Many reports indicate different nonantisense yet sequence-specific effects of antisense phosphorothioate oligonucleotides. Products of enzymatic degradation of the oligonucleotides can also influence cell proliferation. The cytotoxic effects of deoxyribonucleoside-5′-phosphates (dNMPs) and their 5′-phosphorothioate analogs, deoxyribonucleoside-5′-monophosphorothioates (dNMPSs) on 4 human cell types (HeLa, HL-60, K-562, and endothelial cells) were examined, and the effects were correlated with the catabolism of these compounds. The results indicate that differences in cytotoxicity of dNMPs or dNMPSs in these cells depend upon different activity of an ecto-5′-nucleotidase. It has also been found that dNMPSs stimulate proliferation of human umbilical vein endothelial cells and HL-60 cells in a concentration-dependent manner. This stimulation might be caused by the binding of deoxynucleoside-5′-phosphorothioates to as-yet unidentified nucleotide receptor(s) at the cell surface.
Introduction
The rapid degradation of unmodified oligodeoxynucleotides (phosphodiester oligodeoxynucleotides [PO oligos]) by exonucleases and endonucleases limits their application as antisense constructs1 and requires the synthesis and use of modified oligonucleotides. Phosphorothioate analogs of oligonucleotides (PS oligos) containing a sulfur atom at each internucleoside bond still constitute the most promising class of oligonucleotide analogs for potential therapeutic purposes. Although they are much more resistant to nucleolytic degradation than the PO oligos, reports indicating their stereodependent hydrolysis have been published recently.2,3 Moreover, results published by Vaerman et al4 indicate that the cytotoxicity of PO and PS oligos might have been, in part, caused by mononucleotides deoxyribonucleoside-5′-phosphates (dNMPs) or their phosphorothioate analogs, deoxyribonucleoside-5′-monophosphorothioates (dNMPSs) released during enzymatic degradation of the oligonucleotides. The toxicity of the dNMPs to leukemia cell lines depends on their concentration and/or the type of nucleobase. It appeared that 2′-deoxyguanosine 5′-monophosphate (dGMP) was more toxic to the BV 173 cell line than other dNMPs used at the same concentration. This observation indicates that nonantisense mechanisms of the action of oligonucleotides may be sequence-dependent.4 However, Vaerman et al4studied the cytotoxicity of mononucleotides and oligonucleotides using only the leukemia BV 173 cell line bearing the Philadelphia chromosome, and CD34+ bone marrow cells. Some earlier data5-8 have also shown that deoxyribonucleosides influence the growth of leukemia cell lines, but there is no evidence that other types of cells are also sensitive to mononucleotides or their phosphorothioate analogs. Because we have studied the synthesis and therapeutic application of phosphorothioate oligonucleotides,9-11 we have undertaken efforts to explain their potential nonantisense cytotoxicity.
In this study, we have analyzed the influence of mononucleotides (dNMPs and dNMPSs) and oligonucleotides (PO and PS oligos) on 4 different human cell types: the HeLa epitheloid carcinoma cell line, HL-60 promyelocytic leukemia cells, K-562 myelogenous leukemia cells bearing the Philadelphia chromosome, and human umbilical vein endothelial cells (HUVECs). We have examined the cytotoxic effects of mononucleotides, deoxynucleosides, and free purine bases and have correlated the effects with the catabolism of these compounds. It is known that the extracellular metabolism of adenosine 5′-triphosphate (ATP) and adenosine is controlled by enzymes such as ecto-ATP-ases, 5′-nucleotidases, and phosphatases, which are present on the surface of many different cell types.12 Although the biological roles of ATP and adenosine in the different signaling pathways have been studied in detail, the effects of other nucleotides and nucleosides on cell proliferation have not been characterized. Here, we demonstrate that deoxynucleosides, their 5′-phosphates, and their 5′-phosphorothioates can also influence cell proliferation. In the cell types investigated, the cytotoxicity of extracellular dNMPs and dNMPSs depends on the activity of the ecto-5′-nucleotidase (ecto-5′-NT) present at the cell surface. The membrane-bound enzyme can convert dNMPs and dNMPSs to deoxynucleosides, which are, in turn, the substrates for other enzymes (purine nucleoside phosphorylase, adenosine deaminase, guanosine deaminase) or are taken up by the cells. Because the cell lines studied demonstrate different ecto-5′-NT activity, the cytotoxic effects of dNMPs, dNMPSs, and deoxynucleosides are different in each of these cell lines. However, ectonucleotidase activity does not seem to be the only mechanism responsible for the biological activity of these compounds. In the course of our studies, we have also observed that dNMPSs stimulate growth of HUVECs and HL-60 cells in a concentration-dependent manner. This effect might be caused by the binding of deoxynucleoside-5′-phosphorothioates to as-yet unidentified nucleotide receptor(s) at the cell surface.
Materials and methods
Chemicals
The dNMPSs were synthesized by means of the modified method of Murray and Atkinson13 and purified by high-performance liquid chromatography (HPLC). Purine bases (guanine, xanthine, hypoxanthine, and adenine), deoxyguanosine, inosine, and dNMPs were obtained from Sigma (St Louis, MO). Thymidine- and deoxyadenosine-α-thiotriphosphates (TTPαS and dATPαS) were obtained from Pharmacia. Thymidine 3′-phosphorothioate and thymidine 5′,3′-bisphosphorothioate were synthesized by means of the modified oxathiaphospholane method.9 Nucleoside transport inhibitors (nitrobenzylthioinosine [NBTI], dipyridamole, and dilazep); α,β-methylene–adenosine 5′-diphosphate (α,β-methylene–ADP); and α,β-methylene–ATP were also purchased from Sigma. T4 polynucleotide kinase was purchased from Amersham (Buckinghamshire, United Kingdom). Fetal bovine serum (FBS) was obtained from Gibco BRL (Rockville, MD). Before being used in cell cultures, FBS was thermally inactivated by heating for 1 hour at 56°C. Endothelial cell growth factor (ECGF) was obtained from Boehringer Mannheim (Mannheim, Germany).
Oligonucleotides
Cell lines
Endothelial cells (HUVECs) were isolated from navel strings by washing of umbilical veins with 0.1% collagenase solution. The cell lines studied were maintained in RPMI 1640 medium supplemented with 10% (HeLa, K-562) or 20% (HL-60, HUVEC) thermally inactivated FBS, 100 μg/mL streptomycin, and 100 IU/mL penicillin at 37°C in a humidified atmosphere of 5% CO2. In the case of the HUVECs, the medium was additionally supplemented with ECGF (150 μg/mL) and heparin (90 μg/mL). The endothelial cells were cultured in bottles covered with 1% gelatin.
Purine base, deoxynucleoside, deoxynucleotide, or oligonucleotide treatment of cell lines
Cells in exponential growth phase were washed with RPMI 1640 containing FBS and resuspended in this medium at a concentration of 5 × 104 per 200 μL (K-562, HeLa, or HL-60 cells) or 7 × 104 per 200 μL (HUVECs). Guanine, xanthine, deoxynucleosides, or mononucleotides (25 or 100 μM) were added to the cell cultures as sodium salts at time zero of each experiment without any further addition of the reagents. Additionally, the concentration-dependent influence of dGMP and dGMPS on the K-562 and HL-60 cell growth was studied at a 2.5- to 100-μM concentration range. In some experiments, dNMPs or dNMPSs (100 μM concentration) were added to the cells preincubated for 1 to 2 hours with α,β-methylene–ADP (100 μM), or α,β-methylene–ATP (100 μM). NBTI (1 μM), dipyridamole (20 μM), or dilazep (1 μM) was dissolved in water and added to cell cultures for 1 hour before the treatment of cells with nucleosides or mononucleotides. PO and PS oligos were added to the cell culture at 2.5-, 10-, or 25-μM concentration. The number of cells was determined every 24 hours by means of the tetrazolium salt (MTT) method.14
Chromatographic analysis of extracellular catabolism of nucleotides and nucleosides
Each of the studied cell lines was incubated with 200 μM substrate (deoxynucleotide, deoxynucleoside, or purine base) for 96 hours in RPMI 1640 culture medium containing 10% or 20% FBS. For the correlation of catabolism and cytotoxicity of nucleosides, nucleotides, and purine bases, these experiments were carried out under the same conditions as those done to analyze cytotoxicity. Therefore, the assay medium was not supplemented with nucleoside transport inhibitors. At given time points, 250-μL aliquots were removed from the cell cultures, denatured for 30 minutes at 95°C, and spun down. The samples were maintained at −20°C prior to HPLC analysis. The catabolites were identified by means of reverse-phase chromatography on 5 μ BDS Hypersil (Thermo Hypersil, Cheshire, United Kingdom). Purine bases, deoxynucleosides, dNMPs, and dNMPSs were separated with a triethylammonium bicarbonate (TEAB) and CH3CN gradient: 0 to 0.1 M TEAB from 0 to 8 minutes; then gradient 0.1 M TEAB 24% CH3CN/0.1 M TEAB from 8 to 12 minutes; and finally isocratic conditions, 24% CH3CN/0.1 M TEAB (12 to 18 minutes). Under these conditions, products of the enzymatic degradation of mononucleotides and corresponding deoxynucleosides have been readily separated, eg, the dGMP metabolites eluted as follows: xanthine at 4.1 minutes, guanine at 6.5 minutes, deoxyguanosine at 14.6 minutes, and deoxyguanosine 5′-phosphate at 14.1 minutes. In control experiments 5′-mononucleotides and their phosphorothioate analogs were incubated in FBS-supplemented medium (see above) or in the medium from cell cultures from which cells were removed after their 72-hour incubation. These experiments have been carried out to show that ecto-5′-NT activity responsible for mononucleotide dephosphorylation is cell-associated and that this is not present in serum or not released from cells into medium.
Oligodeoxynucleotide degradation in cell culture media
The 5′-32P–radiolabeled oligomers 1 through 4 (2.5 to 10 μM) were incubated at 37°C in RPMI 1640 medium containing 10% or 20% FBS. At various times, 10-μL aliquots were withdrawn and heated for 2 minutes at 95°C. Then, 50 μL water was added to each denatured sample. After vigorous shaking, the protein precipitates were spun down, and the aqueous solutions were dried in a Speed Vac rotary evaporator (Savant Instruments, Farmingdale, NY). The resultant samples were analyzed by 20% polyacrylamide/7 M urea gel electrophoresis. The autoradiograms were scanned by means of an LKB Ultrascan XL densitometer (Pharmacia LKB, Uppsala, Sweden).
Results
Purine-base, nucleoside, or mononucleotide treatment of the K-562 cell line
To analyze the influence of 5′-mononucleotides and their phosphorothioate analogs on the cell growth K, 562 cells were incubated with either dNMPs or dNMPSs for 96 hours. These experiments have shown that dNMPs and dNMPSs are not toxic to the K-562 cell line: after a 72-hour incubation with dNMP or dNMPS, at 100 μM concentration, cell viability was decreased only by 5% to 10%. However, deoxyguanosine and guanine, used at the same concentration, inhibited cell growth by 90% (Table 1). We hypothesized that the different effects of guanine, deoxyguanosine, and dGMP on the K-562 cell line resulted from different catabolism of these compounds, which could be catabolized not only by serum enzymes present in culture medium but also by enzymes anchored to cell membranes. To examine this hypothesis, K-562 cells were incubated with these compounds for 96 hours, and the resultant media supernatants were analyzed by HPLC. HPLC analysis of the control samples (see “Materials and methods”) has shown that after a 72-hour incubation only 10% to 15% of mononucleotides or their 5′-phosphorothioate analogs were converted to the corresponding nucleosides by serum enzymes. Moreover, the cells did not release nucleotidase or phosphatase activities to the medium because dNMPs or dNMPSs were dephosphorylated in the medium taken from these cells cultures to a similar extent as the dephosphorylation in FBS-supplemented medium (Figure 1). These results have shown that an activity responsible for dephosphorylation of dNMPs and dNMPSs is cell membrane–associated. To underline cellular localization of this enzyme, we have used the term ecto-5′-NT.15 The analysis carried out for K-562 cells demonstrated that after a 72-hour incubation only 10% of the dGMP was converted to deoxyguanosine; this could result from phosphatase and/or nucleotidase activity of FBS-supplemented medium (Figure 1). This result indicates very low ecto-5′-NT activity on the K-562 cells. Therefore, dGMP is not efficiently dephosphorylated to deoxyguanosine and remains nontoxic to these cells. Probably because of the low ecto-5′-NT activity, thymidine-5′-phosphate (TMP) is also not toxic to the K-562 cells, although, after a 72-hour incubation, thymidine (100 μM concentration) decreased the number of viable cells by 40%.
The influence of mononucleotides, nucleosides and purine bases on human umbilical vein endothelial cell, HeLa, K 562, and HL-60 cell growth
| . | Cell counts after 72-hour incubation ± SD . | |||
|---|---|---|---|---|
| HUVECs . | HeLa . | K 562 . | HL-60 . | |
| dAMP | 90.3 ± 6.3 | 88.0 ± 9.6 | 97.2 ± 6.9 | 98.0 ± 6.9 |
| TMP | 87.3 ± 12.7 | 71.8 ± 10.8 | 89.2 ± 6.6 | 100.3 ± 8.5 |
| dGMP | 72.8 ± 8.2 | 85.6 ± 6.3 | 97.6 ± 11.3 | 100.0 ± 12.7 |
| dCMP | 99.0 ± 1.0 | 95.0 ± 11.0 | 94.3 ± 8.7 | 98.0 ± 14.5 |
| dAMPS | 124.8 ± 5.7 | 89.2 ± 14.8 | 96.8 ± 5.2 | 120.0 ± 11.2 |
| TMPS | 153.0 ± 11.4 | 98.3 ± 12.8 | 99.5 ± 5.8 | 139.0 ± 10.2 |
| dGMPS | 133.2 ± 12.1 | 98.7 ± 11.2 | 106.3 ± 8.5 | 139.0 ± 10.5 |
| dCMPS | 117.5 ± 10.5 | 94.5 ± 6.5 | 99.7 ± 2.5 | 118.0 ± 10.8 |
| dAdenosine | 90.0 ± 6.0 | 85.5 ± 6.5 | 86.3 ± 11.7 | 103.0 ± 2.2 |
| Thymidine | 92.7 ± 1.7 | 69.0 ± 2.2 | 69.8 ± 9.4 | 103.6 ± 6.5 |
| dGuanosine | 63.5 ± 10.8 | 72.7 ± 11.2 | 11.4 ± 2.3 | 45.5 ± 9.4 |
| dCytidine | 98.5 ± 3.5 | 85.5 ± 1.5 | 94.2 ± 4.0 | 91.5 ± 2.0 |
| Guanine | 68.0 ± 10.0 | 98.0 ± 2.1 | 13.0 ± 5.1 | 39.5 ± 6.5 |
| Xanthine | 96.0 ± 5.2 | 91.0 ± 2.2 | 107.7 ± 5.2 | 92.0 ± 7.1 |
| . | Cell counts after 72-hour incubation ± SD . | |||
|---|---|---|---|---|
| HUVECs . | HeLa . | K 562 . | HL-60 . | |
| dAMP | 90.3 ± 6.3 | 88.0 ± 9.6 | 97.2 ± 6.9 | 98.0 ± 6.9 |
| TMP | 87.3 ± 12.7 | 71.8 ± 10.8 | 89.2 ± 6.6 | 100.3 ± 8.5 |
| dGMP | 72.8 ± 8.2 | 85.6 ± 6.3 | 97.6 ± 11.3 | 100.0 ± 12.7 |
| dCMP | 99.0 ± 1.0 | 95.0 ± 11.0 | 94.3 ± 8.7 | 98.0 ± 14.5 |
| dAMPS | 124.8 ± 5.7 | 89.2 ± 14.8 | 96.8 ± 5.2 | 120.0 ± 11.2 |
| TMPS | 153.0 ± 11.4 | 98.3 ± 12.8 | 99.5 ± 5.8 | 139.0 ± 10.2 |
| dGMPS | 133.2 ± 12.1 | 98.7 ± 11.2 | 106.3 ± 8.5 | 139.0 ± 10.5 |
| dCMPS | 117.5 ± 10.5 | 94.5 ± 6.5 | 99.7 ± 2.5 | 118.0 ± 10.8 |
| dAdenosine | 90.0 ± 6.0 | 85.5 ± 6.5 | 86.3 ± 11.7 | 103.0 ± 2.2 |
| Thymidine | 92.7 ± 1.7 | 69.0 ± 2.2 | 69.8 ± 9.4 | 103.6 ± 6.5 |
| dGuanosine | 63.5 ± 10.8 | 72.7 ± 11.2 | 11.4 ± 2.3 | 45.5 ± 9.4 |
| dCytidine | 98.5 ± 3.5 | 85.5 ± 1.5 | 94.2 ± 4.0 | 91.5 ± 2.0 |
| Guanine | 68.0 ± 10.0 | 98.0 ± 2.1 | 13.0 ± 5.1 | 39.5 ± 6.5 |
| Xanthine | 96.0 ± 5.2 | 91.0 ± 2.2 | 107.7 ± 5.2 | 92.0 ± 7.1 |
HUVEC indicates human umbilical endothelial cell; DAMP, deoxyadenosine-5′-phosphate; TMP, thymidine-5′-phosphate; dCMP, deoxycytidine-5′-phosphate; dAMPS, deoxyadenosine-5′-phosphorothioate; TMPS, thymidine-5′-phosphorothioate; dGMPS, deoxyguanosine-5′-phosphorothioate; dCMPS, deoxycytidine-5′-phosphorothioate; dGMP, 2′-deoxyguanosine 5′-monophosphate; and an initial d, deoxy.
Cell viability was determined with tetrazolium salt assay (expressed as a percentage of control cell counts) 72 hours after addition of mononucleotides, nucleosides, or purine bases at a concentration of 100 μM. Cells were incubated in RPMI 1640 medium supplemented with 10% (K 562, HeLa) or 20% FBS (HUVECs, HL-60). Data points represent means of at least 6 experiments ± SD.
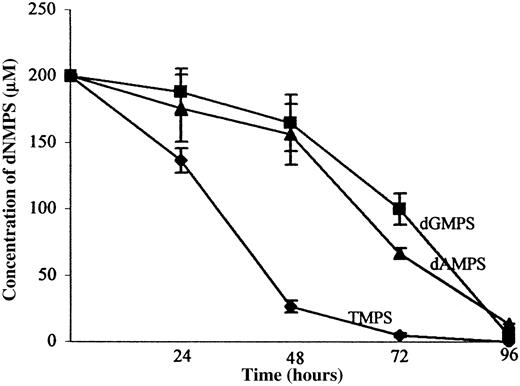
Time course of extracellular dGMP degradation catalyzed by enzymes.
Extracellular dGMP degradation was catalyzed by cell membrane-bound enzymes (A) or serum enzymes (B). The cells were incubated in RPMI 1640 medium supplemented with 10% or 20% FBS and containing 200 μM dGMP. Subsamples of extracellular medium to be used for HPLC analysis were taken at incubation times of 0, 24, 48, 72, and 96 hours and assayed for dGMP and deoxyguanosine. The concentrations were estimated from the peak areas obtained after HPLC. Data points in panel A represent means ± SDs from at least 3 experiments. (B) 2 indicates time course of dGMP degradation in RPMI medium supplemented with 20% heat-inactivated FBS. 1 and 3 indicate dGMP degradation in the medium taken from HeLa and HUVEC cultures, respectively, after their 72-hour incubation.
Time course of extracellular dGMP degradation catalyzed by enzymes.
Extracellular dGMP degradation was catalyzed by cell membrane-bound enzymes (A) or serum enzymes (B). The cells were incubated in RPMI 1640 medium supplemented with 10% or 20% FBS and containing 200 μM dGMP. Subsamples of extracellular medium to be used for HPLC analysis were taken at incubation times of 0, 24, 48, 72, and 96 hours and assayed for dGMP and deoxyguanosine. The concentrations were estimated from the peak areas obtained after HPLC. Data points in panel A represent means ± SDs from at least 3 experiments. (B) 2 indicates time course of dGMP degradation in RPMI medium supplemented with 20% heat-inactivated FBS. 1 and 3 indicate dGMP degradation in the medium taken from HeLa and HUVEC cultures, respectively, after their 72-hour incubation.
Role of nucleoside transporters in cytotoxic effects of nucleosides
The cytotoxic effect of 2′-deoxyguanosine to human leukemic cell lines was reported several years ago.5-8 It was postulated that this effect is caused by intracellular enzymatic phosphorylation of deoxyguanosine to its monophosphate, diphosphate, and, finally, triphosphate (dGTP), which acts as an allosteric inhibitor of ribonucleoside reductase.16-18 Therefore, the cytotoxic effects of mononucleotides on different cell lines may occur not only as the result of their dephosphorylation, but also as a consequence of their effective transport across cell membranes. Some nucleoside carriers responsible for cellular uptake of nucleosides can be inhibited by NBTI, dilazep, or dipyridamole.19Using these inhibitors, we determined their influence on the toxicity of the deoxyguanosine to the K-562 cells. We separately added NBTI, dipyridamole, or dilazep to the K-562 cell cultures, and after 1 hour of incubation, the cells were treated with deoxyguanosine as described above. It appeared that deoxyguanosine was less toxic to the cells preincubated with the inhibitors than to the cells without them (Figure2). After 48-hour incubation with deoxyguanosine, the number of K-562 cells was decreased by 60% as compared with the control, while dipyridamole, dilazep, or NBTI partially protected the K-562 cells from deoxyguanosine toxicity: in the presence of the inhibitors, the cell numbers were decreased by 30% to 40%. These results indicate that the antiproliferative effect of deoxyguanosine on the K-562 cell line is at least partially dependent on the nucleoside transport mechanism. However, some cytotoxic effects of deoxyguanosine have been observed even in the presence of the inhibitors; they decreased the antiproliferative effect of deoxyguanosine by 20% to 30%. The low effectiveness of the inhibitors can be, at least partially, caused by the activity of a purine nucleoside phosphorylase and generation of guanine. Guanine transport across cell membranes cannot be inhibited by NBTI, dilazep, or dipyridamole, and therefore, the decrease of K-562 cell numbers has been observed despite the presence of these inhibitors. Similar experiments using dipyridamole have also been carried out for other cells (HUVECs, HeLa, and HL-60), and their partial protection against deoxyguanosine cytotoxicity has also been observed (data not shown).
The influence of deoxyguanosine on the K-562 cell growth in the presence of nucleoside transport inhibitors.
K-562 cell viability (expressed as a percentage of control cell counts) was determined with MTT assay 24, 48, 72, and 96 hours after addition of 25 μM deoxyguanosine following 1-hour incubation with nucleoside transport inhibitors. Cells (5000 per 200 μL) were growing in RPMI 1640 medium containing 10% FBS. Data points represent means ± SD from 3 experiments. ░ indicates dGuo; ▩, dGuo and NBTI (1 mM); ▪, dGuo and dilazep (1 mM); ▥, dGuo and dipyridamole (20 mM); ■, dipyridamole (20 mM).
The influence of deoxyguanosine on the K-562 cell growth in the presence of nucleoside transport inhibitors.
K-562 cell viability (expressed as a percentage of control cell counts) was determined with MTT assay 24, 48, 72, and 96 hours after addition of 25 μM deoxyguanosine following 1-hour incubation with nucleoside transport inhibitors. Cells (5000 per 200 μL) were growing in RPMI 1640 medium containing 10% FBS. Data points represent means ± SD from 3 experiments. ░ indicates dGuo; ▩, dGuo and NBTI (1 mM); ▪, dGuo and dilazep (1 mM); ▥, dGuo and dipyridamole (20 mM); ■, dipyridamole (20 mM).
Purine base, nucleoside, and mononucleotide treatment of the HL-60 cells
In contrast to the K-562 cells, HL-60 cells do not possess the Philadelphia chromosome. In general, dNMPs (used at 25 or 100 μM concentration) were not toxic to the HL-60 cell line. On the other hand, exposure of a cell suspension to 100 μM deoxyguanosine resulted in a 40% to 50% decrease in cell numbers. HL-60 cells were also sensitive to guanine: it caused a 60% inhibition of cell growth after a 72-hour incubation. HPLC analysis has shown that during a 96-hour incubation, dNMPs (200 μM) were only slightly dephosphorylated to the corresponding deoxynucleosides. TMPs and dGMPs were dephosphorylated by 10%, while none of the dNMPSs were converted to the corresponding deoxynucleosides. These observations indicate that the HL-60 cells are protected from the cytotoxic effect of dGMP owing to the low ecto-5′-NT activity. We have not observed any cytotoxicity of deoxycytidine, deoxyadenosine, or thymidine to the HL-60 cells. Also, none of the dNMPSs inhibited cell growth (Table 1). Interestingly, we have observed that each dNMPS stimulated HL-60 cell growth. After a 72-hour incubation with dNMPS (100 μM, the number of viable cells was increased by 20% to 40%, as compared with the control experiments. The greatest proliferative effects were observed for thymidine-5′-phosphorothioate (TMPS) and dGMPS (40% to 50%) (Table 2). The essential role of the phosphorothioate function in the stimulation of the HL-60 cell growth was confirmed by the addition of a phosphatase-pretreated dGMPS sample to the cell culture. Phosphorothioate anions, released from the dGMPS molecules by the enzyme, did not stimulate cell growth. Additional experiments performed with thymidine 3′-phosphorothioate and thymidine 5′,3′-bisphosphorothioate have shown that the compounds did not influence HL-60 cell growth (data not shown). These results indicate that a target molecule binding 5′-dNMPS possesses significant specificity to phosphorothioate derivatives of deoxyribonucleosides.
Concentration-dependent influence of deoxyguanosine-5′-phosphorothioate on HL-60 cell growth
| Concentration (μM) . | Cell counts ± SD . | |||
|---|---|---|---|---|
| 24 h . | 48 h . | 72 h . | 96 h . | |
| 2.5 | 99.0 ± 9.0 | 92.2 ± 5.2 | 99.1 ± 5.9 | 93.1 ± 4.9 |
| 5.0 | 108.0 ± 15.7 | 116.1 ± 10.1 | 104.7 ± 11.6 | 114.5 ± 8.5 |
| 10.0 | 107.0 ± 18.0 | 120.2 ± 13.1 | 115.3 ± 7.3 | 120.8 ± 17.7 |
| 25.0 | 112.1 ± 11.0 | 136.0 ± 9.0 | 131.4 ± 8.7 | 112.7 ± 9.5 |
| 50.0 | 131.7 ± 15.7 | 148.6 ± 8.8 | 135.0 ± 11.2 | 123.0 ± 12.2 |
| 100.0 | 153.0 ± 18.4 | 151.5 ± 18.8 | 139.0 ± 10.5 | 118.8 ± 21.2 |
| Concentration (μM) . | Cell counts ± SD . | |||
|---|---|---|---|---|
| 24 h . | 48 h . | 72 h . | 96 h . | |
| 2.5 | 99.0 ± 9.0 | 92.2 ± 5.2 | 99.1 ± 5.9 | 93.1 ± 4.9 |
| 5.0 | 108.0 ± 15.7 | 116.1 ± 10.1 | 104.7 ± 11.6 | 114.5 ± 8.5 |
| 10.0 | 107.0 ± 18.0 | 120.2 ± 13.1 | 115.3 ± 7.3 | 120.8 ± 17.7 |
| 25.0 | 112.1 ± 11.0 | 136.0 ± 9.0 | 131.4 ± 8.7 | 112.7 ± 9.5 |
| 50.0 | 131.7 ± 15.7 | 148.6 ± 8.8 | 135.0 ± 11.2 | 123.0 ± 12.2 |
| 100.0 | 153.0 ± 18.4 | 151.5 ± 18.8 | 139.0 ± 10.5 | 118.8 ± 21.2 |
Cell viability was determined with tetrazolium salt assay (expressed as a percentage of control cell counts) 72 hours after addition of deoxyguanosine-5′-phosphorothioate at a concentration-dependent manner. Cells were incubated in RPMI 1640 medium supplemented with 20% FBS. Data points represent means ± SDs of at least 3 experiments.
Mononucleotide treatment of HUVECs
HUVECs were used in these studies as an example of normal nontumor human cells. Because these cells form an endothelium of blood vessels, they may be influenced by mononucleotides and nucleosides released during nucleolytic degradation of antisense oligonucleotides intravenously administered to blood. Among dNMPs and dNMPSs added to the cell culture at a 100 μM concentration for 72 hours, only TMP and dGMP decreased the number of viable cells, by 10% and 30%, respectively. Under the same conditions, deoxyribonucleoside 5′-phosphorothioates (deoxyadenosine-5′-phosphorothioate [dAMPS], TMPS, and dGMPS) stimulated growth of the HUVECs by 20% to 60%. The highest proliferative effect was caused by TMPS after a 24-hour incubation (70% to 90%), but after longer incubation times (48 or 72 hours), the number of viable cells was increased by 50% to 60%. Further increase of dNMPS concentration up to 500 μM caused 300% increase in cell proliferation (data not shown). We supposed that the time dependence of the stimulatory effect was caused by ecto-5′-NT–catalyzed dephosphorylation of TMPS and the subsequent decrease of its concentration in cell culture. Detailed analysis of metabolites from the HUVEC culture revealed significant ecto-5′-NT activity, which is able to dephosphorylate both dNMP and dNMPS (Table3, Figure3). To our best knowledge, this is the first observation that ecto-5′-NT is able to recognize and hydrolyze 5′-phosphorothioate analogs of dNMP. It has been earlier demonstrated that ectonucleotidases in pig smooth-muscle cells dephosphorylated adenosine 5′-monophosphorothioate (AMPS) to adenosine, but ectonucleotidases from endothelial cells could not catabolize adenine nucleotides when sulfur was substituted on the terminal phosphate (ie, AMPS, ADPβS, ATPγS).20,21 In contrast to the ecto-5′-NT from HeLa cells (see below), the HUVEC enzyme dephosphorylates not only TMPS, but also other dNMPSs. In our attempts to inhibit ecto-5′-NT activity and to increase proliferation of HUVECs by dNMPS, we have used α,β-methylene–ADP, which is known to selectively inhibit the enzyme.22 HPLC analysis has confirmed that in the presence of α,β-methylene–ADP, dephosphorylation of dNMP or dNMPS was effectively inhibited (Table 3). However, the inhibition of ecto-5′-NT activity did not increase the proliferative effect of TMPS or other deoxyribonucleoside-5′-phosphorothioates, but rather abolished dNMPS-stimulated proliferation of the HUVECs (Figure4). This effect might be caused by a competition of α,β-methylene–ADP with dNMPS for binding to the same nucleotide receptor. It is known that α,β-methylene–ATP binds to ionotropic (P2X) and metabotropic (P2Y) nucleotide receptors and acts as an agonist.23 It cannot be excluded that α,β-methylene–ADP also possesses some affinity for P2 receptors. It has been found that membranes of WEC cells (endothelial-derived cell line) contain binding sites of high affinity toward α,β-methylene–ATP and α,β-methylene–ADP.24 Although α,β-methylene–ADP has been recommended as a selective inhibitor of ecto-5′-NT activity,22 and α,β-methylene–ATP has been used as a selective P2X agonist,23 some authors suggest that these ADP and ATP analogs can bind to the nucleotide-binding sites of both ectonucleotidases and nucleotide receptors.25 Taking into account the above suggestions,22-25we tested the influence of α,β-methylene–ATP on the dNMPS-stimulated proliferation of HUVECs. If the cells were first treated with 100 μM α,β-methylene–ATP, an addition of TMPS did not stimulate HUVEC growth (Figure 4). Even a 10-μM concentration of α,β-methylene–ATP significantly diminished dNMPS-induced stimulation of HUVEC growth.
Differential rates of extracellular mononucleotide degradation by ecto-5′-nucleotidase from human umbilical vein endothelial cells and HeLa cells
| . | HUVECs . | HUVECs + α,β-methylene–ADP . | HeLa . | HeLa + α,β-methylene–ADP . |
|---|---|---|---|---|
| dAMP | 16.7 ± 7.2 | 200.0 ± 0.5 | 64.0 ± 26.7 | 138.2 ± 24.9 |
| dGMP | 9.3 ± 5.7 | 198.3 ± 5.3 | 128.0 ± 18.0 | 114.0 ± 13.0 |
| TMP | 45.5 ± 13.0 | 197.5 ± 4.5 | 130.5 ± 16.6 | 154.1 ± 25.2 |
| dAMPS | 176.0 ± 25.2 | 200.0 ± 1.0 | 200.0 ± 1.6 | 200.0 ± 2.2 |
| dGMPS | 188.2 ± 17.6 | 199.5 ± 3.4 | 197.1 ± 21.2 | 200.0 ± 1.2 |
| TMPS | 136.7 ± 9.1 | 201.3 ± 6.3 | 177.3 ± 15.1 | 197.2 ± 8.1 |
| . | HUVECs . | HUVECs + α,β-methylene–ADP . | HeLa . | HeLa + α,β-methylene–ADP . |
|---|---|---|---|---|
| dAMP | 16.7 ± 7.2 | 200.0 ± 0.5 | 64.0 ± 26.7 | 138.2 ± 24.9 |
| dGMP | 9.3 ± 5.7 | 198.3 ± 5.3 | 128.0 ± 18.0 | 114.0 ± 13.0 |
| TMP | 45.5 ± 13.0 | 197.5 ± 4.5 | 130.5 ± 16.6 | 154.1 ± 25.2 |
| dAMPS | 176.0 ± 25.2 | 200.0 ± 1.0 | 200.0 ± 1.6 | 200.0 ± 2.2 |
| dGMPS | 188.2 ± 17.6 | 199.5 ± 3.4 | 197.1 ± 21.2 | 200.0 ± 1.2 |
| TMPS | 136.7 ± 9.1 | 201.3 ± 6.3 | 177.3 ± 15.1 | 197.2 ± 8.1 |
Concentrations of the unchanged substrate are given. Ecto-enzymatic degradation of dNMP and dNMPS was measured in RPMI 1640 medium supplemented with 10% (HeLa) or 20% fetal bovine serum (HUVECs) 24 hours after addition of 200 μM mononucleotides or following 2-hour incubation with 100 μM α,β-methylene-ADP. Concentrations of the unchanged substrate in the extracellular medium subsamples were estimated from the peak areas obtained after high-performance liquid chromatography. Data points represent means ± SDs of at least 3 experiments.
ADP indicates adenosine 5′-diphosphate. Other abbreviations are explained in Table 1.
Rates of dNMPS hydrolysis by ecto-5′-NT of HUVECs.
Ecto-enzymatic degradation of dNMPS by HUVECs. The catabolism was determined by reversed- phase HPLC of subsamples taken at 0, 24, 48, 72, and 96 hours after addition of dAMPS, dGMPS or TMPS (200 μM) to adhered cells (40 000 per well per milliliter). The ordinate axis shows the concentration of unchanged substrate. Data points represent means ± SDs from 3 experiments.
Rates of dNMPS hydrolysis by ecto-5′-NT of HUVECs.
Ecto-enzymatic degradation of dNMPS by HUVECs. The catabolism was determined by reversed- phase HPLC of subsamples taken at 0, 24, 48, 72, and 96 hours after addition of dAMPS, dGMPS or TMPS (200 μM) to adhered cells (40 000 per well per milliliter). The ordinate axis shows the concentration of unchanged substrate. Data points represent means ± SDs from 3 experiments.
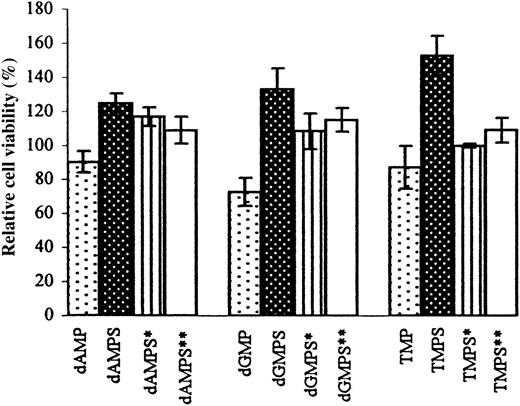
The influence of dNMP, dNMPS alone, and dNMPS in the presence of α,β-methylene–ADP or α,β-methylene–ATP on HUVEC growth.
HUVEC viability (expressed as the percentage of control cell counts) was determined with MTT assay 72 hours after addition of 100 μM dNMP, dNMPS, or dNMPS following 2-hour incubation with 100 μM α,β-methylene–ADP (indicated as dNMPS*) or with 100 μM α,β-methylene–ATP (indicated as dNMPS**). Cells (5000 per 200 μL) were incubated in RPMI 1640 medium supplemented with 20% FBS. Data points represent means ± SDs from at least 3 experiments.
The influence of dNMP, dNMPS alone, and dNMPS in the presence of α,β-methylene–ADP or α,β-methylene–ATP on HUVEC growth.
HUVEC viability (expressed as the percentage of control cell counts) was determined with MTT assay 72 hours after addition of 100 μM dNMP, dNMPS, or dNMPS following 2-hour incubation with 100 μM α,β-methylene–ADP (indicated as dNMPS*) or with 100 μM α,β-methylene–ATP (indicated as dNMPS**). Cells (5000 per 200 μL) were incubated in RPMI 1640 medium supplemented with 20% FBS. Data points represent means ± SDs from at least 3 experiments.
Mononucleotide treatment of HeLa cells
Among dNMPs used in the experiments, only TMP and dGMP were toxic to the HeLa cells: TMP caused a 30% inhibition of cell growth after 72-hour incubation (Table 1). Under similar conditions, TMPS appeared to be nontoxic because it decreased the cell number by only 10%. It should be noted that thymidine alone caused a 30% to 40% inhibition of the HeLa cell growth. Deoxyguanosine decreased the number of viable cells by 40%, while dGMP caused a 20% inhibition of cell growth. These observations suggest that the sensitivity of HeLa cells to extracellular TMP and dGMP could result from the activity of the ecto-5′-NT, releasing the corresponding deoxynucleosides. HPLC analysis has confirmed this hypothesis: after a 48-hour incubation with the HeLa cells, dGMP was completely hydrolyzed to deoxyguanosine, which was, in turn, converted to guanine (15%) and xanthine (85%). Other deoxynucleoside-5′-phosphates (dAMP, dCMP, and TMP) were hydrolyzed by the ecto-5′-NT at rates similar to that of dGMP. Among the deoxyribonucleoside-5′-phosphorothioates, only TMPS was effectively dephosphorylated by the enzyme: 50% conversion of TMPS (200 μM) to thymidine was observed after a 48-hour incubation. However, under the same conditions, dAMPS was not dephosphorylated. It should be noticed that the enzyme from HeLa cell membranes was less sensitive to α,β-methylene–ADP than the ecto-5′-NT from endothelial cells: addition of α,β-methylene–ADP to HeLa cell cultures decreased the rate of dNMP dephosphorylation only slightly (Table 3).
Oligonucleotides (PO and PS oligos) and their antiproliferative effects
To compare biological effects caused by mononucleotides and oligonucleotides, we constructed PO and PS oligomers of the sequences given in Table 4. Preliminary experiments performed with mononucleotides have shown that the cells were more sensitive to dGMP and TMP than to other dNMPs. Therefore, to test the nonantisense effects of oligonucleotide sequences on cell viability, we synthesized oligomers 1 and 2 containing different tetranucleotides: namely, d[TGGT] and d[ACCA] at their 3′-ends. The homo-oligonucleotides d[A]16 and d[T]16(oligomers 3 and 4) were synthesized as additional control oligomers. The stability of phosphate and phosphorothioate oligonucleotides toward serum 3′-exonuclease activity was determined by polyacrylamide gel electrophoresis. We found that the PO oligos were degraded by 50% after a 4-hour incubation in cell culture media, while PS oligos incubated under the same conditions appeared to be much more stable. Even after 24-hour incubation of the PS-d[T]16 in RPMI medium containing 10% heat-inactivated FBS, we did not observe any bands corresponding to shorter oligomers. However, the use of nonthermally inactivated FBS caused partial degradation of the fully phosphorothioated oligonucleotides for which half-life time was approximately 8 hours. In similar experiments carried out by Vaerman et al,4 with the use of nonthermally inactivated 10% fetal calf serum or 2.5% normal human serum, PO oligos were degraded with half-life times of about several minutes. The half-life time for the PO/PS oligo containing a single internucleotide phosphorothioate bond at the 3′-end was about 15 hours. Such experimental conditions generated a significant amount of dNMP and, as interpreted by the authors, dNMPS.4 The oligonucleotides 1 through 4 (used at 10 μM) did not cause any toxic effects to the HL-60 or K-562 cells. Although deoxyguanosine-5′-phosphate was released from oligomer 1a by plasma 3′-exonuclease activity, concentration of the resulting dGMP after a 24-hour incubation was not higher than 15 to 20 μM. Because of the low activity of ecto-5′-NT, dGMP was not dephosphorylated to deoxyguanosine, and therefore, no cytotoxic effect could be observed. For the same reason, the PS oligos 1 through 4b did not influenced the growth of K-562 and HL-60 cells. Only HeLa cells appeared to be sensitive to one of the oligonucleotides used in our experiments. After a 72-hour incubation, the unmodified oligonucleotide d[T]16 (4a) caused 15% to 30% inhibition of cell growth. This effect was clearly concentration-dependent: d[T]16 used at 2.5 μM concentration caused only 15% inhibition, while 10 μM of the same oligomer gave 30% inhibition of the cell growth. The inhibitory effect results from exonucleolytic degradation of the oligonucleotide 4a and TMP release. In our estimation, the concentration of TMP released after 72-hour incubation was approximately 100 μM. Therefore, the inhibitory effects observed for the oligomer 4a used at a concentration of 10 μM and for the free TMP used at 100 μM were almost identical. A further increase of the oligonucleotide concentration (up to 25 μM) did not cause significant enhancement of the antiproliferative effect from what might result from the limited activities of the 3′-exonuclease and/or the ecto-5′-NT. The homo-oligonucleotide d[A]16 (3a) was degraded by the exonuclease with the same efficacy as d[T]16 but dAMP resulting from the cleavage was not toxic to the cells. The phosphorothioate homo-oligonucleotide 4b did not inhibit HeLa cell growth, probably because of its higher resistance to serum 3′-exonuclease activity as compared with the unmodified oligonucleotide d[T]16.
Oligonucleotides used in this study
| ODN . | Sequence (5′ → 3′) . |
|---|---|
| 1a | PO-d[GCTGGAGACATCTGGT] |
| 1b | PS-d[GCTGGAGACATCTGGT] |
| 2a | PO-d[GCTGGAGACATCACCA] |
| 2b | PS-d[GCTGGAGACATCACCA] |
| 3a | PO-d[A]16 |
| 3b | PS-d[A]16 |
| 4a | PO-d[T]16 |
| 4b | PS-d[T]16 |
| ODN . | Sequence (5′ → 3′) . |
|---|---|
| 1a | PO-d[GCTGGAGACATCTGGT] |
| 1b | PS-d[GCTGGAGACATCTGGT] |
| 2a | PO-d[GCTGGAGACATCACCA] |
| 2b | PS-d[GCTGGAGACATCACCA] |
| 3a | PO-d[A]16 |
| 3b | PS-d[A]16 |
| 4a | PO-d[T]16 |
| 4b | PS-d[T]16 |
Sequences of phosphodiester (PO) or phosphorothioate (PS) oligonucleotides used in this study. ODN indicates oligodeoxynucleotide.
Discussion
Vaerman et al4 postulated that dNMP and dNMPS released from PO and PS oligos by plasma exonucleases might inhibit growth of different cell lines. Deoxyribonucleoside-5′-phosphates (especially dGMP and TMP) added to cell cultures caused significant cytotoxic effects to the leukemic BV 173 cell line and to CD34+ bone marrow cells.4 Vaerman et al4 did not examine the toxicity of dNMPS after direct addition to the BV 173 cell cultures. Our experiments, carried out with the use of chemically synthesized dNMPS, have shown that the influence of mononucleotides and their 5′-phosphorothioate analogs on the proliferation of different cell lines is more complex than has been presented so far. Deoxyribonucleoside-5′-phosphates (dGMP and TMP) are toxic to some types of cells, but under the same conditions, their 5′-phosphorothioate analogs do not decrease cell viability. Moreover, in some cases, dNMPSs stimulate cell proliferation independently of the type of nucleobase. A detailed HPLC analysis of media supernatants allowed us to identify ecto-5′-NT activity responsible for dephosphorylation of extracellular mononucleotides to the corresponding nucleosides. The cell lines studied demonstrate different ecto-5′-NT activities. The enzyme from K-562 cells showed very low activity, which resulted in only a 5% yield of dNMP dephosphorylation after a 72-hour incubation, independently of the type of nucleobase. HL-60 cells also degraded the extracellular mononucleotides at a very slow rate. This observation is in agreement with earlier data, because low activity of the ecto-5′-NT from HL-60 cells has been reported by Spychala et al26 and Clifford et al.27 The lack of the ecto-5′-NT activity in K-562 and HL-60 cells results in the high stability of extracellular mononucleotides. They are not dephosphorylated to the corresponding deoxynucleosides and, therefore, are not toxic to these cells. In contrast to the HL-60 and K-562 cells, HUVECs and HeLa cells possess elevated ecto-5′-NT activity, which can dephosphorylate both dNMP and dNMPS. Thymidine 5′-phosphate is slightly toxic to the HeLa cells, because this mononucleotide has been hydrolyzed to thymidine, which can be taken up by the cells and which can inhibit intracellular thymidine kinase.28,29Deoxyguanosine 5′-phosphorothioate is toxic to HUVECs because of its dephosphorylation to deoxyguanosine. To our knowledge, there are no data available on stimulation of mammalian cell growth by 2′-deoxyribonucleoside-5′-monophosphorothioates. However, it is possible that dNMPSs influence HL-60 and HUVEC proliferation by acting on a cell-surface receptor belonging to a P2 nucleotide receptor class activated by nucleoside triphosphates and/or diphosphates. Recent studies reveal much higher diversity among the P2 receptors than was previously thought.30,31 In comparison with ATP or uridine 5′-triphosphate, 5′-mononucleotides usually have much lower agonist activity for the nucleotide receptors.32,33 However, it has been found that AMPS is a weak agonist of a novel P2 receptor on undifferentiated HL-60 cells. Its pharmacological profile is very similar to that of the P2Y11 receptor identified in 2 different cell lines: the 1321N1 astrocytoma and the Chinese hamster over K1 cell line.34-36 One cannot exclude the possibility that HL-60 cells and HUVECs express a similar, as-yet unidentified nucleotide receptor possessing an affinity for 2′-deoxyribonucleoside 5′-phosphorothioates but not for 3′-phosphorothioates or 5′,3′-bisphosphorothioates. The influence of α,β-methylene–ADP and α,β-methylene–ATP on the dNMPS-induced stimulation of the HUVECs may confirm this hypothesis. On the other hand, it cannot be excluded that dNMPSs are phosphorylated by one or more ecto-nucleotide kinases37 to form their diphosphate or triphosphate derivatives, which, in turn, may bind to P2Y or P2X nucleotide receptors. However, although we have found that TTPαS and dATPαS (100 μM) stimulated the growth of HL-60 cells, these triphosphates had no stimulatory effects on HUVECs (data not shown). Many reports indicate different nonantisense yet sequence-specific effects of oligonucleotides.38,39 Aptameric binding of oligonucleotides to proteins or immunostimulatory action of dCG motifs are often responsible for these side effects.40-42Degradation products of the oligonucleotides have also been indicated as a reason for additional biological activity.4Mononucleotide-dependent effects should especially be taken into account in the case of cell culture experiments carried out with the use of significant concentrations (approximately 10 μM) of antisense oligonucleotides in the presence of noninactivated serum. However, it is difficult to estimate what concentrations of an antisense oligonucleotide and, subsequently, of free mononucleotides could generate the effects described above. Taking into account that different dNMPS have shown different stimulatory effects, one can expect that such activity may be dependent on a sequence of a PS oligo used. Also, properties of cells studied in in vitro experiments may be very important: different levels and/or activities of an ecto-5′-NT and a membrane-bound nucleotide receptor may cause cells to have different sensitivities to mononucleoside 5′-phosphorothioates. Smetsers et al43 designed and tested antisense Bcr-Abl methylphosphonate oligonucleotide containing, at the 3′-end, an additional 5-nucleotide–long PO tail. The protein studies and growth determination experiments indicated that the oligonucleotide worked in a nonantisense manner: it inhibited growth of BV 173 and KCL-22 cells, but did not affect the growth of KYO1 cells. Moreover, the same oligomer caused 20% to 40% stimulation of DOHH2 and HL-60 cell growth. It should be noted that all of these cell lines contain the target B2A2 breakpoint. Although the methylphosphonate core of the oligonucleotide is resistant to nucleolytic degradation, the PO pentamer located at the 3′-end could be readily degraded.43 Therefore, in the light of our observations, the cytotoxic or proliferative effects observed for the cell lines studied by Smetsers et al43 might be caused by differences in catabolism of the released nucleotides and/or their binding to nucleotide receptors. Because numerous in vitro experiments were performed with lipid carriers and low concentrations (approximately 1 μM) of PS oligos, the mononucleotide-dependent effects might not play a significant role in these assays. However, the pharmacokinetic properties of PS oligos are being tested in animal models (mice, rats, and monkeys) with single- or multiple-dose administration of 1 to 50 mg/kg, usually without lipid carriers. It has been found that PS oligos are rapidly cleared from the blood, but are accumulated by various organs (especially, kidney and liver).44 Degradation in plasma, kidney, and liver proceeds mainly from the 3′-end, resulting in the appearance of the mononucleoside 5′-phosphorothioates identified in urine from PS-oligo–injected animals.44,45 Although several reports have been published on the biodistribution of PS oligos, little is known about their metabolism in vivo. Therefore, at the moment, any prediction of cytotoxic and/or proliferative effects caused by mononucleotides and demonstrated by different cell types seems to be very difficult. It is important to note that expression of the ecto-5′-NTs does not occur on all cells and varies with cell line and developmental stage.12 On the other hand, membrane-bound nucleotide receptors of many cell types have not been so far identified and characterized. The only way to avoid mononucleotide-dependent effects is to apply antisense phosphorothioate oligonucleotides completely resistant to nucleolytic degradation. Detailed studies on stereocontrolled synthesis of PS oligos9 and their stereoselective degradation by 3′-exonucleases2 allowed us to design a strategy to protect them against these enzymes: even a single internucleotide phosphorothioate bond of the SP-configuration located at the 3′-end of antisense oligonucleotides protects them against exonucleolytic degradation.9 Owing to this protection, dNMPS cannot be released from such SP-tailed phosphorothioate oligonucleotides, and no cytotoxic or proliferative effects should be induced by the mononucleotides. For this reason, the PS oligos containing, at the 3′-end, the internucleotide bonds of the SP configuration are recommended as the best candidates for the studies on evaluation of pure antisense effects.
We thank Dr P. Guga, M. Olesiak, and D. Krajewska for synthesis of mononucleotide 5′-phosphorothioates; Daria Szymanowicz for synthesis of thymidine 3′-phosphorothioate and thymidine 5′,3′-bisphosphorothioate; and an anonymous referee for valuable comments.
Supported by the State Committee for Scientific Research, grant 6 PO4B 014 15 (M.K.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Maria Koziolkiewicz, Department of Bioorganic Chemistry, Centre of Molecular and Macromolecular Studies, Polish Academy of Sciences, Sienkiewicza 112, 90-363 Łódź, Poland; e-mail: mkoziol@bio.cbmm.lodz.pl.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal