Several lines of evidence point to an abnormality in the response of Fanconi anemia cells to reactive oxygen species. To investigate the potential pathologic consequences of an in vivo alteration of redox state in mice lacking one of the Fanconi anemia genes, animals were generated having combined deficiencies of the cytosolic Cu/Zn superoxide dismutase (Sod1) and Fanconi anemia complementation group C (Fancc) genes. Interestingly, hepatocytes of Fancc−/−Sod1−/−mice exhibited a zonal pattern of microvesicular steatosis, possibly as a result of oxidative stress-induced injury to hepatocyte membranes. Consistent with this idea, freshly explantedFancc−/−Sod1−/−hepatocytes demonstrated increased spontaneous production of superoxide in vitro. The second phenotypic feature ofFancc−/− Sod1−/−mice was that of bone marrow hypocellularity accompanied by significant decreases in peripheral blood erythrocyte and leukocyte numbers as compared with wild-type controls. Although flow cytometry analysis with monoclonal antibodies against cell surface antigens revealed normal numbers of primitive hematopoietic progenitor populations inFancc−/−Sod1−/−marrow, lineage-positive progenitor numbers were significantly reduced in these mice. Furthermore, the in vitro clonogenic growth ofFancc−/−Sod1−/−erythroid, myeloid, and early B-lymphoid colonies in semisolid media was profoundly compromised. These results suggested that the altered redox state likely present inFancc−/− Sod1−/−hematopoietic progenitors was responsible for an impairment of cell proliferation or survival.
Introduction
Fanconi anemia (FA) is an autosomal recessive disease of childhood characterized by progressive pancytopenia, various developmental abnormalities, and a predisposition to acute myeloid leukemia.1 Most individuals with FA, however, succumb to the complications of aplastic anemia.2 FA cells demonstrate increased sensitivity to DNA cross-linking agents such as mitomycin C (MMC), diepoxybutane, and cisplatin,2,3 a feature that serves as the basis for an important diagnostic test. FA cells treated with these cross-linking agents show a striking increase in double-strand DNA breaks and inhibited growth with cell cycle arrest in G2.2 To date at least 7 potential FA genes have been indicated by complementation studies, and most of these genes, FANCA, FANCC, FANCD2, FANCE, FANCF, and FANCG, whose mutations account for 6 of the complementation groups, have now been characterized.4-10 Despite the variety of genes involved in this disorder, mutations in FANCA and FANCCaccount for approximately 80% of all patients with FA.11Murine Fancc, being highly similar to the human ortholog, is able to complement human cells deficient in FANCC, restoring MMC resistance.12Fancc-deficient mouse strains were generated through gene targeting. Both had similar phenotypes,13,14 demonstrating compromised gametogenesis, and an increase in the number of chromosomal aberrations, both spontaneously and after exposure to MMC. However, the targeted lines recapitulated neither the developmental nor the hematologic defects typical of human FA.13 14 The reason for this interspecies discordance is unknown, but it has limited the utility of the mutant mice as potential models of FA.
A number of hypotheses regarding the nature of the primary defect in FA have been suggested, including the proposal that FA proteins constitute a DNA damage recognition and signaling pathway, whose impairment is manifested by chromosomal instability and increased sensitivity to interstrand DNA cross-linking agents.15 Although a reduced ability to process DNA cross-links is clearly evident, it has also been proposed that an abnormal reduction of MMC in FA cells leads to the production of reactive species that in turn generate cross-links and other types of oxidative lesions.16 Thus, FA might also result, at least in part, from an abnormal regulation of cell redox state or of the cellular response to oxidative stress or both. In support of this notion, addition of Cu/Zn superoxide dimutase (SOD) to the culture medium of FA cells was reported to attenuate chromosomal breakage as well as MMC cytotoxicity,16 an effect also observed in FA cells overexpressing thioredoxin.17 In keeping with an inability to regulate either production, or the consequences of reactive oxygen species (ROS), some FA cells were shown to be hypersensitive to oxygen.16,18 Thus, cells grew slowly at elevated oxygen levels (eg, 35%) and tended to arrest at G2, whereas at low oxygen concentrations (eg, 5%) growth was normal and accompanied by decreased chromosomal aberrations.18-20 Increased production of ROS by FA cells, such as leukocytes and fibroblasts, has also been reported, suggesting that FANCC might regulate the generation of these species.21,22 A potential endogenous source of superoxide, the NADPH cytochrome P-450 reductase (RED) system, has also been implicated in FA. Not only were chromosomal breaks in FA cells reduced by cytochrome P-450 inhibition, but evidence of a direct physical interaction between FANCC and RED was reported,23 24leading to the hypothesis that FANCC might protect cells from ROS via regulation of RED activity.
Mice with a targeted disruption of the gene encoding the cytosolic Cu/Zn SOD (Sod1) exhibit normal growth and development; however, they show a distinctive motor axonopathy25,26 and impaired gametogenesis.27 The limited spontaneous pathology of Sod1−/− mice suggested that although this enzyme might function to modulate superoxide-mediated effects in some tissues under basal conditions, that it was of critical importance during exposures to specific pro-oxidant stimuli.28,29 In keeping with this,Sod1−/− embryonic fibroblasts exposed to the superoxide-generating herbicide, paraquat, were much more sensitive than wild-type cells.30
We hypothesized that a lack of Sod1 might reveal a role for alterations in redox state with respect to the development of a FA-like syndrome in Fancc-deficient mice. To examine this possibility, we generated mice with combined deficiencies of both theFancc and Sod1 genes. Interestingly,Fancc−/−Sod1−/− mice developed liver pathology as well as bone marrow (BM) hypocellularity and peripheral blood (PB) bicytopenia (erythrocytes and leukocytes), accompanied by a profound reduction of the clonogenic growth of hematopoietic precursors in vitro.
Materials and methods
Generation ofFancc−/−Sod1−/−mice and histologic analysis
Fancc+/− mice14 were crossed with Sod1+/− mice28 to obtain mice that were heterozygous at both loci. Locus-specific polymerase chain reaction (PCR) was used to genotype mice. Brother-sister matings ofFancc+/−Sod1+/−/mice were carried out to produce litters havingFancc−/− Sod1−/− mice. Mice, 8 to 10 weeks of age, were of a mixed genetic background and thus littermate controls were used in all experiments. Viral antibody-free mice were housed in the Center for Molecular Medicine and Therapeutics barrier facility according to protocols approved by the Animal Care Committee at the University of British Columbia. For light microscopy, tissue samples were either frozen or fixed in 4% paraformaldehyde solution and embedded in paraffin and bone sections were first decalcified before processing. For paraffin-embedded sections, hematoxylin and eosin, periodic acid-Schiff with and without diastase, and Masson trichrome were used. Frozen sections were stained with oil-red-O. For electron microscopy, liver blocks were fixed in cold 3% gluteraldehyde and stored at 4°C. Samples were rinsed twice in Millonig buffer (pH 7.4) and were subsequently fixed in 1% osmium tetroxide in Palade solution for 1.5 hours at 4°C. Samples were stained en bloc for 15 minutes with 2% aqueous uranyl acetate and then dehydrated before embedding. Sections were examined with a Phillips 400 electron microscope.
Blood collection, serum alanine aminotransferase measurement, and PB counts
Following Avertin overdose, blood was obtained by cardiac puncture, and either allowed to clot at room temperature, or added to microtainer tubes, pretreated with EDTA, for blood counts. Clotted blood was centrifuged at 14 000 rpm for 5 minutes, and serum was removed and frozen at −80°C. Serum alanine aminotransferase (ALT) levels were determined using a Beckman Synchron CX7. PB counts were performed using a Sysmex 9500 analyzer. Red blood cell (RBC), white blood cell (WBC), platelet, hemoglobin, and mean cell volume (MCV) values were determined.
Tissue isolation
Mice were killed at 8 to 10 weeks of age by intraperitoneal injection of 4% Avertin (0.01 mL/g). Samples were harvested from the same region of the liver in all mice. Single-cell suspensions were prepared by pressing samples through a wire mesh into cold serum-free RPMI 1640 (Life Technologies, Burlington, Ontario, Canada). Cells were then passed through a 40-μ nylon filter to remove clumps and debris. Liver cells were pelleted at 1500 rpm and resuspended in cold RPMI. Total BM cells were collected by flushing femurs from 7- to 8-week old mice with cold Hanks balanced saline solution with 5% fetal calf serum (FCS). Cell viability, more than 90% in all samples, was determined by trypan blue exclusion.
Superoxidequantitation
Isolated liver cells were resuspended, in triplicate, at a density of 5.0 × 105/mL in serum-free RPMI and centrifuged at 1500 rpm, before being resuspended in 100 μL Superoxide Assay Medium (Calbiochem, San Diego, CA). Each culture was then placed into a well of an opaque 96-well polystyrene flat-bottomed microtiter plate (VWR Canlab, Mississauga, Ontario, Canada) kept on ice until analysis. Then, 5.0 μL 4.0 mM luminol solution (Calbiochem), diluted in 95 μL Superoxide Assay Medium, was added simultaneously to all samples. Chemiluminescence was measured at 1 minute after luminol addition using a MLX Microtiter Plate Luminometer (Dynex Technologies, Chantilly, VA). The average intensity of the triplicates was recorded as relative light units (RLUs). Purified SOD (Calbiochem) was added to the cells as a specificity control to show that chemiluminescence was due to superoxide.
Immunoblottingand densitometry
Flash-frozen liver samples were lysed in Nonidet P-40 lysis buffer (1% Nonidet P-40, 150 mM NaCl, 50 mM Tris, pH 7.5, and 10% glycerol) in the presence of multiple protease inhibitors (Boehringer Mannheim, Indianapolis, IN and BDH, Toronto, ON). Lysates were centrifuged for 15 minutes at 14 000 rpm. Liver protein concentration was determined by an assay based on the Bradford method. Lysate volume corresponding to 250 μg total protein was diluted 3:1 with Laemmli sample buffer. Samples were boiled for 5 minutes before electrophoresis. Total cell lysates were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) at 150 V and transferred to nitrocellulose paper by electroblotting at 100 V for 1 hour at room temperature in a solution containing 192 mM glycine, 25 mM Tris, and 20% methanol. Filters were blocked overnight at 4°C in TBST (10 mM Tris, pH 8.0, 150 mM NaCl, and 0.05% Tween-20) containing 5% bovine serum albumin (BSA). Filters were then incubated for 60 minutes at room temperature in TBST with 1% BSA with one of the following antibodies (Stressgen Biotechnologies, Victoria, BC): anti-MnSOD (1:5000), HO-1 (1:2000), or anti–β-tubulin (1:250). After 3 TBST washes, filters were incubated for 1 hour with a horseradish peroxidase–conjugated secondary antibody (Dako Diagnostics, Mississauga, ON). Proteins were detected by chemiluminescence (Amersham, Arlington Heights, IL) using Biomax MR film (Eastman Kodak, Rochester, NY). Densitometry was performed using a GS300 reader (Hoefer Scientific Instruments, San Francisco, CA), and results were analyzed using the GS370 1-D Data System, version 2.0 for Macintosh.
Flow cytometry
A total of 1 × 106 cells was resuspended in 500 μL phosphate-buffered saline (PBS) plus 2% FCS (FACS buffer), blocked on ice with 1 μg anti-FcγRIIb (2.4G2, Pharmingen, Mississauga, ON) for 20 minutes, and then stained with either 0.5 μg anti-CD11b–fluorescein isothiocyanate (FITC; for liver samples) or one of the following FITC-conjugated antibodies for 30 minutes on ice (BM cells): PGP1, B220, Ly6G (Gr-1), 7-4 CD11b, CD14 and TER-119; and (primitive populations): Sca1, c-kit, CD34 (Pharmingen). Cells were washed 3 times with FACS buffer and resuspended in 500 μL FACS buffer before analysis on a FACSort (Becton Dickinson, Mountain View, CA) flow cytometer equipped with CellQuest software (Becton Dickinson). The viable cells that remained unstained represented hepatocytes, whereas the CD11b+ population included Kupffer cells and contaminating PB phagocytes. For BM samples, the percent staining was multiplied by the total cellularity (obtained from one femur) to determine the absolute number of each cell type.
Chromosomeanalysis
For Fancc+/+Sod1+/+, Fancc+/−Sod1+/−, andFancc−/−Sod1−/− BM samples, an aliquot of RPMI + 5% FCS containing 1.5 × 106resuspended BM cells was added to a tube containing 1 mL trypsin-EDTA (Irvine Scientific, Santa Ana, CA) and 0.75 M KCl. The tubes were incubated at 37°C for 25 minutes, spun for 10 minutes at 1000 rpm and the pellet carefully resuspended in Carnoy fixative (3 parts methanol to 1 part glacial acetic acid). The fixative was changed 2 more times and the slides made by air-drying. Approximately 10 metaphases per sample were examined for evidence of chromosomal breaks, gaps, or detectable rearrangements.
Methylcellulose colony-forming assays and lineage depletion of total BM cells
Whole BM cells were plated in 1.1 mL 1% methylcellulose media supplemented with 10% FCS, 2 mM l-glutamine, 10−4 M 2-mercaptoethanol and the following recombinant growth factors: for myeloid assays, methylcellulose was supplemented with 1% BSA, 10 μg/mL bovine pancreatic insulin, 200 μg/mL human transferrin, 3 U/mL recombinant human erythropoietin, 10 ng/mL recombinant mouse interleukin (IL)-3, 10 ng/mL recombinant human IL-6, and 50 ng/mL recombinant mouse stem cell factor (SCF). For pre-B assays 10 ng/mL recombinant human IL-7 was used (Stem Cell Technologies, Vancouver, BC). Cells were dispensed using a blunt-ended needle and cultured at a density of 1.7 × 105 and 5.5 × 104 cells/35-mm dish for pre-B and myeloid colonies, respectively (each sample done in duplicate). Dishes were incubated for 6 (for pre-B) or 12 (for myeloid) days at 37°C, 5% CO2 in air, ≥ 95% humidity. Colonies (> 20 cells) were counted on a gridded stage using an inverted light microscope. Lineage-depleted (Lin−) samples were collected by resuspending the cells at 5.0 × 107 nucleated cells/mL in PBS with 2% FBS, plus 5% rat serum for 15 minutes at 4°C. Samples were first incubated with an antibody cocktail (CD5, CD11b, CD45R, GR1, 7-4, and TER-119) and subsequently with an antibiotin tetrameric antibody (both antibody cocktails from Stem Cell Technologies) complex (each step for 15 minutes at 4°C); then a magnetic colloid was added for cell separation as recommended (Stem Cell Technologies). To isolate Lin− populations, the suspension was applied to a primed 0.3-inch magnetic column and washed 3 times with PBS containing 2% FBS. The cells in the flow-through were enumerated and trypan blue exclusion used to determine viability (>95%).
Statisticalmethods
The Student t test (Microsoft Excel) was used when analyzing the results. A P value less than .05 was considered significant.
Results
Fancc−/−Sod1−/−mice develop zonal microvesicular hepatic steatosis
No developmental defects or gross skeletal abnormalities were seen in Fancc−/−Sod1−/− mice. Body weights of Fancc−/−, Sod1−/−, Fancc+/−Sod1+/−,Fancc+/+Sod1+/+, andFancc−/−Sod1−/− mice, both male and female, were not statistically different from one another (data not shown). Liver and spleen weights were not increased in any of the mutants as compared toFancc+/+Sod1+/+ controls. However, necropsy and histologic analysis ofFancc−/− Sod1−/− mice revealed abnormalities of the liver and BM.
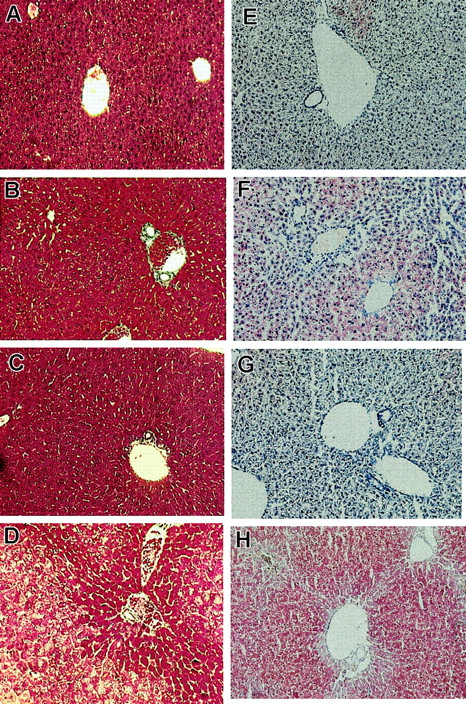
On inspection, livers ofFancc−/−Sod1−/− mice (n = 6) were pale and exhibited a yellow reticular surface pattern (data not shown). Liver sections were examined by light and electron microscopy, with a typical sample shown in Figure 1. Liver sections from Fancc−/− (Figure 1A,E),Sod1−/− (Figure 1B,F),Fancc+/−Sod1+/− (Figure 1C,G), andFancc−/−Sod1−/− (Figure 1D,H) mice were stained with Mason trichrome (Figure 1A-D). Whereas periportal (zones 1 and 2) hepatocytes were unremarkable, zone 3 cells were distended by numerous periodic acid-Schiff–negative (data not shown) cytoplasmic vacuoles that did not displace the nuclei. No inflammatory cell infiltrates were present in the liver, and trichrome stain did not reveal evidence of hepatic fibrosis or increased collagen deposition. Staining with oil red-O (Figure 1E-H) demonstrated microvesicular steatosis (Figure 1H) in zone 3 hepatocytes ofFancc−/−Sod1−/− mice.Fancc−/− mice (Figure 1E) revealed no increase in lipid staining over controls, whereas Sod1−/−mice (Figure 1F) revealed modest amounts of oil red O-positive droplets distributed in a nonzonal pattern. Transmission electron microscopy, performed onFancc+/−Sod1+/− (Figure2A,C) andFancc−/−Sod1−/− (Figure 2B,D) liver samples, revealed no morphologic abnormalities of Kupffer cells or endothelial sinusoidal cells, and aside from the obvious lipid-filled vacuoles, the structure of hepatocyte smooth endoplasmic reticulum and mitochondria was unremarkable (Figure 2C-D).
Histologic examination reveals zonal hepatic microvesicular steatosis inFancc−/−Sod1−/−mice.
Histology of Fancc−/−Sod1−/−and control livers (magnification × 400). Sections ofFancc−/−(A,E),Sod1−/−(B,F),Fancc+/−Sod1+/−(C,G),and Fancc−/−Sod1−/−(D,H) livers were stained with Masson trichrome (A-D) and oil red-O (E-H). WhereasFancc−/−, Sod1−/−, andFancc+/−Sod1+/−cells show normal morphology, Fancc−/−Sod1−/−hepatocytes demonstrate a zone 3 abnormality characterized by abundant cytoplasmic vacuolation. Staining with oil red-O demonstrates prominent microvesicular zone 3 lipid accumulation without nuclear displacement in Fancc−/−Sod1−/−mice. Small amounts of lipid droplets are present inSod1−/−mice.
Histologic examination reveals zonal hepatic microvesicular steatosis inFancc−/−Sod1−/−mice.
Histology of Fancc−/−Sod1−/−and control livers (magnification × 400). Sections ofFancc−/−(A,E),Sod1−/−(B,F),Fancc+/−Sod1+/−(C,G),and Fancc−/−Sod1−/−(D,H) livers were stained with Masson trichrome (A-D) and oil red-O (E-H). WhereasFancc−/−, Sod1−/−, andFancc+/−Sod1+/−cells show normal morphology, Fancc−/−Sod1−/−hepatocytes demonstrate a zone 3 abnormality characterized by abundant cytoplasmic vacuolation. Staining with oil red-O demonstrates prominent microvesicular zone 3 lipid accumulation without nuclear displacement in Fancc−/−Sod1−/−mice. Small amounts of lipid droplets are present inSod1−/−mice.
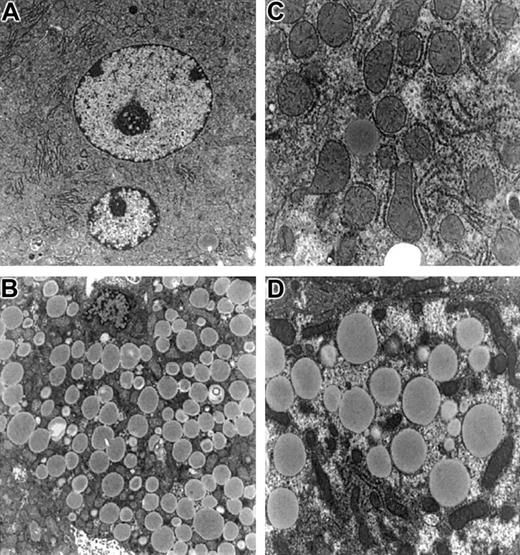
Electron microscopy of hepatocytes from
Fancc−/−Sod1−/− mice reveals no increase in organelle damage. Hepatocytes fromFancc+/−Sod1+/−(A,C) andFancc−/−Sod1−/−(B,D) mice stained with osmium tetroxide. (A) A normal multinucleated centrilobular hepatocyte (× 5400) and (C) a higher magnification showing normal organelles (× 11 750). (B) Microvesicular steatosis in a centrilobular Fancc−/−Sod1−/−hepatocyte (× 5400) and (D) (× 11 750).
Electron microscopy of hepatocytes from
Fancc−/−Sod1−/− mice reveals no increase in organelle damage. Hepatocytes fromFancc+/−Sod1+/−(A,C) andFancc−/−Sod1−/−(B,D) mice stained with osmium tetroxide. (A) A normal multinucleated centrilobular hepatocyte (× 5400) and (C) a higher magnification showing normal organelles (× 11 750). (B) Microvesicular steatosis in a centrilobular Fancc−/−Sod1−/−hepatocyte (× 5400) and (D) (× 11 750).
To search for evidence of hepatocyte injury, serum ALT and TUNEL were used. ALT levels were as follows:Sod1−/−, 55.3 ± 8.67;Fancc−/−, 45.5 ± 2.87;Fancc+/+Sod1+/+, 41.3 ± 7.98;Fancc+/− Sod1+/−, 22.7 ± 4.2; and Fancc−/−Sod1−/−, 126.8 ± 52.0. Although the serum ALT level fromFancc−/−Sod1−/− mice was increased (∼3-fold) over littermate controls, suggesting that low levels of hepatocyte damage may have been present, these data are not statistically significant (P = .12). TUNEL assay of liver sections revealed no difference with respect to the rare apoptotic cells seen when Fancc−/− Sod1−/−and littermate mice were compared (data not shown).
Primary Fancc−/−Sod1−/−liver cell cultures generate increased levels of superoxide
To search for evidence of oxidant stress inFancc−/−Sod1−/− livers, we assayed spontaneous superoxide production from primary liver cell cultures. Luminol, which undergoes chemiluminescence when oxidized by superoxide, enabled quantitation of the relative amounts of this species. The average intensity of the samples was recorded as RLUs, with the RLU values being proportional to the level of superoxide in the samples. Figure 3 shows the average RLU values for 5 mice per group, each mouse sample assayed in triplicate, taken immediately after luminol addition. In all samples, the luminol signal was ablated when SOD protein was added to the culture medium (data not shown).Fancc+/+Sod1+/+, Fancc−/−, andFancc+/−Sod1+/− controls all had statistically similar RLU values of 0.33, 0.38, and 0.44, respectively.Sod1−/− samples showed a marginally elevated RLU value of 0.52 that was statistically different fromFancc+/+Sod1+/+ mice (P = .01). In contrast, there was a 4.8-fold increase in the RLU value obtained fromFancc−/−Sod1−/− cells (1.62) compared with Fancc+/+Sod1+/+controls (P = .0008). Although hepatocytes were likely the source of the increased levels of superoxide inFancc−/−Sod1−/− cells, we are unable to define the potential contribution of Kupffer cell-derived superoxide. FACS analysis demonstrated that the percentage of CD11b+ cells was similar in all samples (data not shown).
Primary hepatocytes have an increase in superoxide levels detected by luminol-dependent chemiluminescence.
The level of superoxide is reflected by the RLU value. Each bar represents the mean ± SEM for 5 mice per group, with each sample in triplicate. *, P < .05; **, P < .001 by the Student t test.
Primary hepatocytes have an increase in superoxide levels detected by luminol-dependent chemiluminescence.
The level of superoxide is reflected by the RLU value. Each bar represents the mean ± SEM for 5 mice per group, with each sample in triplicate. *, P < .05; **, P < .001 by the Student t test.
Increased expression of manganese SOD and heme-oxygenase-1 inFancc−/−Sod1−/−livers
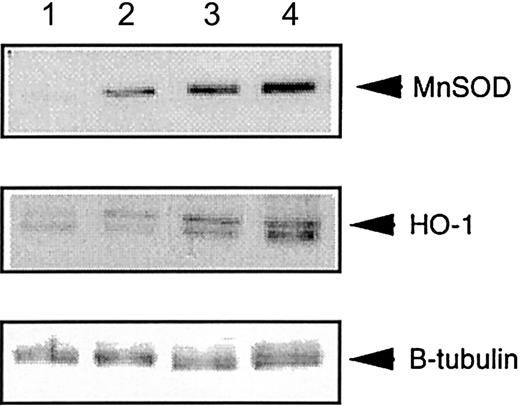
Heme-oxygenase-1 is induced by various cellular stressors, including ROS.31,32 Similarly, manganese SOD (MnSOD) can also be induced by ROS, including superoxide.33 34 Thus, an increased level of MnSOD or heme-oxygenase-1 (HO-1) in total liver cell lysates, as assessed by immunoblotting with anti-MnSOD and HO-1 antibodies, would be predicted to accompany the putative pro-oxidant state inFancc−/−Sod1−/− hepatocytes. Figure 4 represents protein levels of HO-1 and MnSOD in lysates fromFancc+/+Sod1+/+, Fancc+/−Sod1+/−, andFancc−/−Sod1−/− mice. Densitometric analysis of a number of immunoblotting experiments was carried out, with the ratio of protein band intensities for MnSOD or HO-1 normalized according to band intensities following stripping and reimmunoblotting of the same filters with an anti–β-tubulin antibody. The results demonstrate increased levels of MnSOD and HO-1 of about 4-fold and 10-fold, respectively, inFancc−/− Sod1−/− livers, as compared with Fancc+/+Sod1+/+littermates, consistent with in vivo oxidative stress.
Liver-specific expression of MnSOD and HO-1 is increased in
Fancc−/−Sod1−/− mice.Autoradiographs showing total liver lysates immunoblotted with antibodies against MnSOD and HO-1, and normalized for loading with anti–β-tubulin antibody. Lane 1 control (Stressgen Biotech, Victoria, British Columbia, Canada), lane 2Fancc+/+Sod1+/+, lane 3Fancc+/−Sod1+/−, and lane 4Fancc−/−Sod1−/− liver lysates.
Liver-specific expression of MnSOD and HO-1 is increased in
Fancc−/−Sod1−/− mice.Autoradiographs showing total liver lysates immunoblotted with antibodies against MnSOD and HO-1, and normalized for loading with anti–β-tubulin antibody. Lane 1 control (Stressgen Biotech, Victoria, British Columbia, Canada), lane 2Fancc+/+Sod1+/+, lane 3Fancc+/−Sod1+/−, and lane 4Fancc−/−Sod1−/− liver lysates.
PB and BM abnormalities ofFancc−/−Sod1−/−mice
To search for evidence of BM dysfunction, we evaluated PB cells from Fancc+/+Sod1+/+, Fancc+/−Sod1+/−,Fancc−/−, Sod1−/−, and Fancc−/−Sod1−/− mice (Table1). Significant decreases were observed in the RBC (P = .005) and WBC (P = .03) compartments of Fancc−/−Sod1−/−mice, as compared withFancc+/+ Sod1+/+ littermates. WBC values from Fancc−/−,Sod1−/−, andFancc−/−Sod1−/− mice, however, were not significantly different from one another (P = .18 and P = .12, respectively).Fancc−/− Sod1−/− differentials (n = 4) revealed that the WBC decrease was due to a reduction in both neutrophils and lymphocytes. There was no indication of a granulocyte maturation arrest in any of the samples. PB smears revealed that lymphocytes from Fancc−/− Sod1−/−blood were often larger with more immature nuclear chromatin then either Fancc+/+Sod1+/+ orFancc+/−Sod1+/− controls. Erythrocyte MCV was significantly increased (P < .00001), and there were higher numbers of polychromatic RBCs inFancc−/− Sod1−/− mice compared with controls (data not shown). Fancc−/− mice demonstrated a trend toward reduced WBC counts; however, this decrease was not significant (P = .07). Platelet counts were normal in Fancc−/−Sod1−/− mice, consistent with the normal megakaryocyte numbers observed in the marrow. Interestingly, 8-week-old, but not older (platelet count 587 ± 12.9) Fancc−/− mice showed a significant (P = .007) decrease in platelet numbers. Furthermore, there were reductions in both RBC (P = .03) and hemoglobin (P = .04) values inSod1−/− mice, as compared withFancc+/+Sod1+/+ controls. Peripheral counts were obtained from mice up to the age of 3 months. With age, WBC values from Fancc−/− mice (10.5 ± 1.5) increase to Fancc+/+Sod1+/+ levels (10.21 ± 0.86), ceasing to be statistically similar to WBC values from Fancc−/−Sod1−/− mice (5.9 ± 0.86). Thus, in contrast toFancc−/−Sod1−/− mice, the reductions of WBCs and RBCs seen in 8- to 10-week-oldFancc−/− mice normalize over time.
Peripheral blood values from mice aged 8 to 10 weeks
| Genotype . | n . | CBC Values‡ . | ||||
|---|---|---|---|---|---|---|
| RBCs (1012/L) . | WBCs (109/L) . | Hgb (g/L) . | PLTs (109/L) . | MCV (fL) . | ||
| Fancc+/+Sod1+/+ | 15 | 10.02 ± 0.27 | 3.69 ± 1.09 | 163.4 ± 4.47 | 605.3 ± 47.2 | 52.8 ± 0.69 |
| Fancc+/−Sod1+/− | 18 | 9.66 ± 0.27 | 6.59 ± 0.86 | 161.9 ± 4.50 | 708 ± 49.3 | 52.3 ± 0.62 |
| Fancc−/− | 9 | 9.68 ± 0.20 | 1.55 ± 0.23 | 158.6 ± 4.05 | 435 ± 26.9* | 53.5 ± 0.42 |
| Sod1−/− | 10 | 8.72 ± 0.42* | 1.94 ± 0.56 | 147 ± 3.96* | 745.5 ± 49.1 | 54.1 ± 1.0 |
| Fancc−/−Sod1−/− | 9 | 7.9 ± 0.36† | 0.96 ± 0.20* | 136 ± 4.52† | 719 ± 93.7 | 58.5 ± 0.68† |
| Genotype . | n . | CBC Values‡ . | ||||
|---|---|---|---|---|---|---|
| RBCs (1012/L) . | WBCs (109/L) . | Hgb (g/L) . | PLTs (109/L) . | MCV (fL) . | ||
| Fancc+/+Sod1+/+ | 15 | 10.02 ± 0.27 | 3.69 ± 1.09 | 163.4 ± 4.47 | 605.3 ± 47.2 | 52.8 ± 0.69 |
| Fancc+/−Sod1+/− | 18 | 9.66 ± 0.27 | 6.59 ± 0.86 | 161.9 ± 4.50 | 708 ± 49.3 | 52.3 ± 0.62 |
| Fancc−/− | 9 | 9.68 ± 0.20 | 1.55 ± 0.23 | 158.6 ± 4.05 | 435 ± 26.9* | 53.5 ± 0.42 |
| Sod1−/− | 10 | 8.72 ± 0.42* | 1.94 ± 0.56 | 147 ± 3.96* | 745.5 ± 49.1 | 54.1 ± 1.0 |
| Fancc−/−Sod1−/− | 9 | 7.9 ± 0.36† | 0.96 ± 0.20* | 136 ± 4.52† | 719 ± 93.7 | 58.5 ± 0.68† |
Values represent average ± SEM for the indicated number of animals per group.
RBC indicates red blood cells; WBC, white blood cells; CBC, complete blood count; Hgb, hemoglobin; PLTs, platelets; MCV, mean cell volume.
P < .05.
P < .0005.
CBCs were quantified using a Sysmex 9500 automated blood analyser.
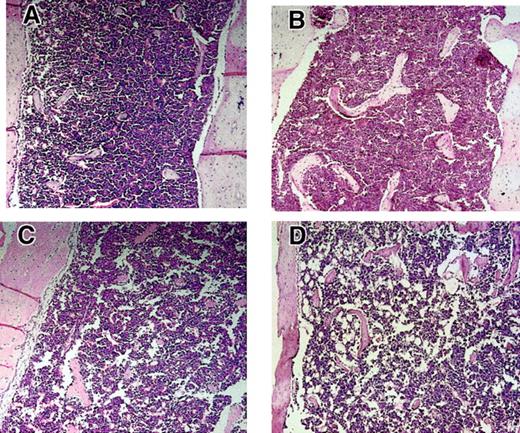
The BMs of Fancc+/−Sod1+−, Fancc−/−, Sod1−/−, and Fancc−/− Sod1−/− femurs were next assessed (representative examples are shown in Figure5). Decreased cellularity was present inFancc−/− Sod1−/−, suggested by increased fat cell numbers, particularly in the long bone metaphyses. In 5 of 5 Fancc−/−Sod1−/− femurs analyzed, a large increase in the amount of BM fat was present, whereas in only 1 of 4 Fancc+/+Sod1+/+ and 1 of 4 Fancc+/− Sod1+/− controls there was an increase in fat, and when present this was less pronounced than that of Fancc−/−Sod1−/−mice. Total BM cell numbers per femur were obtained fromFancc+/+Sod1+/+,Fancc+/−Sod1+/−,Fancc−/−, Sod1−/−, andFancc−/−Sod1−/− mice (Table2; n is as shown). Cellularity was decreased in Fancc−/− Sod1−/−mice (2.33 × 107 ± 0.19) compared withFancc+/+ Sod1+/+ controls (3.98 × 107 ± 0.62) by 58%; however, this was not significant (P = .06). There was also no statistical difference in total BM cellularity betweenFancc+/−Sod1+/−,Fancc−/− , and Sod1−/−controls. To determine whether any specific BM cell type might be differentially affected, flow cytometry was carried out on total BM samples with the following monoclonal antibodies: PGP1, B220, Ly6G, 7-4, CD11b, CD14, and Ter-119. As shown in Table 2, the average number of Fancc−/−Sod1−/− cells of each type was decreased by at least 40%, as compared withFancc+/+ Sod1+/+ controls. To investigate whether these reductions were mirrored by reduced numbers of committed (Lin+) progenitors, Lin+Sca1+, Lin+ckit+and Lin+CD34+ values were obtained fromFancc+/−Sod1+/− andFancc−/−Sod1−/− mice (n = 3). The average absolute number of these progenitor populations forFancc+/− Sod1+/− mice was 1.8 × 106, 3.6 × 106, and 1.7 × 106, and forFancc−/−Sod1−/−mice, 0.9 × 106, 1.26 × 106, and 0.8 × 106, respectively, demonstrating that committed progenitor populations were decreased inFancc−/−Sod1−/− BMs. There was no evidence of increased extramedullary hematopoiesis, because spleens obtained from 2 sets of animals revealed no change in cellularity:Fancc−/−Sod1−/−(9.65 × 107 ± 0.45) andFancc+/−Sod1+/−(13 × 107 ± 3.85).
Hypocellularity and increased fat accumulation in Fancc−/−Sod1−/−BM.
Metaphyseal sections of leg bones fromFancc+/+Sod1+/+(A),Fancc−/−(B), Sod1−/−(C), and Fancc−/−Sod1−/−(D) mice (magnification × 400). Marrow fat content (clear areas) is increased in Fancc−/−Sod1−/−mice compared with Fancc+/+ Sod1+/+ or toFancc−/−. Controls revealed only rare fat cells,whereas Sod1−/−mice did show some increase in fat spaces.
Hypocellularity and increased fat accumulation in Fancc−/−Sod1−/−BM.
Metaphyseal sections of leg bones fromFancc+/+Sod1+/+(A),Fancc−/−(B), Sod1−/−(C), and Fancc−/−Sod1−/−(D) mice (magnification × 400). Marrow fat content (clear areas) is increased in Fancc−/−Sod1−/−mice compared with Fancc+/+ Sod1+/+ or toFancc−/−. Controls revealed only rare fat cells,whereas Sod1−/−mice did show some increase in fat spaces.
Total bone marrow cellularity and absolute number of cell types from mice aged 8 to 10 weeks
| Genotype . | n . | Average cellularity/femur (107) . | mAb (Absolute cell no. 107) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PGP1 . | B220 . | Ly6G . | CD11b . | CD14 . | PMN . | Ter-119 . | |||
| Fancc+/+Sod1+/+ | 6 | 3.98 ± 0.62 | 2.89 | 0.86 | 1.28 | 1.33 | 0.05 | 1.21 | 1.36 |
| Fancc+/−Sod1+/− | 7 | 4.08 ± 0.57 | 3.14 | 1.21 | 1.48 | 1.42 | 0.14 | 1.46 | 1.30 |
| Fancc−/− | 6 | 3.41 ± 0.33 | 2.21 | 0.77 | 0.87 | 0.77 | 0.04 | 0.73 | 1.2 |
| Sod1−/− | 5 | 3.53 ± 0.57 | 2.7 | 0.88 | 1.23 | 1.2 | 0.06 | 1.1 | 1.05 |
| Fancc−/−Sod1−/− | 6 | 2.33 ± 0.19 | 1.68 | 0.56 | 0.79 | 0.73 | 0.01 | 0.85 | 0.72 |
| Genotype . | n . | Average cellularity/femur (107) . | mAb (Absolute cell no. 107) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PGP1 . | B220 . | Ly6G . | CD11b . | CD14 . | PMN . | Ter-119 . | |||
| Fancc+/+Sod1+/+ | 6 | 3.98 ± 0.62 | 2.89 | 0.86 | 1.28 | 1.33 | 0.05 | 1.21 | 1.36 |
| Fancc+/−Sod1+/− | 7 | 4.08 ± 0.57 | 3.14 | 1.21 | 1.48 | 1.42 | 0.14 | 1.46 | 1.30 |
| Fancc−/− | 6 | 3.41 ± 0.33 | 2.21 | 0.77 | 0.87 | 0.77 | 0.04 | 0.73 | 1.2 |
| Sod1−/− | 5 | 3.53 ± 0.57 | 2.7 | 0.88 | 1.23 | 1.2 | 0.06 | 1.1 | 1.05 |
| Fancc−/−Sod1−/− | 6 | 2.33 ± 0.19 | 1.68 | 0.56 | 0.79 | 0.73 | 0.01 | 0.85 | 0.72 |
Average BM cellularity/femur is represented as the average ± SEM. Fancc−/−Sod1−/−cellularity was decreased by approximately 52% compared withFancc+/−Sod1+/− (P= .08). Absolute numbers represent the average % staining × average cellularity and was consistantly reduced by a minimum of 40% compared with Fancc+/−Sod1+/−controls. Cellularity was decreased in Sod1−/−and Fancc−/− mice as well.
Fancc−/−Sod1−/−total BM cells fail to show increased apoptosis or chromosomal aberrations
Because BM hypocellularity inFancc−/−Sod1−/− mice may have been due to an increased level of apoptosis, total BM samples were analyzed by flow cytometry and propidium iodide/annexinV (PI/A) staining. However, this revealed no gross increase in apoptotic cells in Fancc−/−Sod1−/− mice, as compared with Fancc+/−Sod1+/−controls (Table 3). Although suggesting that increased apoptosis might not be the explanation for the BM hypocellularity, the possibility of increased apoptosis within a progenitor subset was not excluded by this procedure. Because gross cytogenetic abnormalities would impair hematopoietic cell development, we evaluated metaphase chromosome spreads fromFancc+/+Sod1+/+, Fancc+/−Sod1+/−, andFancc−/−Sod1−/− BM cells. However, there was no evidence of increased chromosomal aberrations (breaks, gaps, or detectable rearrangements) inFancc−/−Sod1−/− mice (n = 2) as compared with control mice (n = 3) on examination of 10 metaphase cells per mouse (data not shown).
PI/Annexin FACS Analysis from total bone marrow samples
| Genotype . | n . | % Staining . | |
|---|---|---|---|
| Annexin+ PI− . | Annexin+PI+ . | ||
| Fancc+/−Sod1+/− | 5 | 25.49 ± 4.59 | 15.52 ± 3.08 |
| Fancc−/−Sod1−/− | 5 | 28.38 ± 3.83 | 16.44 ± 6.12 |
| Genotype . | n . | % Staining . | |
|---|---|---|---|
| Annexin+ PI− . | Annexin+PI+ . | ||
| Fancc+/−Sod1+/− | 5 | 25.49 ± 4.59 | 15.52 ± 3.08 |
| Fancc−/−Sod1−/− | 5 | 28.38 ± 3.83 | 16.44 ± 6.12 |
FACS Staining was used to quantitate the number of apoptotic cells from total BM samples of 8-9 week old mice. Annexin+/PI− values represent cells initiating the apoptotic process and Annexin+/PI+ values represent cells that have already undergone apoptosis and death.
In vitro hematopoietic colony growth is severely impaired inFancc−/−Sod1−/−mice
Because BM hypocellularity might result from inadequate growth of hematopoietic progenitors inFancc−/−Sod1−/− mice, we examined the in vitro clonogenic potential of committed myeloid (granulocyte-macrophage colony-forming units [CFU-GMs]) and lymphoid (pre-B colony-forming units [CFU-pre-Bs]) progenitors fromSod1−/−, Fancc−/−,Fancc+/+Sod1+/+, Fancc+/−Sod1+/−, andFancc−/−Sod1−/− mice. Figure6A represents the average number of progenitors/femur ± SEM from myeloid (dark bars) and pre-B (hatched bars) methylcellulose assays for n = 6 to 8 animals per genotype, with each experiment done in duplicate (forSod1−/− pre-B cultures, n = 4). These data clearly show that the numbers of colonies from myeloid and pre-B progenitors/femur fromFancc−/− Sod1−/− mice (P = .0002 for both) was severely depressed, as compared with Fancc+/+Sod1+/+ controls. The data indicate that the number of myeloid and pre-B progenitors/femur from Fancc−/− Sod1−/− mice was approximately 75-fold lower than that fromFancc+/+Sod1+/+ controls. Interestingly, the number of colonies obtained fromSod1−/− and Fancc−/−BM samples was also significantly reduced (P = .04 and P = .01, respectively) for both the myeloid and pre-B assays when compared withFancc+/+ Sod1+/+ andFancc+/−Sod1+/− controls.
Colony-forming assays.
(A) Colony-forming assays reveal decreased numbers of progenitors inFancc−/−Sod1−/−BM samples. Myeloid (dark bars) and pre-B (hatched bars) CFUs were determined forSod1−/−, Fancc−/−, Fancc+/+ Sod1+/+, Fancc+/−Sod1+/−, andFancc−/−Sod1−/−mice. The decrease in the number of progenitors/femur is highly significant (P = .0002) whenFancc−/−Sod1−/−mice are compared to Fancc+/+Sod1+/+controls. Values represent the average number of progenitors/femur of 6 mice per group ± SEM. * = P < .05, ** = P < .001. (B)Fancc−/−Sod1−/−progenitors fail to generate normal ratios of CFU-GEMM, CFU-GM/G/M, and BFU-E. Myeloid colonies from panel A were assessed morphologically to determine the cell types contributing to the colonies. CFU-GEMM (dark bars), CFU-GM/G/M (light gray bars), and BFU-E (hatched bars) colonies were scored by eye and the values represent average percent of cell type ± SEM (n = 6-8 mice/group). Ratios of CFU-GM/G/M, and CFU-GEMM from Fancc−/−Sod1−/−samples were significantly different fromFancc+/+Sod1+/+ controls (P = .002 and P = .003, respectively).
Colony-forming assays.
(A) Colony-forming assays reveal decreased numbers of progenitors inFancc−/−Sod1−/−BM samples. Myeloid (dark bars) and pre-B (hatched bars) CFUs were determined forSod1−/−, Fancc−/−, Fancc+/+ Sod1+/+, Fancc+/−Sod1+/−, andFancc−/−Sod1−/−mice. The decrease in the number of progenitors/femur is highly significant (P = .0002) whenFancc−/−Sod1−/−mice are compared to Fancc+/+Sod1+/+controls. Values represent the average number of progenitors/femur of 6 mice per group ± SEM. * = P < .05, ** = P < .001. (B)Fancc−/−Sod1−/−progenitors fail to generate normal ratios of CFU-GEMM, CFU-GM/G/M, and BFU-E. Myeloid colonies from panel A were assessed morphologically to determine the cell types contributing to the colonies. CFU-GEMM (dark bars), CFU-GM/G/M (light gray bars), and BFU-E (hatched bars) colonies were scored by eye and the values represent average percent of cell type ± SEM (n = 6-8 mice/group). Ratios of CFU-GM/G/M, and CFU-GEMM from Fancc−/−Sod1−/−samples were significantly different fromFancc+/+Sod1+/+ controls (P = .002 and P = .003, respectively).
In vitro colony-forming assays provide additional information about the quality of committed progenitors because both the size of the colonies as well as the frequency of different cell types arising from a myeloid progenitor can be evaluated. For example, most colonies scored fromFancc−/−Sod1−/− mice just met the criteria for colony size (> 20 cells/colony), as compared with colonies from Fancc+/+Sod1+/+controls, which were highly cellular. Furthermore, the colonies described in Figure 6A were scored by cell morphology into granulocyte, erythrocyte, macrophage, megakaryocyte colony-forming unit (CFU-GEMM), CFU-GM/G/M, and erythroid burst forming unit (BFU-E) groups. Figure 6B represents the frequency of progenitors/105 BM cells. We found that colonies enumerated fromFancc−/−Sod1−/− samples were mostly erythroid in origin, 57.4% ± 20.44% (P = 0.12) with very few CFU-GM/G/M, 8.78% ± 8.0%, and CFU-GEMM, 0.46% ± 0.21%, colonies being present (P = .002 andP = .003, respectively). Sod1−/−, Fancc−/−,Fancc+/+Sod1+/+, Fancc+/−Sod1+/− progenitors, on the other hand, all gave rise to the different cell types at similar frequencies. It was also important to determine whether plating increased numbers of cells would augment colony formation byFancc−/−Sod1−/− BM samples. However, plating at concentrations of 2, 5, or 10 times the original cell number did not augment growth ofFancc−/−Sod1−/− colony-forming cells (data not shown), suggestive of toxicity, or an inadequate response to exogenous growth factors. Although cells from individuals with FA can be sensitive to ambient oxygen, subjecting theFancc−/−Sod1−/− BM cultures to 5% O2 also did not augment colony growth (data not shown). The latter 2 experiments represent 3 mice for each ofFancc−/−Sod1−/− andFancc+/−Sod1+/−, with each experiment performed in duplicate.
Primitive progenitor numbers are normal in Fancc−/−Sod1−/−mice
Although BM hypocellularity could result from reduced levels of early progenitors, Fancc−/−Sod1−/−BM samples did not exhibit a significant reduction in the Lin− compartment. Thus, the absolute number of Lin− cells, as determined by flow cytometry of nonfractionated total BM samples, was similar forFancc+/− Sod1+/−(1.7 × 105 ± 0.3/femur) andFancc−/−Sod1−/− mice (1.3 × 105 ± 0.7/femur); n = 3 in each case. Furthermore, the absolute number of Lin−cells (obtained after Lin+ cell depletion-column experiments) from Fancc+/−Sod1+/−controls (7.6 × 105 ± 1.52) was similar to that ofFancc−/−Sod1−/− mice (5.2 × 105 ± 1.05); n = 6 (values represent cell numbers obtained from both femurs and tibiae per mouse). Flow cytometry of Lin− cells, obtained following Lin+depletion, using monoclonal antibodies against CD34, Sca1 and c-kit revealed no significant differences. The values below represent experiments using 5 animals per group and are the average absolute number ± SEM. Thus, the absolute number of Lin−Sca1+c-kit− and Lin−Sca1+c-kit+ cells fromFancc−/−Sod1−/− mice was 1.3 × 104 ± 0.3 and 5.1 × 104 ± 2.0, whereas forFancc+/−Sod1+/− controls, values were 2.0 × 104 ± 0.9 and 3.4 × 104 ± 0.9, respectively. The similarity is also observed in the Lin−CD34+Sca1−compartment, where bothFancc−/−Sod1−/− mice andFancc+/−Sod1+/− controls had 13 × 105 ± 0.7 cells. We conclude that the absolute number of cells within the Lin− compartment ofFancc−/−Sod1−/− BM is similar to controls and that progenitor subpopulations within the Lin− compartment of these mice are also similar to controls.
Discussion
Herein we show that mice having combined deficiencies of Fancc and the primary cytosolic superoxide-detoxifying enzyme, Sod1, exhibit 2 novel phenotypes: fatty liver and an impairment of hematopoietic cell development.
Fancc−/−Sod1−/− liver pathology was characterized primarily by zone 3 microvesicular steatosis, possibly a manifestation of in vivo superoxide toxicity as evidenced by increased superoxide production. In keeping with superoxide-mediated pathology, we found increases in the oxidative stress-inducible enzymes, MnSOD and HO-1, withinFancc−/−Sod1−/− livers. Although microvesicular fatty liver is often encountered as a result of mitochondrial dysfunction, it can also result from impaired egress of lipids from hepatocytes as seen following specific hepatotoxin exposures.35 ROS, such as superoxide, either alone, or in combination with nitric oxide (NO) to yield peroxynitrite (ONOO−),36 are able to react with a variety of cellular macromolecules, including membrane lipids.37Lipid peroxidation and accumulated hydroxy fatty acids38within membranes of the endoplasmic reticulum, for example, can interfere with transport of lipids or components of very-low-density lipoprotein particles that are responsible for removing lipids from hepatocytes.35 Interestingly, and possibly in keeping with superoxide-mediated organelle, and possibly plasma membrane damage, the livers of mice lacking MnSOD (Sod2) also demonstrated microvesicular steatosis39 and increased levels of serum ALT. It should also be noted that ROS can function as second messengers, modulating the activities of intracellular signaling molecules and transcription factors.40 41 Thus, liver pathology inFancc−/−Sod1−/− mice might stem from abnormal gene expression patterns secondary to elevated superoxide levels or reduced dismutation of this species into hydrogen peroxide.
Increased superoxide production byFancc−/−Sod1−/− liver cell cultures was interesting given the reports of elevated ROS generation by FA cells,21,22 and the reported protective effects of SOD, where extrinsic SOD reduced the high rates of chromosomal breakage in FA cells, and also diminished MMC cytotoxicity.16,42,43Interestingly, reduced SOD1 levels have been reported in FA erythrocytes.44-46 Unlike theFancc−/−Sod1−/− cross, hepatic steatosis has not been reported in human FA. Assuming that a similar pathogenetic mechanism were involved in FA, this discordance might be explained by the species-specific differences in CYP P-450 genes or xenobiotic exposure.
Because lipid accumulation in hepatocytes can be accompanied by necrosis47 and inflammatory infiltrates, we searched for evidence of hepatocyte damage and cellular infiltrates. Only the modest elevations of serum ALT were suggestive of hepatocyte damage, and this occurred in the absence of overt necrosis or pathologic collagen deposition. Activation of Kupffer cells, which also leads to ROS, NO, as well as proinflammatory cytokine production, can injure hepatocytes, and is often accompanied by neutrophil infiltration.37 The lack of infiltrates, the normal percentages of CD11b+ cells in Fancc−/−Sod1−/− liver samples, and the zonal liver pathology, however, would suggest a defect intrinsic to the hepatocytes ofFancc−/−Sod1−/− mice.
The second phenotype ofFancc−/−Sod1−/− mice was that of marrow hypoplasia, accompanied by a striking impairment of in vitro hematopoietic colony formation. Given the normal levels of primitive precursors, this was suggestive of a growth or survival defect in committed progenitor populations. Similar toFancc−/− Sod1−/− mice, in vitro colony generation by FA BM samples was impaired at both the multipotential and differentiated progenitor levels,48-50with the mean CFU-GM values for human FA colony-forming cells being approximately 15-fold lower than controls.50 In contrast to humans with FANCC mutations, however,Fancc−/− mice do not show spontaneous permanent cytopenias or decreased clonogenic potential.13,14 Like their human counterparts, however, hematopoietic cells from Fancc−/− show increased sensitivity to interferon-γ, tumor necrosis factor-α, and macrophage inflammatory protein-1α, as well as deregulated apoptosis.51Fancc−/− BM cells also exhibit a decrease (7- to 12-fold) in short-term and long-term multilineage repopulating ability.52 The mild thrombocytopenia we observed in young (8-10 week), but not older (3 month) Fancc−/− mice had not been reported previously, and is likely attributable to differences in the genetic backgrounds of the mice. This variable may also account for the modest reductions in myeloid and lymphoid colony formation in ourFancc−/− mice. Unlike individuals with FA, platelet counts of youngFancc−/−Sod1−/− mice were normal. It is possible that thrombocytopenia would occur over time were BM failure progressive in these mice. Interestingly, increased MCV ofFancc−/−Sod1−/− RBCs was analogous to the macrocytosis commonly observed in patients with FA53; however, this morphology has many causes, including liver dysfunction.54
The finding of impaired hematopoiesis inFancc−/−Sod1−/− mice was most strongly supported by our functional studies of in vitro growth of committed progenitors. CFU-GEMMs, CFU-GMs, and CFU-preBs fromFancc−/−Sod1 BM samples failed to grow. Furthermore, despite normal levels of the earliest (Lin−) progenitors, there were considerably lower numbers of Lin+progenitors/femur in Fancc−/−Sod1−/−mice. In addition, the ability of BM cells to produce colonies of normal size and containing the usual range of cell types was compromised. In keeping with a markedly reduced proliferative potential (or an increased rate of apoptosis) CFU numbers in vitro were not increased by simply plating more cells (up to 10-fold). Thus, poorFancc−/−Sod1−/− colony formation did not appear to result from the plating of lower progenitor numbers, but instead pointed to a progenitor cell growth or survival defect. Although Fancc−/−Sod1−/− total BM samples did not reveal evidence of overt apoptosis, increased death restricted to a progenitor subset(s) would readily explain the lack of growth in the Fancc−/−Sod1−/−colony-forming assays. This might be attributable to superoxide-mediated genotoxicity superimposed on a background of reduced DNA repair capacity due to the lack of Fancc. Although oxygen-dependent toxicity did not appear to play a role in colony-forming assay inhibition (growth in 5% oxygen did not “rescue” growth), it is possible that committed progenitors have pro-oxidant intracellular environments that result in toxicity even at reduced oxygen tensions, or that marrowFancc−/−Sod1−/− progenitors were damaged by ambient oxygen during harvesting and initial colony-forming assay plating procedures.
Alternatively, because ROS appear to be required for the normal proliferative response to various growth factors,55 it is possible that Sod1 deficiency led to loss of a positive growth signal. There is evidence that ROS, such as superoxide and hydrogen peroxide, can act as second messengers for a variety of stimuli, including growth factors.55 GM-colony-stimulating factor stimulation, for example, led to rapid increases in cellular hydrogen peroxide levels, accompanied by elevated levels of tyrosine phosphorylation.56 The latter may be due to the transient inhibition of protein-tyrosine phosphatases by this species, an event predicted to favor protein-tyrosine kinase-dependent signaling.55 Furthermore, alterations in redox potential affect a wide range of cellular processes.40,55 The balance between ROS and antioxidant systems may thus regulate cellular responses to external stimuli40,56,57; for example, interfering with hydrogen peroxide generation attenuated the proliferative response of hematopoietic cells to colony-stimulating factors. A lack of Sod1 would be predicted to inhibit growth factor–mediated cell growth by reducing conversion of superoxide to hydrogen peroxide. Thus, hematopoietic progenitors fromFancc−/−Sod1−/− mice might be intrinsically hyporesponsive to growth factor stimulation. Perhaps in keeping with this, we observed a consistent reduction in colony formation in vitro when Sod1−/− BM samples were plated. It is also notable that the abnormalities ofFancc−/−Sod1−/− mice are analogous to those of W/Wv mice that lack normal stem cell factor receptor kinase (c-kit) activity and demonstrate a marked suppression of CFU growth in vitro.58 There is evidence that FANCC is required for normal STAT1 activation following growth factor stimulation.59 This intriguing finding raises the possibility that a “2-hit” signaling abnormality might account for the hematopoietic defect ofFancc−/−Sod1−/−mice: namely, that a decreased level of growth factor-induced ROS (specifically, hydrogen peroxide), together with a defect in STAT1-mediated signaling, act synergistically to inhibit proliferation and/or survival of hematopoietic progenitors.
We are indebted to Dr R. K. Humphries of the Terry Fox Laboratory (Vancouver, BC) for his critical review of our data, and to S. Middler and J. Patterson of the British Columbia Children's and Women's Hospital Pathology Laboratory for preparing samples for electron microscopy and for the analysis of blood samples. We also thank L. Spence who performed the genotyping, S. Smith and T. McKernan in the Centre for Molecular Medicine and Therapeutics vivarium, and N. Makhani for her assistance.
Supported by grants no. 010265 (to F.R.J.) and 007223 (to M.B.) from the National Cancer Institute of Canada, with funds from the Canadian Cancer Society. S.H. was supported by a Studentship from the Cancer Research Society of Canada.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Frank R. Jirik, Department of Biochemistry and Molecular Biology, University of Calgary, 3330 Hospital Dr NW, Calgary, Alberta, T2N 4N1 Canada; e-mail: jirik@ucalgary.ca.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal