Expression of multidrug resistance (MDR) features by acute myeloid leukemia (AML) cells predicts a poor response to many treatments. The MDR phenotype often correlates with expression of P-glycoprotein (Pgp), and Pgp antagonists such as cyclosporine (CSA) have been used as chemosensitizing agents in AML. Gemtuzumab ozogamicin, an immunoconjugate of an anti-CD33 antibody linked to calicheamicin, is effective monotherapy for CD33+ relapsed AML. However, the contribution of Pgp to gemtuzumab ozogamicin resistance is poorly defined. In this study, blast cell samples from relapsed AML patients eligible for gemtuzumab ozogamicin clinical trials were assayed for Pgp surface expression and Pgp function using a dye efflux assay. In most cases, surface expression of Pgp correlated with Pgp function, as indicated by elevated dye efflux that was inhibited by CSA. Among samples from patients who either failed to clear marrow blasts or failed to achieve remission, 72% or 52%, respectively, exhibited CSA-sensitive dye efflux compared with 29% (P = .003) or 24% (P < .001) among samples from responders. In vitro gemtuzumab ozogamicin–induced apoptosis was also evaluated using an annexin V–based assay. Low levels of drug-induced apoptosis were associated with CSA-sensitive dye efflux, whereas higher levels correlated strongly with achievement of remission and marrow blast clearance. In vitro drug-induced apoptosis could be increased by CSA in 14 (29%) of 49 samples exhibiting low apoptosis in the absence of CSA. Together, these findings indicate that Pgp plays a role in clinical resistance to gemtuzumab ozogamicin and suggest that treatment trials combining gemtuzumab ozogamicin with MDR reversal agents are warranted.
Introduction
A novel anti-CD33 antibody–calicheamicin conjugate, gemtuzumab ozogamicin (CMA-676, Mylotarg, Wyeth-Ayerst Laboratories, Philadelphia, PA), was recently shown to be safe and effective for the treatment of relapsed acute myeloid leukemia (AML).1 The selectivity of this agent is based on the fact that CD33, a member of the sialic acid–binding receptor family, is expressed on blast cells from more than 90% of AML cases2but not on pluripotent hematopoietic stem cells or nonhematologic tissues. In the phase I study of gemtuzumab ozogamicin, leukemic blasts were cleared from the blood and marrow of a portion of patients whose cells were highly saturated with antibody and expressed a low level of multidrug resistance (MDR) function, as indicated by low efflux of the dye 3,3′-diethyloxacarbocyanine iodide (DiOC2).3 Because DiOC2 is a substrate of the membrane transporter P-glycoprotein (Pgp), the product of the MDR1 gene, Pgp was implicated as a potential mediator of gemtuzumab ozogamicin resistance. In vitro studies of drug-resistant CD33+ cell lines have also suggested that calicheamicin is exported by Pgp.4 To date, the roles of PgP and non-Pgp resistance mechanisms in the clinical response to gemtuzumab ozogamicin have been poorly defined. Such information is important given the high prevalence of the MDR phenotype in elderly, relapsed, and secondary AML and the association of MDR expression with a poor prognosis in de novo AML.5-12
Of additional significance, antibody-directed therapy represents an ideal setting for testing agents that reverse the MDR phenotype. Cyclosporine (CSA) and PSC 833 are Pgp antagonists that have been used as chemosensitizing agents in AML treatment trials. To date, the combination of conventional chemotherapeutic drugs with CSA or PSC 833 has resulted in improved clinical responses in some studies but not in others.13-18 The lack of efficacy using standard agents and schedules relates in part to a failure of the MDR modulator to substantially enhance the therapeutic index. Both CSA and PSC 833 alter the pharmacokinetics of anthracyclines and epidophyllotoxins, resulting in higher systemic drug levels and increased nonhematologic toxicities. Thus, in most studies combining CSA or PSC 833 with chemotherapy, the dose of chemotherapy has been substantially reduced to avoid excess toxicity. Theoretically, such limitations would be avoided in regimens combining MDR reversal agents with antibody-targeted therapies. In that case, the chemosensitizing effect would augment cytotoxicity against the targeted tumor cells; however, toxicity to normal tissues should be decreased because of the lack of systemic drug exposure.
In the present study, we set out to characterize the role of Pgp and the MDR phenotype in clinical gemtuzumab ozogamicin resistance. Positive findings would support the rationale to combine gemtuzumab ozogamicin with an MDR reversal agent to treat high-risk AML. For these studies, pretreatment blast cell samples from relapsed AML patients eligible for phase II gemtuzumab ozogamicin clinical trials were analyzed for Pgp surface expression, Pgp function as indicated by DiOC2 efflux and modulation by CSA, and in vitro gemtuzumab ozogamicin–induced apoptosis. In a subset of samples, the ability of CSA to increase in vitro apoptosis was also tested. Among the patients who underwent treatment on study, blast cell phenotypic features and in vitro drug susceptibility were correlated with the achievement of complete hematologic remission (CR), CR in the absence of platelet recovery to 100 ×109/L (100 000/μL) (CRp), and the clearance of marrow blasts after 2 gemtuzumab ozogamicin doses. This latter criterion was analyzed to assess in vivo drug sensitivity among patients whose outcome was not affected by early death and nonleukemic complications.
Our results indicate that residual marrow leukemia after gemtuzumab ozogamicin treatment and failure to achieve CR or CRp correlated highly with blast cell Pgp function and low in vitro drug-induced apoptosis. However, blasts from a subset of nonresponder patients exhibited discordant blast cell phenotypic profiles; this included cases with low Pgp surface expression and increased DiOC2 efflux that was not inhibited by CSA and cases without detectable Pgp function but low in vitro apoptosis. Thus, other drug transporters and/or nontransporter mechanisms may also contribute to clinical gemtuzumab ozogamicin resistance. Nevertheless, we further found that low baseline levels of blast cell in vitro drug-induced apoptosis could be increased by CSA in a portion of cases, supporting the notion that MDR reversal agents could be useful adjuncts to gemtuzumab ozogamicin therapy.
Patients, materials, and methods
Patients and treatment
Adult patients with untreated first relapse of AML (at least 3 months after first CR) were eligible for 1 of 3 open-label, multicenter phase II protocols designed to evaluate the safety and efficacy of gemtuzumab ozogamicin (Mylotarg; Wyeth Laboratories, Radnor, PA).1 Eligible patients were required to have leukemic blast cells that expressed surface CD33 at 4 times above background in more than 80% of the blasts, as determined by cytofluorometric immunofluorescence. Patients with secondary AML were excluded from these studies. Planned therapy was for gemtuzumab ozogamicin to be given at 9 mg/m2 for up to 3 doses with at least 14 days, but no more than 28 days, between doses. A total of 142 patients, including 80 patients aged 60 years or older, were treated with 1 dose (28 patients), 2 doses (109 patients), or 3 doses (5 patients). Twenty-eight patients received only 1 gemtuzumab ozogamicin dose primarily because of disease progression and/or infection and not because of treatment-induced toxicity. Because prolonged cytopenias occurred in some patients following 3 doses, most patients received only 2 doses. A summary of the safety and efficacy results from the phase II studies has been published.1 On the basis of the phase II clinical results, the Food and Drug Administration approved the use of gemtuzumab ozogamicin for relapsed AML in elderly patients, recommending 2 doses at 9 mg/m2given at least 14 days apart.
In the phase II studies, a clinical CR was defined as: (1) leukemic blasts absent from peripheral blood; (2) blast percentage in the bone marrow 5% or less as measured by morphologic studies; (3) peripheral blood counts with hemoglobin at least 90 g/L (9 g/dL), absolute neutrophil count at least 1.5 ×109/L (1500/μL), platelets at least 100 ×109/L (100 000/μL); and (4) red blood cell and platelet transfusion independence. An additional response designation, called CRp, included patients with all CR criteria except for failure to recover a platelet count at least 100 × 109/L (100 000/μL) prior to additional postremission therapy. This designation was used because delayed platelet recovery was seen with gemtuzumab ozogamicin in some responding patients.1 At initial analysis, the relapse-free and landmark survival of patients achieving CRp were not statistically different from survival durations of patients achieving CR.1 Therefore, in this study blast cell phenotypic data from patients with CR and CRp were combined and compared with data from patients with no response. For the current studies, an additional subgroup analysis was carried out on the patients who received 2 gemtuzumab ozogamicin doses and also had adequate posttreatment marrow samples evaluated by blinded central independent morphology and histologic review (J.M.B.).
All patients participating in the phase II trials signed informed consents, and the institutional review boards of the participating institutions approved all protocols.
Cell samples
Heparinized marrow samples, obtained within 14 days prior to the first gemtuzumab ozogamicin treatment, were used for isolation of mononuclear cells (MNCs) for in vitro studies. Samples collected at the Fred Hutchinson Cancer Research Center were processed either immediately or the following day. Samples collected at other centers were sent at 4°C to 8°C by overnight express and were processed the following day. Preliminary studies determined that viability, Pgp surface expression, and dye efflux were not adversely affected by the delayed processing required for most samples. The MNC fraction was isolated by density centrifugation over Ficoll/Hypaque at 1.077 gm/mL, and contaminating red cells were lysed in an aqueous buffer containing 0.15 M NH4Cl, 12 mM NaHCO3, and 0.13 mM ethylenediaminetetraacetic acid. Cell viability was confirmed by trypan blue dye exclusion, and CD33 expression was confirmed by immunofluorescence staining using phycoerythrin (PE)-conjugated murine anti-CD33 antibody (Becton Dickinson, San Jose, CA) as previously described.3 The CD33+ cells were identified by PE fluorescence staining intensity that was greater than the 99th percentile upper limit of staining intensity on MNCs incubated with a nonbinding isotype control PE-conjugated antibody.
Analysis of Pgp surface expression
The surface expression of Pgp on freshly isolated blast samples was determined by multiparameter cytofluorometric immunofluorescence staining using methods adapted from those previously described.19 Briefly, for Pgp surface expression, MNCs were incubated first with a murine immunoglobulin (Ig) G2aanti-Pgp antibody, 4E3.16 (provided by R. Arceci, University of Cincinnati, OH), followed by a secondary fluorescein isothiocyanate (FITC)–conjugated rat antimouse IgG2a antibody (BD PharMingen, San Diego, CA). For a staining negative control, samples were incubated with the nonbinding IgG2a murine monoclonal antibody, 19E12. For a Pgp-positive cellular control, TF1 cells, which constitutively overexpress Pgp, were incubated with 4E3.16 or 19E12 followed by secondary antibody. Each patient MNC sample was analyzed in parallel with a TF1 positive control sample. Before analysis, Pgp-stained clinical MNC samples were also incubated with PE-conjugated anti-CD33, to identify the blast cell population, and with propidium iodide (PI) (Sigma, St Louis, MO) to detect nonviable cells. All samples were assayed on a FACScan flow cytometer (Becton Dickinson), and results were analyzed using the Cellquest software (Becton Dickinson). Data analysis was carried out on PI−, CD33+ cells with size and granularity properties consistent with blast cells. For Pgp surface expression, a 4E3.16 FITC fluorescence staining intensity greater than the 95th percentile upper limit of the intensity of staining with 19E12 isotype control antibody was considered positive. To quantitate the level of surface expression within the Pgp+ population, the mean FITC fluorescence intensity of cells stained with 4E3.16 was divided by the mean fluorescence intensity of cells stained with 19E12 to obtain a staining intensity ratio.
Analysis of Pgp function by dye efflux
Because calicheamicin has no intrinsic fluorescence capacity, it cannot be used to directly assess drug uptake and extrusion by AML blasts. Therefore, the fluorescent dye DiOC2 was used as a surrogate substrate to assess Pgp function. DiOC2 is felt to be relatively specific for Pgp; it is not exported by another MDR transporter found in some cases of AML, the multidrug resistance–associated protein 1 (MRP1).11 In the current studies, multiparameter cytofluorometric analyses were carried out to determine DiOC2 efflux within the CD33+, PI− MNC blast cell populations using methods similar to those previously described.19 To load the cells with dye, 4 × 105 MNCs were incubated in the dark in a 10-ng/mL solution of DiOC2 for 30 minutes at 37°C. The cells were then washed and resuspended in fresh media without dye. Aliquots were transferred to 4°C for subsequent analysis of dye loading or were returned to 37°C for 90 minutes to allow time for active efflux. Prior to analysis of DiOC2 fluorescence, cells were stained for CD33 and PI. After dye loading, parallel samples were also incubated in the presence of 2500 ng/mL CSA (Novartis Pharmaceuticals, East Hanover, NJ), a concentration that inhibits Pgp-mediated dye efflux and is achievable in vivo by continuous infusion of CSA.13-15 As reported by others,6,11 19 non-Pgp, non-MRP transporters appear to extrude DiOC2 in some AML samples with low Pgp surface expression. Therefore, CSA inhibition of dye efflux was correlated with the level of Pgp surface display, as reflected by 4E3.16 staining intensity, to assess potential alternative drug transport mechanisms. All samples were analyzed on the FACScan flow cytometer, gating on the CD33+, PI− blast cell populations. The mean DiOC2 fluorescence intensities, in linear fluorescence channel numbers, were calculated from histograms obtained after dye loading and after efflux in the presence or absence of CSA. Efflux ratios in the presence or absence of CSA were calculated by dividing the loading fluorescence intensity by the intensities obtained after incubation for 90 minutes.
Analysis of drug-induced apoptosis
Density gradient-isolated MNCs containing leukemic blast cells were cultured at 106 cells per milliliter for 2 hours at 37°C in 96-well round bottom plates in 300 μL Iscoves modified Dulbecco medium (IMDM; Gibco, Grand Island, NY) with 2% fetal bovine serum (Summit Biotechnology, Fort Collins, CO) in the presence of either 10 ng/mL of gemtuzumab ozogamicin or the drug equivalent concentration of a control immunoconjugate consisting of calicheamicin linked to an antibody that recognizes the MUC1 antigen on epithelial tumors (hCTM01-calicheamicin)20 (both provided by Wyeth-Ayerst Research, Radnor, PA). After the 2-hour incubation, the cells were washed twice and resuspended without immunoconjugate in fresh IMDM with 20% fetal bovine serum and 100 ng/mL of each of the following recombinant human cytokines: granulocyte-macrophage colony-stimulating factor, stem cell factor, interleukin-3, and granulocyte colony-stimulating factor (all obtained from PeproTech, Rocky Hill, NJ). Cultures were maintained at 37°C in 5% CO2 in humidified air until assayed for apoptosis at 48 to 96 hours. The surface display of phosphatidylserine, as detected by annexin V binding, was used as the indicator of drug-induced apoptosis. For this assay, cells were stained with FITC-conjugated annexin V (BD PharMingen) using the instructions provided by the manufacturer. Cells were also stained with PI to detect nonviable cells. All samples were analyzed on the FACScan flow cytometer. Cells that had undergone necrotic death were identified as PI+, annexin V+. Cells undergoing apoptosis were identified as annexin V+, PI−. Specific apoptosis was calculated as the percentage of maximal gemtuzumab ozogamicin–induced apoptosis minus the percentage of apoptosis in parallel cultures exposed to hCTM01-calicheamicin. In additional studies, the ability of CSA to increase in vitro drug-induced apoptosis was tested in 91 samples from study-eligible patients. For these assays, blast cell samples were incubated with gemtuzumab ozogamicin or hCTM01-calicheamicin control in the presence of 2500 ng/mL CSA for 2 hours, followed by washing to remove the immunoconjugate and reculturing in complete media with 1000 ng/mL CSA for 48 to 96 hours prior to determination of annexin V binding by cytofluorometric immunofluorescence assay. Results of annexin V binding assays were calculated as percentages of apoptosis compared with control cultures incubated with CSA and hCTM01-calicheamicin. These results were then compared with gemtuzumab ozogamicin–induced apoptosis in parallel cultures exposed to immunoconjugate without CSA.
Statistical analysis
The statistical significance of the differences between distributions of continuous values for in vitro blast cell parameters was determined using the Wilcoxon rank sum test. Correlations between continuous variables were established using Pearson correlation coefficients. Odds ratios were calculated from 2 × 2 tables, and associated confidence intervals were taken from logistic regression models.
Results
Pgp features correlate with clinical remission and marrow blast clearance
Assays for 4E3.16 staining intensity, baseline DiOC2efflux, and inhibition of DiOC2 efflux by CSA were carried out on 85, 126, and 124 pretreatment blast cell samples, respectively, from patients who went on to receive gemtuzumab ozogamicin on study. We first compared the baseline DiOC2 efflux ratio and inhibition of efflux by CSA with 4E3.16 staining intensity to determine the level of inhibition that most closely correlated with Pgp surface expression and, in turn, the level most likely to be indicative of Pgp function. Similar statistical correlations of dye transport modulation by CSA with Pgp expression have been used by others to designate Pgp function.8,10 Overall, a close correlation was found between the baseline DiOC2 efflux ratio and 4E3.16 staining intensity ratio (R = 0.674, P < .0001, n = 85, data not shown). When baseline efflux ratios were plotted against staining intensity for groups of samples with varying degrees of CSA inhibition, the group that yielded the highest correlation coefficient with the strongest statistical power consisted of samples with greater than or equal to 50% efflux inhibition by CSA (R = 0.796, P < .0001, n = 36, Figure1). Therefore, we designated a level of greater than or equal to 50% inhibition of baseline dye efflux as indicative of CSA sensitivity and representative of Pgp function in these AML samples. Of note, similar to observations by others,6,10,11 19 blast cell populations with discordant Pgp features were seen, including samples with elevated baseline dye efflux ratios but with lower Pgp surface expression and/or lack of inhibition by CSA (Figure 1). Thus, some less common populations of blast cells appear to extrude DiOC2 through non-Pgp transporters.
Correlations of pretreatment blast cell Pgp surface expression and Pgp function.
Pgp surface expression, as determined by the immunofluorescence staining intensity ratio using the antibody 4E3.16, correlated significantly with Pgp function, as indicated by the efflux ratio of the Pgp substrate dye DiOC2 in the 36 patient samples where dye efflux was inhibited to levels greater than or equal to 50% by CSA (black circles). In comparison, surface expression of Pgp correlated poorly with the baseline dye efflux ratio in the 23 patient samples that were insensitive to CSA inhibition (ie, < 50% inhibition of efflux [white circles]) despite exhibiting elevated levels of baseline efflux at or above the levels of samples that were sensitive to CSA.
Correlations of pretreatment blast cell Pgp surface expression and Pgp function.
Pgp surface expression, as determined by the immunofluorescence staining intensity ratio using the antibody 4E3.16, correlated significantly with Pgp function, as indicated by the efflux ratio of the Pgp substrate dye DiOC2 in the 36 patient samples where dye efflux was inhibited to levels greater than or equal to 50% by CSA (black circles). In comparison, surface expression of Pgp correlated poorly with the baseline dye efflux ratio in the 23 patient samples that were insensitive to CSA inhibition (ie, < 50% inhibition of efflux [white circles]) despite exhibiting elevated levels of baseline efflux at or above the levels of samples that were sensitive to CSA.
We next compared the baseline DiOC2 efflux ratio with CSA sensitivity. A trend was noted when the baseline efflux data were analyzed by quartiles. Only 1 (3%) of 31 samples from the lowest quartile, which included baseline DiOC2 efflux ratios less than 1.21, was sensitive to CSA. By comparison, 12 (39%) of 31 samples in the low-intermediate quartile (baseline efflux ratios 1.21 to 1.45), 17 (55%) of 31 in the high-intermediate quartile (baseline ratios 1.46 to 1.99), and 26 (84%) of 31 in the highest quartile (baseline efflux ratios ≥ 2) were inhibited by CSA. Overall, 56 (45%) of 124 samples exhibited CSA-reversible DiOC2efflux, indicative of Pgp function.
We then asked whether Pgp surface expression, DiOC2 efflux ratio, and CSA inhibition of dye efflux correlated with clinical responses. Because the survival of patients with CRp was not statistically different from the survival of patients with CR,1 those groups were combined and compared with patients who failed to respond. In addition, exploratory multivariate analyses found no influence of cytogenetics, age, number of chemotherapy cycles to achieve first CR, duration of first CR, and CD34 expression on the rates of CR and CRp.1 Therefore, those clinical and biological parameters would not be expected to confound the assessment of the effects of MDR features on response.
Among the 142 patients treated on the phase II gemtuzumab ozogamicin trials, the overall response rate (CR plus CRp) was 30%. The rates of CR and CRp among the patients with blast cell data for 4E3.16 staining intensity ratio and DiOC2 efflux ratio were 25% and 30%, respectively; thus, these cohorts are representative of the group as a whole.
The achievement of CR or CRp correlated with low dye efflux. The median baseline DiOC2 efflux ratio of blast cell populations from 38 patients with CR and CRp was 1.24 (range 1.04 to 3.02), compared with 1.58 (range 1.06 to 5.01) in 88 samples from nonresponders (Wilcoxon rank sum test, P = .002) (Figure2A). Elevated dye efflux was reversed by CSA in 46 (52%) of 88 nonresponder blast cell samples and in only 9 (24%) of 38 samples from patients who achieved CR or CRp (P = .003; odds ratio 3.5; 95% confidence interval, 1.5 to 8.3). In contrast, surface expression of Pgp as indicated by 4E3.16 staining intensity was not significantly different among samples from patients with CR and CRp compared with samples from nonresponders (Figure 2B). This may be due to the relatively lower sensitivity of this assay in clinical AML samples and/or to the smaller number of patients evaluated by this method.
Correlations of blast cell Pgp features with achievement of complete remission (CR and CRp) or clearance of marrow blasts as an indication of in vivo drug susceptibility.
(A) The median baseline DiOC2 efflux ratio (indicated by the horizontal line) was significantly higher among the group of samples from patients who failed to respond to gemtuzumab ozogamicin compared with samples from patients who achieved a CR or CRp (1.58 vs 1.24; *P = .002). In addition, samples with CSA-sensitive DiOC2 efflux (black circles) were more common in the group without a response (52%) compared with responders (24%; P = .003). (B) The median levels of Pgp surface expression as indicated by 4E3.16 staining intensity ratios were not significantly different between samples from patients with CR or CRp and samples from nonresponders. (C) In comparison, Pgp surface expression was significantly higher among samples from patients who failed to clear marrow blasts compared with patients who achieved 5% or fewer blasts after 2 doses of gemtuzumab ozogamicin (1.11 vs 1.03; *P = .03). (D) The median baseline DiOC2efflux ratio among samples from patients with residual leukemia was significantly higher than the median value among samples from patients who cleared marrow blasts (1.73 vs 1.27; *P = 0.0002), and nonresponder patient samples more frequently exhibited inhibition of dye efflux by CSA (72%) compared with samples from patients with 5% or fewer marrow blasts after 2 doses of gemtuzumab ozogamicin (29%;P < .001).
Correlations of blast cell Pgp features with achievement of complete remission (CR and CRp) or clearance of marrow blasts as an indication of in vivo drug susceptibility.
(A) The median baseline DiOC2 efflux ratio (indicated by the horizontal line) was significantly higher among the group of samples from patients who failed to respond to gemtuzumab ozogamicin compared with samples from patients who achieved a CR or CRp (1.58 vs 1.24; *P = .002). In addition, samples with CSA-sensitive DiOC2 efflux (black circles) were more common in the group without a response (52%) compared with responders (24%; P = .003). (B) The median levels of Pgp surface expression as indicated by 4E3.16 staining intensity ratios were not significantly different between samples from patients with CR or CRp and samples from nonresponders. (C) In comparison, Pgp surface expression was significantly higher among samples from patients who failed to clear marrow blasts compared with patients who achieved 5% or fewer blasts after 2 doses of gemtuzumab ozogamicin (1.11 vs 1.03; *P = .03). (D) The median baseline DiOC2efflux ratio among samples from patients with residual leukemia was significantly higher than the median value among samples from patients who cleared marrow blasts (1.73 vs 1.27; *P = 0.0002), and nonresponder patient samples more frequently exhibited inhibition of dye efflux by CSA (72%) compared with samples from patients with 5% or fewer marrow blasts after 2 doses of gemtuzumab ozogamicin (29%;P < .001).
Because failure to achieve a CR or CRp may result from infection or other morbidities rather than from failure of gemtuzumab ozogamicin to kill leukemic cells, we evaluated clearance of blasts from the marrow after 2 doses of gemtuzumab ozogamicin as a more accurate indicator of in vivo drug susceptibility. Adequate blast cell counts, determined by a central review of marrow aspirates, were available on 52 patients with data for 4E3.16 staining and 74 patients with data for DiOC2 efflux. The rate of blast clearance among all patients with adequate marrow counts was 66%, whereas the rates among patients with data for 4E3.16 staining and dye efflux were 65% and 66%, respectively. Marrow blast clearance was associated with a lower median 4E3.16 staining intensity ratio (1.03 for patients with marrow clearance [range 0.89 to 1.22, n = 34] vs 1.11 for patients with residual marrow blasts [range 0.95 to 1.97, n = 18],P = .03, Figure 2C). Also, marrow blast clearance was strongly associated with a lower median baseline DiOC2efflux ratio (1.27 for patients with marrow clearance [range 1.04 to 3.02, n = 49] vs 1.73 for patients with residual marrow blasts [range 1.14 to 3.73, n = 25], P = .0002, Figure 2D). Inhibition of dye efflux by CSA occurred in 18 (72%) of 25 samples from patients with residual leukemia, compared with only 14 (29%) of 49 samples from patients who achieved 5% or fewer marrow blasts (P < .001; odds ratio 6.4; 95% confidence interval, 2.2 to 18.8).
Although most samples with higher baseline DiOC2efflux ratios were also inhibited by CSA, thus likely indicating Pgp function, some were not. For example, 14 samples from nonresponders and 5 from patients with CR or CRp with baseline efflux ratios in the high-intermediate to high ranges (ie, ≥ 1.46) were not sensitive to CSA inhibition. Further studies will be required to determine if non-Pgp transporters are responsible for dye efflux in these samples and whether this mechanism has an effect on clinical response.
In vitro drug-induced apoptosis correlates with clinical remission and marrow blast clearance
In vitro blast cell susceptibility to gemtuzumab ozogamicin was characterized in samples from 75 patients. Apoptosis was used as the indicator of in vitro drug susceptibility. For this assay, the percentage of annexin V+, PI− cells was determined following a 2-hour in vitro exposure to gemtuzumab ozogamicin. Maximal apoptosis was usually seen at 72 hours after in vitro exposure. The percentage of cells with specific annexin V binding following exposure to gemtuzumab ozogamicin ranged from 0% to 36%, with a median value of 6%.
The level of apoptosis was first compared with blast cell Pgp features to investigate an association of MDR phenotype with in vitro drug resistance. Lower levels of apoptosis were associated with higher Pgp surface expression, as indicated by 4E3.16 staining; however, this trend did not reach statistical significance (Figure3A). In comparison, lower levels of apoptosis were significantly associated with higher baseline DiOC2 efflux ratios that were reversed by CSA (Figure 3B). Among the 28 samples with CSA-sensitive dye efflux, the median level of apoptosis was 4% (range 0% to 23%). Furthermore, only 5 of those 28 samples achieved a level of apoptosis greater than 7%, and 4 of those 5 samples exhibited baseline DiOC2 efflux ratios in the low-intermediate range in the absence of CSA (ie, 1.21 to 1.45). In comparison, no correlation was seen between apoptosis and baseline dye efflux among samples with baseline ratios less than 1.21 or elevated efflux that was not sensitive to CSA (ie, absence of Pgp function) (Figure 3B). This latter group was more susceptible to gemtuzumab ozogamicin, with a 10% median level of apoptosis (range 0% to 36%) and 28 (60%) of 47 achieving a level greater than 7%. Together, these data indicate that lower in vitro drug susceptibility is linked to Pgp function, as reflected by CSA-sensitive dye efflux.
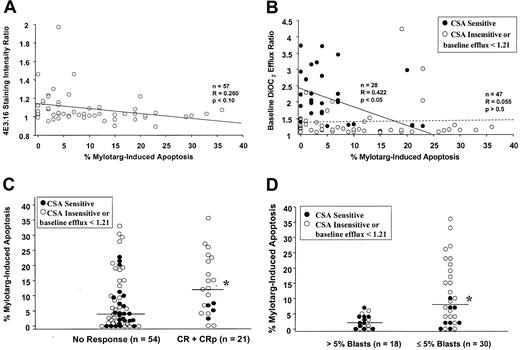
Correlations of in vitro drug-induced apoptosis with blast cell Pgp features and with achievement of complete remission (CR and CRp) or clearance of marrow blasts after treatment with gemtuzumab ozogamicin.
(A) In vitro blast cell susceptibility to gemtuzumab ozogamicin, as indicated by drug-induced apoptosis, was greater among samples with lower Pgp surface expression; however, the regression coefficient did not reach statistical significance. (B) Higher levels of drug-induced apoptosis were observed predominantly in blast cell samples with lower baseline DiOC2 efflux ratios. This correlation was statistically significant among samples with baseline dye efflux ratios greater than or equal to 1.21 and sensitivity to CSA inhibition of dye efflux (black circles and solid line). In contrast, no correlation was seen among samples either insensitive to CSA or with baseline efflux ratios less than 1.21 (dotted line). (C) Blast cell samples from patients with CR or CRp were more susceptible to drug in vitro, as indicated by a significantly higher median percentage of gemtuzumab ozogamicin–induced apoptosis (12%), compared with samples from patients who failed to achieve remission (median 4% apoptosis; *P = .03). CSA-sensitive DiOC2 efflux (black circles) was more common among the nonresponder patient samples (46%) than samples from responders (19%). (D) Similarly, the median value for the percentage of gemtuzumab ozogamicin–induced apoptosis among samples from patients who cleared marrow blasts was significantly higher than the median value among the samples from patients with residual leukemia (8% vs 2%; *P = .0009). Of note, a level of apoptosis greater than 7% was observed exclusively in samples from patients with marrow blast clearance, whereas lower levels of apoptosis were observed in samples from both responders and nonresponders. CSA-sensitive DiOC2 efflux was more common in nonresponder samples (61%) compared with samples from patients who achieved 5% or fewer marrow blasts (27%).
Correlations of in vitro drug-induced apoptosis with blast cell Pgp features and with achievement of complete remission (CR and CRp) or clearance of marrow blasts after treatment with gemtuzumab ozogamicin.
(A) In vitro blast cell susceptibility to gemtuzumab ozogamicin, as indicated by drug-induced apoptosis, was greater among samples with lower Pgp surface expression; however, the regression coefficient did not reach statistical significance. (B) Higher levels of drug-induced apoptosis were observed predominantly in blast cell samples with lower baseline DiOC2 efflux ratios. This correlation was statistically significant among samples with baseline dye efflux ratios greater than or equal to 1.21 and sensitivity to CSA inhibition of dye efflux (black circles and solid line). In contrast, no correlation was seen among samples either insensitive to CSA or with baseline efflux ratios less than 1.21 (dotted line). (C) Blast cell samples from patients with CR or CRp were more susceptible to drug in vitro, as indicated by a significantly higher median percentage of gemtuzumab ozogamicin–induced apoptosis (12%), compared with samples from patients who failed to achieve remission (median 4% apoptosis; *P = .03). CSA-sensitive DiOC2 efflux (black circles) was more common among the nonresponder patient samples (46%) than samples from responders (19%). (D) Similarly, the median value for the percentage of gemtuzumab ozogamicin–induced apoptosis among samples from patients who cleared marrow blasts was significantly higher than the median value among the samples from patients with residual leukemia (8% vs 2%; *P = .0009). Of note, a level of apoptosis greater than 7% was observed exclusively in samples from patients with marrow blast clearance, whereas lower levels of apoptosis were observed in samples from both responders and nonresponders. CSA-sensitive DiOC2 efflux was more common in nonresponder samples (61%) compared with samples from patients who achieved 5% or fewer marrow blasts (27%).
We next addressed the association of in vitro drug-induced apoptosis with clinical response. The rate of clinical response (CR and CRp) among the 75 patients with apoptosis data was 28%. The median percentage of apoptosis among the 21 samples from patients with CR and CRp was 12% (range 0% to 36%), compared with 4% among the 54 samples from nonresponding patients (range 0% to 33%,P = 0.03, Figure 3C). Central review marrow blast cell counts were available on 48 patients with apoptosis data, of whom 63% achieved 5% or fewer blasts after treatment. Marrow blast clearance was similarly found to correlate strongly with a higher median percentage of in vitro gemtuzumab ozogamicin–induced apoptosis (8% for patients with marrow clearance [range 0% to 36%, n = 30] vs 2% for patients with residual marrow blasts [range 0% to 7%, n = 18], P = .0009, Figure 3D). Higher levels of apoptosis were seen only in samples from patients who cleared marrow blasts after gemtuzumab ozogamicin treatment. Specifically, apoptosis greater than 7% occurred in 15 (50%) of 30 samples from patients with 5% or fewer marrow blasts, compared with 0 (0%) of 18 samples from nonresponding patients. These differences in drug susceptibility in culture were not attributable to variations in input blast cell populations, because the median pretreatment blast percentage in the responding cohort was 80% (range 0% to 100%) and the median in the nonresponding patient samples was 88% (range 6% to 100%). Thus, specific apoptosis above 7% predicted for blast cell clearance in this group of patients. However, levels of apoptosis of 7% or less were not predictive; half of the samples from responding patients and all of the nonresponder samples showed this lower level of in vitro susceptibility.
Lower in vitro apoptosis was also associated with Pgp function, as indicated by CSA-sensitive dye efflux, in nonresponder blast cell samples. Further analysis revealed that among the patients failing to achieve CR or CRp, 25 (46%) of 54 blast cell samples exhibited CSA-sensitive dye efflux and 19 (76%) of those 25 samples were associated with 7% or less apoptosis (Figure 3C). In comparison, only 4 (19%) of the 21 samples from responding patients exhibited CSA-sensitive dye efflux, and all of those were associated with 7% or less drug-induced apoptosis. Of note, 15 blast cell samples from nonresponders had 7% or less apoptosis and no evidence of Pgp function, suggesting that non-Pgp mechanisms mediate drug resistance in vitro and may play a role in clinical response. Among the patients who failed to clear marrow blasts after treatment, the lower levels of apoptosis were associated with CSA-sensitive dye efflux in 11 (61%) of 18 samples, whereas 8 (27%) of 30 samples from patients who achieved 5% or fewer marrow blasts exhibited CSA-sensitive dye efflux (Figure3D).
Enhancement of in vitro gemtuzumab ozogamicin–induced apoptosis by cyclosporine
Pretreatment blast cell samples were incubated with and without CSA during in vitro gemtuzumab ozogamicin exposure to determine whether an MDR reversal agent could augment drug-induced apoptosis. Data were available on samples from 91 study-eligible patients.
Of particular interest was the ability of CSA to increase apoptosis to greater than 7%, because patients whose cells exhibited that level of apoptosis achieved marrow blast clearance after treatment. Forty-nine patient samples with baseline levels of gemtuzumab ozogamicin–induced apoptosis less than or equal to 7% (median 2%, range 0% to 7%) were evaluated for an effect of CSA on drug susceptibility. When those samples were coincubated with CSA and gemtuzumab ozogamicin, apoptosis levels were increased to greater than 7% in 14 (29%) of 49. Among those 14 responsive samples, the median level of apoptosis in the presence of CSA was 11% (range 8% to 25%) and the median increase from the baseline level without CSA was 7%. In comparison, among the 35 samples with low baseline apoptosis that was not enhanced by CSA, the median level of apoptosis with CSA was 3% (range 0% to 7%) and the median increase from baseline was only 1%. Thus, CSA can improve gemtuzumab ozogamicin cytotoxicity in at least a subset of blast cell populations in vitro and may therefore be of benefit in vivo. Because only 30 of the 91 study-eligible patients with apoptosis data were subsequently treated and/or evaluable on treatment protocols, and only 13 of those 30 samples exhibited low baseline apoptosis in the absence of CSA, definitive correlations regarding CSA chemosensitization and clinical outcome could not be made.
Discussion
In this study of patients with relapsed AML, we found that blast cell Pgp function, as indicated by CSA-sensitive dye efflux, was associated with a poorer clinical response to targeted therapy with gemtuzumab ozogamicin. Because other clinical and biological features, such as cytogenetics, age, number of chemotherapy cycles required to achieve first CR, duration of first CR, and CD34 expression, did not correlate with response in this patient population,1 the MDR phenotype appears to represent an important independent prognostic variable. Pgp features were more commonly observed in samples from patients who failed to clear marrow blasts and failed to achieve CR or CRp, compared with cells from patients with responses. Because DiOC2 efflux was effectively modulated by CSA in most of the drug-resistant samples from nonresponding patients, CSA could be a potent chemosensitizing adjunct in vivo.
To further investigate the relationship of MDR features and drug susceptibility, we evaluated in vitro gemtuzumab ozogamicin–induced apoptosis. We found that drug-induced levels of apoptosis greater than 7% predicted for marrow blast clearance in vivo; however, low levels of apoptosis were not predictive because they did not distinguish between responders and nonresponders. Significantly, low levels of drug-induced apoptosis were associated with CSA-sensitive dye efflux and, in a portion of samples, CSA increased gemtuzumab ozogamicin–induced apoptosis. Thus, CSA may augment gemtuzumab ozogamicin–induced cytotoxicity against MDR-expressing leukemic blasts in vivo. Furthermore, this combination should avoid the enhanced toxicity seen when MDR reversal agents are combined with nontargeted conventional chemotherapy.
The poor response to gemtuzumab ozogamicin in patients with high functional expression of Pgp mimics the adverse prognostic impact of MDR features in AML treated with conventional chemotherapy.5-12 In de novo AML, blast cells from roughly 20% to 40% of younger patients and from more than 70% of older patients express Pgp.5,6,8,10-12,21 A high frequency of expression is also seen in secondary and relapsed adult AML5-7 and in cases that coexpress CD34.8,19Pgp expression is linked to lower CR rates, higher rates of refractory disease and, in some studies, shorter overall survival after treatment with cytarabine and daunorubicin, mitoxantrone, idarubicin, or etoposide.6,8,10-12 Drug resistance is presumed to be due to the active export of anthracyclines and epidophyllotoxins by Pgp, thereby preventing drug-induced cell death; however, alternative mechanisms may be involved in some cases.11,19Observations with cell lines suggest that CSA-sensitive, non-Pgp membrane transporters might prevent drug-induced cytotoxicity.22 Other investigations with cell lines and primary AML samples have demonstrated that Pgp can inhibit apoptosis by an efflux-independent mechanism.23 In our studies, 45% of relapsed AML patient blast cell samples exhibited CSA-sensitive dye efflux, and only 24% of those patients achieved CR or CRp after receiving gemtuzumab ozogamicin. In comparison, 52% of patients whose blasts exhibited low baseline dye efflux or lack of CSA sensitivity responded. Given the lower expected frequency of MDR expression in de novo AML, our observations suggest that gemtuzumab ozogamicin may show greater efficacy in those patients than in patients with relapsed, refractory, or secondary disease.
Although blast cell CSA-sensitive dye efflux correlated with Pgp surface expression and in vitro drug susceptibility in most patient samples, some discordant phenotypic features and clinical responses were seen. For example, lower Pgp surface expression and/or lack of dye efflux inhibition by CSA were associated with moderate to high baseline DiOC2 efflux ratios in a number of samples. Similar examples of discordance between AML cell dye efflux and membrane Pgp display have been reported by others.10,11 19 We further found that low in vitro drug-induced apoptosis (ie, <7% apoptosis) was associated with lack of evidence for Pgp function in 7 samples from patients who failed to clear marrow blasts and in 15 samples from patients who failed to achieve CR or CRp. Together, these observations suggest that non-Pgp transporters and mechanisms other than drug efflux may contribute to gemtuzumab ozogamicin resistance in vitro and in vivo.
Assays for in vitro gemtuzumab ozogamicin–induced apoptosis were carried out to determine whether they were predictive of in vivo response and whether cytotoxicity was impaired by Pgp function, enabling further exploration of Pgp mechanisms. Prior studies of conventional chemotherapeutic agents have similarly used in vitro apoptosis or cytotoxicity assays to correlate with clinical response.24-31 Although some have demonstrated a direct association between drug susceptibility in culture and in vivo drug activities24,25 or clinical responses,26,31 others have observed discrepant results attributed to either heterogeneity within the AML blast cell populations30 and/or the multifactorial nature of drug resistance.27-30 Data from these and other studies indicate that a variety of mechanisms can contribute to the MDR phenotype in AML32,33 and that in vitro drug susceptibility assays must be interpreted in the context of potential mediators affecting drug transport, detoxification, and cell survival. In our studies, higher levels of drug-induced apoptosis in vitro were highly predictive of in vivo gemtuzumab ozogamicin susceptibility, as indicated by marrow blast clearance. We also observed a negative correlation between Pgp function and in vitro gemtuzumab ozogamicin–induced apoptosis, consistent with reports suggesting that free calicheamicin is a Pgp substrate and that overexpression of Pgp renders AML cell lines resistant to gemtuzumab ozogamicin cytotoxicity.4
We extended our observations further by showing that, in a portion of samples, CSA enhances gemtuzumab ozogamicin–induced apoptosis, achieving a level of in vitro susceptibility that was associated with improved clinical responses. Although these results support the use of MDR reversal agents in clinical trials with gemtuzumab ozogamicin, further studies are clearly needed to better understand the mechanisms of drug-induced cell death, the precise role of Pgp in this process, and the potential contributions of other resistance factors. In addition, because low levels of gemtuzumab ozogamicin–induced apoptosis were seen with samples from both responders and nonresponders, our apoptosis assay was not sufficiently powerful to recommend it for clinical decision-making.
In summary, our findings demonstrate a role for Pgp in clinical gemtuzumab ozogamicin resistance in patients with first relapse of AML. Based on these observations, treatment trials combining gemtuzumab ozogamicin with MDR reversal agents are warranted. Given the potential for hepatotoxicity with gemtuzumab ozogamicin,1,34combination protocols using reversal agents that might also affect liver function, such as infusional CSA,13 should be carefully designed and closely monitored for safety. Additional studies to define the cellular mechanisms of cytotoxicity and Pgp-mediated resistance will be important both for devising optimal reversal strategies and for identifying potential mechanisms relevant to other antibody-based targeted therapies. Finally, further studies are needed to characterize non-Pgp transporters and nontransporter resistance mechanisms, because they appear to be important in some patients who fail to respond to gemtuzumab ozogamicin.
M.S.B. and L.H.L. are employees of Wyeth-Ayerst Research, whose product was studied in this article.
Supported in part by research funding from Genetics Institute/Wyeth-Ayerst to I.D.B. I.D.B. is supported by the American Cancer Society F. M. Kirby Clinical Research Professorship.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Michael Linenberger, Seattle Cancer Care Alliance, Div of Hematology, Mailstop G6-800, 825 Eastlake Ave E, PO Box 19023, Seattle, WA 98109; e-mail: linen@u.washington.edu.

![Fig. 1. Correlations of pretreatment blast cell Pgp surface expression and Pgp function. / Pgp surface expression, as determined by the immunofluorescence staining intensity ratio using the antibody 4E3.16, correlated significantly with Pgp function, as indicated by the efflux ratio of the Pgp substrate dye DiOC2 in the 36 patient samples where dye efflux was inhibited to levels greater than or equal to 50% by CSA (black circles). In comparison, surface expression of Pgp correlated poorly with the baseline dye efflux ratio in the 23 patient samples that were insensitive to CSA inhibition (ie, < 50% inhibition of efflux [white circles]) despite exhibiting elevated levels of baseline efflux at or above the levels of samples that were sensitive to CSA.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/4/10.1182_blood.v98.4.988/6/m_h81611429001.jpeg?Expires=1768107310&Signature=bm3WWxWLsg9uNPGaEkUGPF3aTeW7K3mtTd--q1lPEQUEmudMSITAoayFv9cJq60RG0Uls6VXSgsrtsCp2mVLh6xVAhy~pLTwGoSrHpmK7bAaLnM7x8E76uI2lp50fIiRFyM-ltb6cZl8TZmQczJAk9CHJnDGCgk9bDKSjo0OBdaU-r9fG5AH3rtUBd3wg6Zjrk~Kf4AcD2S~D8UYIxamTqdbtdTwczmaMpAkBta~obH132gZC7AwwZZ4F0w0C0v6lOChIqO592hGmhyh0bt2k9JiApPdQ~0A15h7qeyk--Vgb01qZELGG0bucgCpFToYQjY~QpnCnZPKHnp~p-pLyQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)