To better understand how humans adapt to hypoxia, the levels of hemoglobin (Hb), serum erythropoietin (Epo), and vascular endothelial growth factor (VEGF) were measured in 106 patients with severe obstructive sleep apnea-hypopnea syndrome. The results indicated that temporal hypoxic stimulation increases Hb. Furthermore, a minor increase in Epo and a substantial increase in VEGF were found. The induction in patients with severe sleep apnea was greater than that reported in other types of hypoxia.
Introduction
Remarkable progress has been made in understanding the molecular basis of oxygen sensing and transcriptional regulation of physiologically relevant genes, including those encoding erythropoietin (Epo) and vascular endothelial growth factor (VEGF).1Induction of these genes confers multiple responses for maintenance of oxygen hemostasis. At the transcriptional level, these genes are all under the control of hypoxia-inducible factor-1 (HIF-1).2There is an HIF-1 binding site in the enhancer of the Epogene2 and in the promoter of the VEGFgene.3 Both of these genes are induced by hypoxia in vivo and in vitro by means of a common oxygen and signaling pathway.1 HIF-1 is a widely expressed heterodimeric protein composed of HIF-1α and aryl hydrocarbon nuclear translocator (ARNT) subunits, both of which belong to the rapidly growing PAS family of basic helix-loop-helix (bHLH) transcription factors.4At the messenger RNA (mRNA) level, both HIF-1 andARNT genes are constitutively expressed and not significantly up-regulated by hypoxia. Whereas changes in oxygen tension do not affect ARNT protein abundance, hypoxia markedly increases the levels of HIF-1α protein.5 The oxygen-dependent degradation (ODD) of HIF-1α is mediated by an internal 200-residue ODD domain via the ubiquitin-proteasome pathway.6 Despite these findings in vitro, very little is known about the steps underlying the activation of HIF-1 through the oxygen sensor by hypoxia in humans. Plasma Epo increases exponentially with the degree of hypoxia in humans.7 High altitude stimulates Epo production in humans.8 Obstructive sleep apnea is a recognized cause of sleep-associated hypoxemia.9 Nocturnal oxygenation correlates with daytime awake arterial oxygen saturation, but it cannot be accurately predicted from awake measurements of oxygenation in patients with obstructive sleep apnea or chronic obstructive pulmonary disease.10Intermittent nocturnal hypoxia in patients with obstructive sleep apnea was not accompanied by elevated serum Epo or erythrocytosis.11 However, the number of the subjects in this study was small (n = 26) and did not include severely affected patients. In the present study, the responses of VEGF and Epo to temporal hypoxic stimulation were assayed in patients with severe obstructive sleep apnea-hypopnea syndrome (OSAHS).
Study design
We measured the levels of hemoglobin (Hb), serum VEGF, and Epo in patients with severe OSAHS (n = 106) and compared them with the levels in controls (n = 45). Individuals with anemia (Hb < 12.0 g/dL), renal or liver disease, and coronary artery disease were excluded. Assays of serum VEGF and Epo were performed by enzyme-linked immunosorbent assay. Serum samples were obtained from the patients when they first came to the clinic. The patients, all of whom had severe OSAHS, were divided into 5 groups according to the apnea-hypopnea index (AHI; 30-49, 50-69, 70-89, 90-109, and > 110) and the controls had an AHI of less than 5, as shown in Table 1.
Results and discussion
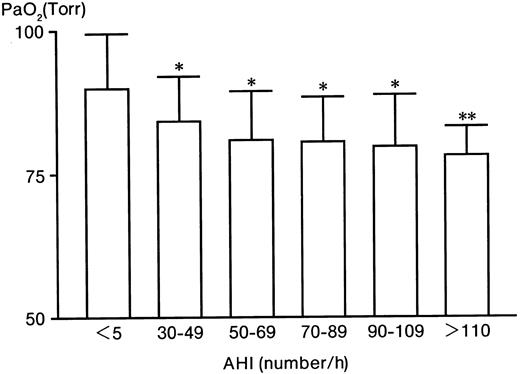
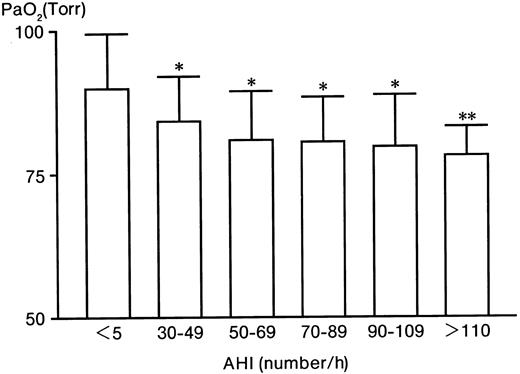
With increases in the AHI, PaO2 significantly decreased from 89.8% ± 9.4% (in the control group) to 78.2% ± 5.1% (in the AHI > 110 group) (Figure1). In contrast to PaO2, Hb significantly increased from 14.5 ± 1.4 g/dL (control) to 17.2 ± 0.3 g/dL (AHI > 110 group) (Table 1). Serum VEGF levels significantly (P < .005) increased from 150 ± 111 (control) to 755 ± 182 pg/mL (AHI > 110 group), 5 times higher than the control level (Table 1). The serum Epo level in the control group was 10 ± 5 mU/mL (Table 1). Compared with the control level, Epo levels in the AHI 30 to 49, 50 to 69, and 70 to 89 groups were increased to 17, 13, and 16 mU/mL, respectively (P < .025), 1.6 times higher than the control level (P < .025) (Table 1). However, the levels in the AHI 90 to 109 and AHI greater than 110 groups were not increased (P > .05) (Table 1). Furthermore, there were no significant relationships between Epo and Hb, between VEGF and Hb, or between Epo and VEGF (data not shown).
The level of PaO2 in patients with severe OSAHS and controls.
* indicates P < .005; **,P < .025.
The level of PaO2 in patients with severe OSAHS and controls.
* indicates P < .005; **,P < .025.
Moore-Gillon and Cameron demonstrated that 2 hours of hypoxia (12% oxygen) per day leads to a rise in red cell mass in rats and that there is a dose-response relationship between the duration of hypoxia and red cell mass.12 Other workers have shown that 1 hour of hypoxia (10% oxygen) per day leads to a rise in hematocrit in rats.13 However, despite substantial nocturnal hypoxemia in some patients in the former study, there was no significant effect on serum Epo, and no significant change occurred when nocturnal hypoxemia was corrected by nasal continuous positive airway pressure.11 Also, no patient had a serum Epo level more than 48 mU/mL, which was the upper limit of the normal range for the assay system used.11 Thus, intermittent nocturnal hypoxemia in the patients was not accompanied by significantly elevated serum Epo levels. This finding conflicted with those of Cahan and associates,14 who demonstrated that serum Epo levels in patients with obstructive sleep apnea were approximately 2-fold higher than those in normal subjects. Daytime hypoxemia appears to be an important determinant of serum Epo and red cell mass15in patients with chronic lung disease, but nocturnal hypoxemia does not appear to exert an appreciable independent influence on erythrocyte production.16
We found an increase in Hb, a minor increase in Epo, and a substantial increase in VEGF in the patients with OSAHS. The 1.6-fold increase in Epo in our study was compatible with that in a previous report.14 This result indicates that a small increase in Epo allows for a corresponding increase in red cell mass. The resultant enhanced delivery of oxygen to tissues then dampens the hypoxic signal, thereby shutting off further stimulus for Epo gene transcription. This represents the closing of a negative feedback loop.
As to the response of VEGF by hypoxia, Gunga and colleagues reported reduced VEGF concentrations immediately after an ultramarathon run at high altitude.17 Asano and coworkers measured a transient decrease of serum VEGF 10 days after the beginning of altitude training at 1886 m, followed by an increase, reaching maximum values on day 19.18 Schobersberger and associates reported that VEGF in a group of runners was significantly elevated after they ran the Swiss Alpine Marathon of Devos (distance 67 km, altitude difference 2300 m) and further increased 2.4-fold until day 5 after exposure. Epo was also increased after exercise but reached a maximum 2 hours after the run (2-fold increase) and decreased thereafter.19 They concluded that the increase of VEGF was due to both the stimulation of hypoxia and exercise. Especially after exercise, the tissue damage that occurred as a result of running increased the levels of cytokines such as interleukin 6 (IL-6) and tumor necrosis factor-α (TNF-α) which, in turn, may have stimulated the production of VEGF mRNA.20 It is possible, though unlikely, that changes in IL-6 and TNF-α in patients with OSAHS contribute to the observed increase in VEGF and Epo.
Supported by grants-in-aid for scientific research from the Ministry of Education, Science and Culture of Japan, Renal Anemia Foundation, and the Chugai Foundation, Tokyo, Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Shigehiko Imagawa, Division of Hematology, Institute of Clinical Medicine, University of Tsukuba, Tsukuba, Ibaraki 305-8575, Japan; e-mail: simagawa@md.tsukuba.ac.jp.