both significantly higher than at 0 weeks (Wilcoxon rank sum test, P < .001) and show an increasing time trend (Cuzick nonparametric trend test, P < .01). Data are expressed as a mean ± 1 SD.
Engraftable stem cells expressed as fold decreases compared with nonirradiated controls.
The data presented in Figure 6 are presented here as a fold decrease in engraftment of post–1 Gy marrow at different times after 1 Gy compared with engraftment seen with nonirradiated marrow. Engraftments at 6 and 12 weeks are both significantly higher than at 0 weeks (Wilcoxon rank sum test, P < .001) and show an increasing time trend (Cuzick nonparametric trend test, P < .01). Data are expressed as a mean ± 1 SD.
Engraftment of bone marrow from donors irradiated 0 or 12 months prior to harvest (1 Gy).
Male bone marrow was harvested 0 or 12 months after WBI and transplanted in 1 Gy–treated female hosts. Engraftment was measured by FISH for Y chromosomes at 8 weeks after transplantation. Controls consisted of nonirradiated donors transplanted in 1 Gy–treated recipients. Engraftments measured for time 0 and 12 months were significantly different from each other and from controls. Mean ± SE is shown (n = 5). There is no age-matched control for these studies.
Engraftment of bone marrow from donors irradiated 0 or 12 months prior to harvest (1 Gy).
Male bone marrow was harvested 0 or 12 months after WBI and transplanted in 1 Gy–treated female hosts. Engraftment was measured by FISH for Y chromosomes at 8 weeks after transplantation. Controls consisted of nonirradiated donors transplanted in 1 Gy–treated recipients. Engraftments measured for time 0 and 12 months were significantly different from each other and from controls. Mean ± SE is shown (n = 5). There is no age-matched control for these studies.
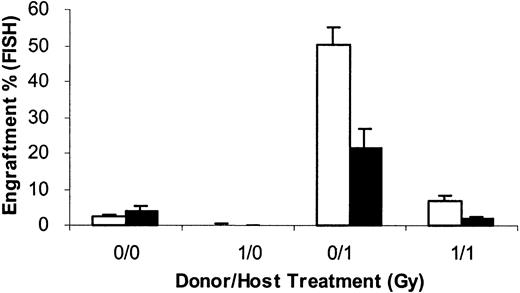
To further evaluate the stem cell nature of cells engrafting into 1 Gy–treated hosts, we evaluated the ability of marrow harvested 8 weeks postengraftment of 40 × 106 male BALB/c cells into 1 Gy–treated female BALB/c hosts to serially repopulate in 10 Gy–treated secondary and tertiary hosts at 8-week intervals (Figure3). Engraftment in each case was determined by FISH, and for the secondary and tertiary transplants 20 × 106 cells were infused. The groups for the initial experiment consisted of (1) 40 × 106 nonirradiated male BALB/c marrow cells infused into nonirradiated hosts (designated 0 Gy/0 Gy); (2) 40 × 106 1 Gy–exposed marrow cells infused into nonirradiated hosts (1 Gy/0 Gy); (3) 40 × 106 1 Gy–exposed marrow cells infused into 1 Gy–exposed hosts (1 Gy/1 Gy); and (4) 40 × 106 nonirradiated marrow cells infused into 1 Gy–exposed hosts (0 Gy/1 Gy). At 8 weeks after this primary transplant, 20 × 106 chimeric cells were harvested and then infused into 5 secondary 10 Gy–exposed female BALB/c recipients; this was again repeated in 8 weeks, and engraftment assessed 8 weeks after each transplant. In the primary transplant, there were 55.6% ± 6% male cells in marrow 8 weeks after transplantation. This was the marrow inoculum for the 20 × 106 cells used for the secondary transplant. (Figure10).
Serial transplantation of male/female chimeric marrow from 1 Gy– engrafted hosts.
Cells harvested from 0- or 1 Gy–exposed female hosts 8 weeks after infusion of nonirradiated or 1 Gy–exposed marrow were serially transplanted at 8-week intervals at 20 × 106 marrow cells into 10 Gy–exposed secondary or tertiary female BALB/c hosts. Marrow engraftment was determined in each instance 8 weeks after transplantation. The first category (* /) represents the treatment of the marrow in the initial transplant into 0- or 1 Gy–treated hosts. The second category (/*) represents the treatment of the original female BALB/c host. Data are expressed as a mean percentage engraftment (FISH) ± 1 SD. ▪ indicates secondary; ■, tertiary.
Serial transplantation of male/female chimeric marrow from 1 Gy– engrafted hosts.
Cells harvested from 0- or 1 Gy–exposed female hosts 8 weeks after infusion of nonirradiated or 1 Gy–exposed marrow were serially transplanted at 8-week intervals at 20 × 106 marrow cells into 10 Gy–exposed secondary or tertiary female BALB/c hosts. Marrow engraftment was determined in each instance 8 weeks after transplantation. The first category (* /) represents the treatment of the marrow in the initial transplant into 0- or 1 Gy–treated hosts. The second category (/*) represents the treatment of the original female BALB/c host. Data are expressed as a mean percentage engraftment (FISH) ± 1 SD. ▪ indicates secondary; ■, tertiary.
These data show the stem cell nature of the male cells engrafting into 1 Gy hosts but also show that with serial transplantation the male cells seem to compete less well with the female cells in the pooled marrow mixtures, possibly indicating a deleterious effect of the initial engraftment. It is unlikely that secondary or tertiary recipient female cells contributed significantly to these populations 8 weeks after 10 Gy conditioning.
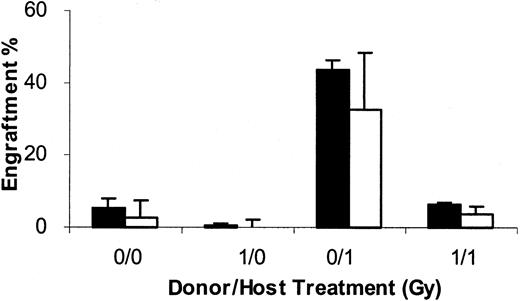
The possibility existed that treatment with 1 Gy reduced marrow stem cell engraftability by altering non–stem cell facilitator or stimulatory cells rather than directly affecting the stem cells. Thus, we evaluated whether adding 40 × 106 nonirradiated female cells to the 40 × 106 1 Gy–exposed male cells and infusing these into female BALB/c hosts would enhance engraftment of the male cells. We evaluated groups of mice in which hosts and donors were nonirradiated (0 Gy/0 Gy), in which donors were irradiated but not hosts (1 Gy/0 Gy), in which donors and hosts were irradiated (1 Gy/1 Gy) and, finally, in which donors were nonirradiated and hosts irradiated (0 Gy/1 Gy). In each instance, results for 8-week engraftment were determined in cohorts of mice either receiving only 40 × 106 male cells (without female) or 40 × 106 male and 40 × 106 female marrow cells (with female) (Figure 11). One Gy exposure of host marrow resulted in the expected decrease in male engraftment. Additional female marrow, rather than increasing engraftment, resulted in an apparent dilutional decrease of male engraftment. This indicated that the 1 Gy WBI was probably acting directly on male-engraftable stem cells.
Engraftment at 8 weeks when female BALB/c marrow was added to 1 Gy–exposed male BALB/c marrow.
BALB/c female mice, either nonirradiated (___/0 Gy) or irradiated (___/1 Gy), were infused with nonirradiated (0 Gy/___) or irradiated (1 Gy/___) male marrow (40 × 106 cells) without the addition of female marrow cells (without female) or with the addition of 40 × 106 nonirradiated female BALB/c marrow cells (with female). Data are presented as the mean male engraftment in marrow ± 1 SD at 8 weeks after cell infusion. There were 4 to 5 individual mice per experimental group. ■ indicates without female BM; ▪, with female BM.
Engraftment at 8 weeks when female BALB/c marrow was added to 1 Gy–exposed male BALB/c marrow.
BALB/c female mice, either nonirradiated (___/0 Gy) or irradiated (___/1 Gy), were infused with nonirradiated (0 Gy/___) or irradiated (1 Gy/___) male marrow (40 × 106 cells) without the addition of female marrow cells (without female) or with the addition of 40 × 106 nonirradiated female BALB/c marrow cells (with female). Data are presented as the mean male engraftment in marrow ± 1 SD at 8 weeks after cell infusion. There were 4 to 5 individual mice per experimental group. ■ indicates without female BM; ▪, with female BM.
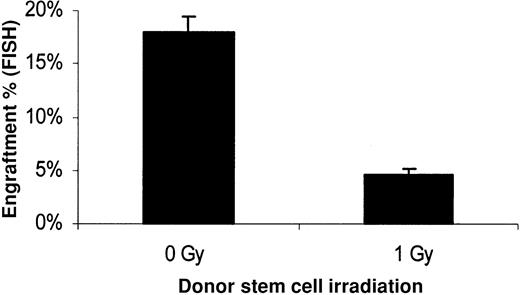
Furthermore, we evaluated the direct effect of 1 Gy irradiation at the stem cell level. Purified Lin− HoechstlowRhodaminelow quiescent stem cells were obtained by high-speed sorting. These cells were then treated with 0 or 1 Gy and immediately transplanted into 1 Gy–treated female recipients. Engraftment at 8 weeks after transplantation was 17.9% ± 1.5% for the nonirradiated stem cells and 4.6% ± 0.6% for the 1 Gy–exposed stem cells (Figure 12). This represents a 3.9 ± 0.8-fold decrease of engraftability in the 1 Gy–irradiated Lin− Hoechstlow Rhodaminelow group compared with the nonirradiated ones. These data confirm a direct effect of low-dose irradiation on the engraftability of purified hematopoietic stem cells independently from the accompanying cells.
Stem cell toxic effect of low-dose irradiation on donor stem cells.
Lin− Hoechst 33342lowRhodamine123low–sorted stem cells from male BALB/c mice (1.8 × 104 per mouse) were injected intravenously in 1 Gy–treated female BALB/c recipients. Prior to transplantation, half of the sorted stem cells were exposed to 1 Gy irradiation, and the other half received no treatment. Engraftment was measured in bone marrow 8 weeks after transplantation with FISH using a Y-chromosome painting probe. Cells were counted using a Rhodamine filter to give the percentage of engraftment. Mean ± SE is shown (n = 5,P ≤ .001).
Stem cell toxic effect of low-dose irradiation on donor stem cells.
Lin− Hoechst 33342lowRhodamine123low–sorted stem cells from male BALB/c mice (1.8 × 104 per mouse) were injected intravenously in 1 Gy–treated female BALB/c recipients. Prior to transplantation, half of the sorted stem cells were exposed to 1 Gy irradiation, and the other half received no treatment. Engraftment was measured in bone marrow 8 weeks after transplantation with FISH using a Y-chromosome painting probe. Cells were counted using a Rhodamine filter to give the percentage of engraftment. Mean ± SE is shown (n = 5,P ≤ .001).
Discussion
Engraftment of BALB/c male marrow cells into 1 Gy–treated female hosts has validated the model of stem cell engraftment based on host donor stem cell competition forwarded by studies on transplantation in nonmyeloablated hosts.
The present results extend information on this model, showing that the engrafting cells have secondary and tertiary engrafting capacity, ie, they are true stem cells, although the relative decrease in male chimerism over time suggests that male stem cells may have been damaged by the initial transplant. In addition, the question of whether the defective engraftment of marrow from mice exposed to 1 Gy WBI could be due to a depletion of accessory cells or enrichment of inhibitory cells rather than a direct effect on engrafting stem cells was answered. Addition of normal female BALB/c marrow to irradiated male BALB/c marrow did not increase engraftment but, rather, resulted in a dilutional decrease in male cell engraftment. The 1 Gy treatment of purified murine Lin− HoechstlowRhodaminelow stem cells free of accessory cells resulted in a significant decrease of engraftability, confirming a direct toxic effect of irradiation on the stem cells.
A striking finding was the very slow alteration in host engraftability at prolonged times after 1 Gy host WBI. Engraftment, as compared with that seen in nonirradiated hosts, was still significantly elevated at 3, 6, and 12 months postirradiation. In previous studies, we have shown that peripheral blood counts have returned to normal by 2 weeks after 1 Gy.15 Thus, the present data suggest interesting strategies for allogeneic transplantation. The “cytokine storm” seen immediately after high-dose chemoradiotherapy exerts an adverse effect on graft-versus-host disease.18 Perhaps delaying allogeneic transplantation in 1 Gy–treated hosts to a time when steady-state lymphohematopoiesis has been achieved may allow for less graft-versus-host disease and superior results with allogeneic marrow transplantation. Work by Xun and colleagues19 has, in fact, shown that delaying marrow infusion to 4 days after 8.5 Gy (850 cGy) total body irradiation in BALB/c mice markedly reduced acute graft-versus-host mortality in these hosts after major histoincompatible transplantation. Perhaps a further delay of 2 to 3 weeks postirradiation with a lower irradiation dose might further improve on these results. Whether alteration of the timing of transplantation in a syngeneic transplant setting employing low-dose irradiation might improve engraftment results remains an open question.
The present results with host stem cell depletion and engraftment immediately after 1 Gy remain consistent with the concept of stem cell competition determining donor chimerism. However, the observation that stem cell recovery after 1 Gy was more rapid and complete than alterations in host engraftability suggests that factors in addition to straight stem cell competition may be operative in the 1 Gy–exposed host. Although studies by Hendrikx et al20 suggest that high-dose irradiation may actually impair stem cell homing, it is possible that lower-dose host irradiation may facilitate homing by altering cytokines, cytokine receptors, or adhesion proteins—to cite a noninclusive list.
Exposure of BALB/c mice to 1 Gy resulted in a profound deficit of engraftable stem cells as measured in 1 Gy hosts. Although there was recovery, this deficit persisted out to 6 months (and probably out to 1 year) postirradiation. This suggests that significant stem cell expansion, an elusive goal at best in vitro,21,22 may be very difficult in animals or patients previously treated with radiation or radiomimetic agents and is consistent with data indicating the existence of a proliferative defect in murine primitive hematopoietic stem cells after exposure of mice to higher levels of ionizing radiation or to various chemotherapeutic agents.23-25 This also resonates with data indicating a decline of stem cell capacity on transplantation and telomeric DNA loss in in vitro “expansion” cultures.26,27 However, the in vitro data indicating difficulty in expanding long-term engraftable stem cells need to be balanced against recent reports of in vitro expansion of long-term engraftable stem cells in the presence of thrombopoietin, horse serum, and murine stroma.28
Supported by National Institutes of Health grants P01-HL-56920, PO1-DK-5022, RO1-DK-49650, and R01-DK2742.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Peter J. Quesenberry, Chief, Dept of Research, Roger Williams Medical Center, 825 Chalkstone Ave, Providence, RI 02908-4735; e-mail: petques9@aol.com.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal