Hematopoietic stem cell (HSC) homing is believed to rely heavily on adhesion interactions between stem cells and stroma. An in vitro assay was developed for adhesion of engraftable HSCs in bone marrow suspensions to pre-established Dexter-type long-term bone marrow culture stromal layers. The cell numbers in the adherent layer and supernatant were examined, along with the engraftment capability of adherent layer cells to indicate the number of HSCs that homed to in vitro stroma. The cell number in the supernatant declined over the 24-hour period. The number of test cells adhering to the stromal layer increased during the first hour and then fell at 6 and 24 hours. The number of test HSCs adhering to the stromal layer was substantial at 20 minutes, increased during the first hour, and then remained constant at 1, 6, and 24 hours of adhesion. These data indicate that adhesion of engraftable HSCs occurs quickly and increases during the first hour of contact with pre-established stroma, that adhesion plateaus within 1 hour of contact, and that HSCs maintain their engraftment capability for at least 24 hours of stromal adhesion. Long-term engraftment from test cells at more than 1 hour of adhesion represents 70.7% of the predicted engraftment from equivalent numbers of unmanipulated marrow cells, indicating that 2 of 3 test engraftable HSCs adhered. These findings demonstrate the usefulness of this model system for studying stem-stromal adhesion, allowing further dissection of the mechanism of HSC homing and exploration of possible manipulations of the process.
Introduction
Hematopoietic stem cell (HSC) homing is the process by which HSCs, infused intravenously in the transplantation setting, specifically extravasate in the bone marrow to engraft and proliferate there. Homing has been studied extensively both in vivo and in vitro and is believed to rely on adhesion molecule interactions between stromata, which consist of stromal cells and extracellular matrix, and stem cells.1-18 In vivo homing studies typically rely on infusion of a labeled or detectable HSC population and subsequent detection at various times after the transplantation.16,19 In this approach, the recipients must be examined early enough (approximately 24 hours or earlier) that only infused cells, and not their progeny, are detected. However, testing this early can make it difficult to ascertain that the cells are truly homed and not migratory. Also, to dissect the mechanisms of homing, it would be desirable to conduct various manipulations, such as adding an adhesion receptor blocking antibody, and this can be cumbersome or even impossible in vivo. On the other hand, in vitro systems are relatively easy to manipulate, but the assay result can be difficult to interpret. Most in vitro homing studies examine adhesion of a hematopoietic cell population or cell line to a test substrate, such as purified extracellular matrix molecule coatings or stromal cell lines, under various conditions.20-29 While these systems are more manageable, they do not accurately reflect the in vivo condition. Further, the outcome is most often measured by counting adherent cells, or counting cells remaining in the supernatant and subtracting them from the total, and this is limiting because it is impossible to determine the functionality of the adhered cells.
A set of reports20,22,28 has described an assay that groups populations of hematopoietic progenitors according to their adhesion to plastic and/or stroma, quantified by release of granulocytic-macrophage colony forming unit cells into the supernatant. The most primitive cells adhere to both plastic and stroma, whereas stroma-only-adherent cells are less primitive, but more primitive than nonadherent cells. The adherent cells are quiescent and drug resistant, suggesting qualities of HSCs. In another study,24 mouse bone marrow was fractionated by adhesion to plastic only and then assayed by engraftment, separating a very early primitive HSC population from in vitro colony-forming cells and spleen colony-forming cells. However, plastic adhesion is not relevant to in vivo homing. Another model system26 was described as a cytoadhesion assay for the binding of cloned hematopoietic progenitor cells to stroma, assayed by 51Cr labeling of hematopoietic cells. However, this system again used cell lines for both the hematopoietic progenitor and stromal populations. Perhaps the closest in vitro model to in vivo homing reported to date27 characterized fibronectin-adhering cells by in vitro repopulation of irradiated stroma by cobblestone area–forming cells, and demonstrated maximum adhesion at 4 hours.
Thus, no model system has been reported for studying in vitro homing of primitive engraftable stem cells and the interaction of primary stem cells with a complex stroma. Here we report the development of a system for testing adhesion of primitive engraftable HSCs to pre-established stromal layers in vitro. Cell populations are allowed to adhere to pre-established Dexter-type long-term bone marrow culture (LTBMC) stromal layers, and then the entire adherent cell population is transplanted into irradiated recipients. LTBMCs are characterized by the formation of a complex adherent layer, composed of various types of stromal cells and extracellular matrix, and the maintenance of hematopoiesis in vitro for many weeks.30,31 In these cultures, hematopoiesis proceeds without the addition of exogenous growth factors, but is dependent on interaction between hematopoietic progenitor cells and the stroma.30-42 Thus, LTBMCs can act as an in vitro model of bone marrow, particularly for studying stem cell–stromal interactions. Our model assumes that HSC adhesion to the stromal layer in LTBMCs reflects the in vivo interactions that form the basis of homing. While transplantation is somewhat time consuming and demanding compared with cell counts or colony assays, engraftment is still the truest measure of functional stem cells.43 This approach has several major advantages compared with previously described systems. The adhesion step is conducted in vitro where manipulation and testing are relatively easy, yet both the hematopoietic and the stromal populations are complex explant populations, which best reflects the in vivo situation. All tightly adherent cells are assayed directly, and thus there is no problem with degrees of adhesion or bias due to selective separation. Finally, our approach allows determination of the short- and long-term engraftment capability of the adhering cells, for a true stem cell adhesion assay.
Materials and methods
Animals
All studies were approved by the University of Massachusetts Medical School Institutional Animal Care and Use Committee. We purchased 6- to 8-week-old (18 to 22 g) BALB/c mice from Taconic Laboratories (Germantown, NY). Mice were maintained in conventional clean conditions at the Animal Facility of the University of Massachusetts Medical Center, Biotech II building, and were given mouse food and acidified water ad libitum. All animals were acclimated for at least 1 week prior to experimental use.
Long-term bone marrow cultures
Cultures were performed according to the method of Dexter,30 with modifications as described previously.32 37 Fresh whole bone marrow was harvested by flushing bones from female BALB/c mice in Fischer medium (Gibco BRL, Grand Island, NY) with 100 U/mL penicillin, 100 μg/mL streptomycin (Gibco BRL), 0.0125 μg/mL fungizone (Gibco BRL), 10−7 M hydrocortisone sodium succinate (Upjohn, Kalamazoo, MI), and 20% horse serum (lot AFG5429) (Hyclone, Logan, UT). Pooled harvested cells were counted in crystal violet on a Neubauer hemacytometer and suspended at a concentration of 4 × 106/mL, then seeded into either 25-cm2 (10 mL cell suspension per flask) or 75-cm2 (30 mL cell suspension per flask) vent-cap tissue-culture flasks (Falcon, Franklin Lakes, NJ). Cultures were incubated at 33°C in 5% CO2 in air, and fed weekly by removal of one half the supernatant medium and cells and replacing it with fresh medium (demidepopulation). At every feeding, the nonadherent cells in the supernatant medium were counted (data not shown) in trypan blue as an index of the health of the cultures.
Adhesion assay
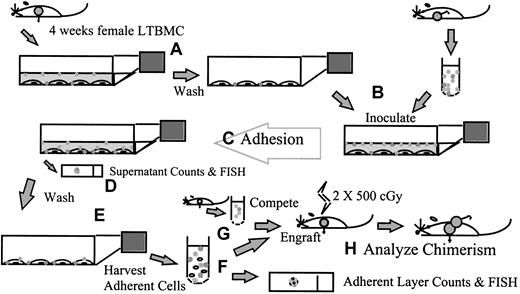
Figure 1 is a schematic representation of the adhesion assay approach. LTBMCs were established and maintained for 4 weeks as described above. The supernatant cells were removed by rocking the flask, followed by removal of the media, and the stromal layers were washed once with phosphate-buffered saline (PBS). Fresh male BALB/c marrow was harvested by flushing into LTBMC medium, suspended to 2 × 106 per mL in medium, and inoculated onto the pre-established stromal layers. The cultures were then incubated at 33°C in 5% CO2 in air during the adhesion step. After the adhesion time specified for the particular experiment, the supernatant media and cells were removed. Aliquots of the supernatant cells were taken for cell counts in crystal violet on a Neubauer hemacytometer. The adherent layers were then washed with PBS, 3 times unless noted otherwise, and the adherent cells harvested and studied. Adherent cells were harvested by mechanical dissociation of the stromal layer by scraping in PBS with disposable cell scrapers (Falcon) and vigorous pipetting. Cells were counted in crystal violet on a Neubauer hemacytometer, and the percentage of male cells in the adherent layer was determined by FISH (described below). When 75-cm2 flasks were used, the cell counts were divided by 3 to give the cell count per 25-cm2 flask equivalent. The engraftment capability of adherent male cells was determined by transplantation to indicate the number and quality of test stem cells adhering (below).
A schematic summary of the adhesion assay.
LTBMCs are established from female BALB/c mice and maintained for 4 weeks. (A) The supernatant cells are removed and the stromal layers washed. (B) Fresh male BALB/c marrow is harvested into LTBMC medium and inoculated onto the pre-established stromal layers. (C) The cultures are incubated for the adhesion step, during which time any experimental manipulations to explore the in vitro homing process can be carried out. (D) After the adhesion time, the supernatant media and cells are removed and counted; samples can be taken for fluorescence in situ hybridization (FISH) for other analyses. (E) The stromal layers are washed; then the adherent cells are harvested by mechanical dissociation and studied. (F) Cells are counted, and the percentage of male cells in the adherent layer is determined by FISH. (G) Cells are transplanted into lethally irradiated female BALB/c recipients, in competition with freshly harvested female marrow. (H) The percentage of male chimerism in female recipients is used to determine the engraftment capability of adherent male cells.
A schematic summary of the adhesion assay.
LTBMCs are established from female BALB/c mice and maintained for 4 weeks. (A) The supernatant cells are removed and the stromal layers washed. (B) Fresh male BALB/c marrow is harvested into LTBMC medium and inoculated onto the pre-established stromal layers. (C) The cultures are incubated for the adhesion step, during which time any experimental manipulations to explore the in vitro homing process can be carried out. (D) After the adhesion time, the supernatant media and cells are removed and counted; samples can be taken for fluorescence in situ hybridization (FISH) for other analyses. (E) The stromal layers are washed; then the adherent cells are harvested by mechanical dissociation and studied. (F) Cells are counted, and the percentage of male cells in the adherent layer is determined by FISH. (G) Cells are transplanted into lethally irradiated female BALB/c recipients, in competition with freshly harvested female marrow. (H) The percentage of male chimerism in female recipients is used to determine the engraftment capability of adherent male cells.
Transplantation
The engraftment capability of male cells in the adherent layer was assayed by competitive repopulation of lethally irradiated female recipients; therefore, BALB/c mice were used for all experiments because of their very low degree of immunoreactivity to the H-Y antigen.44 The cell suspension being studied was injected in competition with a set proportion of fresh female cells to quantitate the engraftment capability of the test cells in comparison with a standard population.45 46 Cell suspensions were strained through 40-μm pore size strainers (Falcon) to prevent embolization of recipients and were suspended for injection in PBS at the concentration indicated for each individual experiment. Recipient female mice were irradiated to 10.0 Gy in 2 fractions of 5.0 Gy, 3 hours apart, from a Gammacell 40 cesium source irradiator (92.6 to 96 cGy/min) 1 hour before injection. A single injection of cells in 0.5 mL PBS was given by the lateral tail vein.
Recipients were killed at different times after transplantation, to determine short- and long-term engraftment. Bone marrow was harvested by flushing the femurs of the recipients in PBS, and the resulting cell suspensions were used for FISH and DNA preparation for Southern blot analysis to determine the percentage of male chimerism.
Fluorescence in situ hybridization
Cytospin preparations of harvested marrow suspensions were analyzed. After fixation in Carnoy solution (75% methanol, 25% acetic acid), cells were treated with 0.2 μg/mL proteinase K (Sigma Chemical, St Louis, MO) in 20 mM Tris buffer at 37°C for 90 seconds, dehydrated through an ethanol series, denatured for 3 minutes in 70% formamide (Gibco BRL) at 71°C, and dehydrated again. Digoxigenin-labeled Y-chromosome painting probe47 was applied for overnight incubation at 45°C. Slides were then washed in 50% formamide, 2 × SSC, and 4 × SSC, stained with rhodamine-labeled antidigoxigenin antibody (Boehringer Mannheim, Indianapolis, IN), and washed again in 4 × SSC. Cell preparations were mounted in Vectashield mounting medium (Vector Laboratories, Burlingame, CA) with 0.4 μM DAPI (4, 6-diamidino-2-phenylindole) (Sigma Chemical). Female (negative) and male (positive) controls were included in every batch. Cells were scored by visual microscopic counting, and at least 100 cells in at least 4 separate fields were counted for each sample to determine the percentage of bone marrow cells expressing the Y chromosome (percentage of male chimerism).
Southern blot analysis
DNA was extracted from cell suspensions by means of proteinase K (Sigma Chemical) and RNase (Sigma Chemical) digestion, phenol and chloroform extraction, and ethanol precipitation; then the concentration and purity of DNA were determined by spectrophotometry with the 260:280 nm ratio. Then, 5 μg DNA was analyzed by restriction endonuclease Dra-I (Boehringer Mannheim) digestion followed by gel electrophoresis in 0.8% agarose (Gibco BRL) and Southern blotting onto Zeta-Probe nylon membranes (BioRad, Hercules, CA) with 0.4 M sodium hydroxide. Membranes were probed with a 32P-labeled pY2 complementary DNA (cDNA) probe for repetitive sequences on the Y chromosome48 (courtesy of Dr Ihor Lemischka, Princeton University, Princeton, NJ) and with a 32P-labeled interleukin-3 (IL-3) cDNA probe as a loading control (courtesy of J. Ihle and DNAX, Palo Alto, CA). Probes were labeled by means of a random primed labeling kit (Boehringer Mannheim) using 32P-dCTP (New England Nuclear, Boston, MA). Hybridization was performed in bags containing 10% dextran sulfate, 1% sodium dodecyl sulfate, 1 × Denhardt solution, and 4 × SSCP (salt, sodium citrate, phosphate) at 65°C for 12 to 24 hours. Blots were analyzed by means of a BioRad Molecular Imager to quantitate the intensity of the radioactivity, and the proportion of male cells present in each sample (percentage of male chimerism) was calculated by comparison with 2 male and 2 female controls on each comb of each gel, followed by correction by means of the IL-3 probing intensity. For confirmation, autoradiography was performed by means of Kodak XRP film (Eastman Kodak, Rochester, NY) with intensifying screens at −70°C for varying times.
Data analysis
To compare cell count data at different adhesion times, the 2-tailed z test was used, with results considered significant whenP < .05. To evaluate engraftment results, the nonparametric Kruskal-Wallis test was used, with results considered significant when P < .05.
To compare engraftment results in experiments, the actual percentage of male chimerism of each female recipient was converted to the ratio of actual to expected engraftment to reflect the proportion of engraftable male HSCs in the test cell suspension that adhered to the stroma in each experiment; it was calculated as follows (Table1). First, the number of adherent layer cells harvested from the flasks, called flask yield, was determined. The number of adherent layer cells injected in each experiment was divided by flask yield to determine the flask equivalent injected. The flask equivalent was multiplied by the number of fresh male cells inoculated onto the flask to determine the male input equivalent injected, under the assumption that all HSCs in the test marrow suspension adhered. Then, the competition was determined as the number of fresh competitive female marrow cells injected plus the number of adherent layer female cells injected, calculated by means of the percentage of female (by FISH) multiplied by the number of adherent layer cells. Then, on the assumption that all recipient marrow would be of transplant origin and that male and female cells would compete equally, the expected male chimerism was calculated by dividing the number of male cells injected by the total number of cells injected (ie, if 2 × 106 male cells and 2 × 106female cells were injected, the expected engraftment would be 2 ÷ 4 = 50%). The assumption that Dexter-type culture cells competitively engraft similarly to fresh marrow is based on our findings in other experiments.49 Finally, the ratio of actual to expected engraftment was calculated by simply dividing the actual percentage of male chimerism for each recipient by the engraftment expected on the basis of the cell mixture injected. This ratio thus reflects the proportion of male HSCs inoculated onto a given flask that actually adhered to the stromal layer. To compare ratios of actual to expected engraftment in different groups, the 2-tailed Student t test was used, with results considered significant when P < .05.
Method for calculation of ratio of actual to expected engraftment
| Step . | Calculation . | Result . |
|---|---|---|
| 1 | No. adherent layer cells harvested from 25-cm2 flask | Flask yield |
| 2 | No. adherent layer cells injected ÷ flask yield | Flask equivalent injected |
| 3 | No. fresh male cells inoculated onto 25-cm2 flasks initially × flask equivalent injected | Male input equivalent injected |
| 4 | (No. adherent layer cells injected × % female in adherent layer [by FISH]) + no. fresh female cells injected | Total female competition injected |
| 5 | Male input equivalent injected ÷ (male input equivalent injected + total female competition injected) | Expected engraftment |
| 6 | Actual % male chimerism for each recipient ÷ expected engraftment | Ratio of actual to expected engraftment |
| Step . | Calculation . | Result . |
|---|---|---|
| 1 | No. adherent layer cells harvested from 25-cm2 flask | Flask yield |
| 2 | No. adherent layer cells injected ÷ flask yield | Flask equivalent injected |
| 3 | No. fresh male cells inoculated onto 25-cm2 flasks initially × flask equivalent injected | Male input equivalent injected |
| 4 | (No. adherent layer cells injected × % female in adherent layer [by FISH]) + no. fresh female cells injected | Total female competition injected |
| 5 | Male input equivalent injected ÷ (male input equivalent injected + total female competition injected) | Expected engraftment |
| 6 | Actual % male chimerism for each recipient ÷ expected engraftment | Ratio of actual to expected engraftment |
FISH indicates fluorescence in situ hybridization.
Results
Number of washes
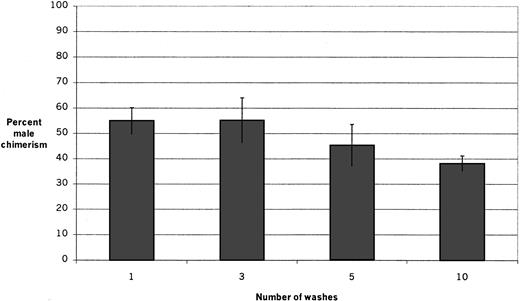
To determine the optimum number of washes for the assay system, the male engraftment capability of the adherent layer was determined after allowing male whole bone marrow suspensions to adhere to pre-established female stromal layers for 1 hour and washing the adherent layers once, 3 times, 5 times, and 10 times (Figure2). The resulting chimerism in irradiated female recipients 6 weeks after transplantation of adherent layer cells in the different groups (mean ± SEM) was 55.0% ± 5.2%, 55.2% ± 8.8%, 45.3% ± 8.3%, and 38.1% ± 3.1% after 1, 3, 5, and 10 washes, respectively. The groups are not significantly different, although there appears to be a trend for decreased adhesion of HSCs after 10 washes. In all subsequent experiments, the layers were washed 3 times.
Engraftment after varying numbers of washes.
Fresh male marrow suspensions were inoculated onto pre-established stromal layers and allowed to adhere for 1 hour; then the layers were washed once, 3 times, 5 times, or 10 times before cells were harvested for transplantation. The percentage of male chimerism in irradiated female recipients 6 weeks after transplantation in 2 experiments (4 to 5 recipients per group per experiment) is shown. There is no significant difference among the groups; however, there appears to be a trend toward decreased adhesion of engraftable HSCs after 10 washes.
Engraftment after varying numbers of washes.
Fresh male marrow suspensions were inoculated onto pre-established stromal layers and allowed to adhere for 1 hour; then the layers were washed once, 3 times, 5 times, or 10 times before cells were harvested for transplantation. The percentage of male chimerism in irradiated female recipients 6 weeks after transplantation in 2 experiments (4 to 5 recipients per group per experiment) is shown. There is no significant difference among the groups; however, there appears to be a trend toward decreased adhesion of engraftable HSCs after 10 washes.
Cell counts at different adhesion times
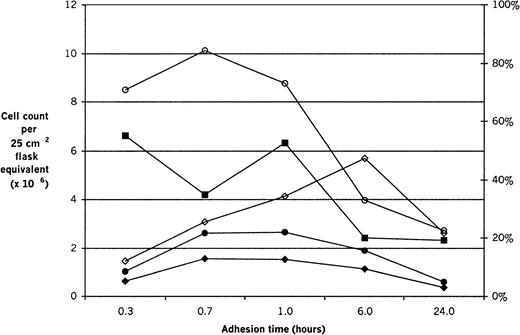
Cell counts per 25-cm2 flask equivalent in the adherent layer and supernatant and the male percentage in the adherent layer (by FISH) were determined after male bone marrow cells were allowed to adhere to pre-established female stromal layers for 20 minutes (0.3 hours), 40 minutes (0.7 hours), 1 hour, 6 hours, and 24 hours (Figure 3). Using the cell counts, FISH results, and the number of male cells fed on, we calculated the adherent layer male cell count and the percentage of test male cells recovered in the adherent layer (Figure3). As expected, supernatant cell counts declined over the 24-hour period, but even at the 20-minute time point, they were substantially lower (6.6 × 106) than in the test suspension (2.0 × 107); by the end of the 24-hour period, only approximately one tenth of the test cells remained in suspension (2.3 × 106). The counts at 6 and 24 hours were significantly lower than at the other time points (P = .01 and P = .004, respectively). The adherent layer cell count remained stable for the first hour, with a small nonsignificant rise between 20 and 40 minutes, and then it also declined dramatically over the 24 hours from 8.5 × 106 to 2.7 × 106. Again, the counts at 6 and 24 hours were significantly lower than at the other time points (P = .004 andP < .0001, respectively). The percentage of male cells in the adherent layer was already 12% after 20 minutes of adhesion; it continued to climb to a peak of 47% at 6 hours and then decreased to 22% by 24 hours of adhesion. The only significantly different value was at 20 minutes (P = .0002). Thus, the number of adherent male cells and the percentage of the test male cells that were recovered in the adherent layer both increased moderately from 20 minutes to 40 minutes and to 1 hour and then also decreased at 6 and 24 hours to less than the 20-minute value. For both of those parameters, the 20-minute and 24-hour time points were significantly lower than the others (P = .03 and P < .001, respectively, for both).
Cell counts at different adhesion times.
Fresh male marrow suspensions were inoculated onto pre-established female stromal layers and allowed to adhere for 20 minutes (0.3 hours), 40 minutes (0.7 hours), 1 hour, 6 hours, and 24 hours. Eight experiments are combined. Cell numbers per 25-cm2 flask equivalent in the adherent layer (○) and supernatant (■) were counted in crystal violet on a Neubauer hemacytometer. Both the adherent layer and the supernatant cell counts are significantly lower at 6 and 24 hours than at other time points (P = .004 andP < .0001, respectively, for the adherent layer andP = .01 and P = .004, respectively, for the supernatant). The male percentage in the adherent layer (◊) was determined by FISH; it is lower at 20 minutes than at the other time points (P = .0002). The absolute number of male cells present in the adherent layer (●) and the percentage of the test male cells that were recovered in the adherent layer (⧫) were calculated on the basis of the cell counts, the FISH results, and the number of male cells initially fed on. The male percentage in the adherent layer (◊) and the percentage of test male cells recovered in the adherent layer (⧫) refer to the right-hand y-axis. For both of these, the values at 20 minutes and 24 hours were significantly lower than at the other time points (P = .03 and P < .0001, respectively, for both).
Cell counts at different adhesion times.
Fresh male marrow suspensions were inoculated onto pre-established female stromal layers and allowed to adhere for 20 minutes (0.3 hours), 40 minutes (0.7 hours), 1 hour, 6 hours, and 24 hours. Eight experiments are combined. Cell numbers per 25-cm2 flask equivalent in the adherent layer (○) and supernatant (■) were counted in crystal violet on a Neubauer hemacytometer. Both the adherent layer and the supernatant cell counts are significantly lower at 6 and 24 hours than at other time points (P = .004 andP < .0001, respectively, for the adherent layer andP = .01 and P = .004, respectively, for the supernatant). The male percentage in the adherent layer (◊) was determined by FISH; it is lower at 20 minutes than at the other time points (P = .0002). The absolute number of male cells present in the adherent layer (●) and the percentage of the test male cells that were recovered in the adherent layer (⧫) were calculated on the basis of the cell counts, the FISH results, and the number of male cells initially fed on. The male percentage in the adherent layer (◊) and the percentage of test male cells recovered in the adherent layer (⧫) refer to the right-hand y-axis. For both of these, the values at 20 minutes and 24 hours were significantly lower than at the other time points (P = .03 and P < .0001, respectively, for both).
Engraftment
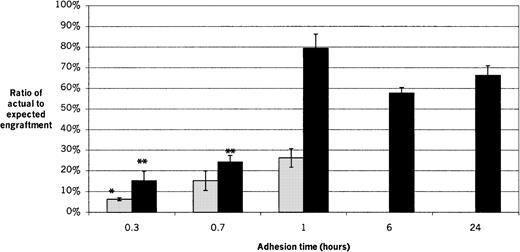
The actual chimerism values of transplant recipients for each experimental condition are shown and compared with the cell numbers injected and the resulting ratios of actual to expected engraftment in Table 2. The short-term (2-week) engraftment capability of adherent male cells was determined after adhesion times of 20 minutes (0.3 hours), 40 minutes (0.7 hours), and 1 hour (Figure 4). The ratio of actual to expected engraftment for recipients of 20-minute adherent cells was significantly lower than for 1 hour (P = .014), while there was no significant difference between the 20-minute and 40-minute groups or between the 40-minute and 1-hour groups, with values (mean ± SEM) of 6.3% ± 0.7%, 15.2% ± 4.7%, and 26.2% ± 4.5%, respectively.
Comparison of cell numbers injected and actual chimerism results to ratio of actual to expected engraftment
| Adhesion time . | Adherent layer cells injected (× 106) . | Ratio of adherent layer cells to fresh female cells* . | Flask equivalent injected . | Ratio of input male equivalent cells to total female cells† . | Actual % male chimerism‡ . | Ratio of actual to expected engraftment‡ . |
|---|---|---|---|---|---|---|
| Short engraftment time | ||||||
| 20 minutes | 15.0 | 15 | 1.8 | 2.5 | 4.5 ± 0.5 | 6.3 ± 0.7 |
| 40 minutes | 17.8 | 17.8 | 1.8 | 2.5 | 10.8 ± 3.3 | 15.2 ± 4.7 |
| 1 hour | 19.5 | 19.5 | 1.8 | 2.4 | 18.5 ± 3.2 | 26.2 ± 4.5 |
| Long engraftment time | ||||||
| 20 minutes | 15.0 | 15 | 1.8 | 2.5 | 10.9 ± 3.3 | 15.2 ± 4.6 |
| 40 minutes | 17.8 | 17.8 | 1.8 | 2.5 | 17.2 ± 2.3 | 24.2 ± 3.2 |
| 1 hour | 12.3 | 17.3 | 1.7 | 6.0 | 63.7 ± 3.0 | 79.4 ± 6.9 |
| 6 hours | 6.7 | 16 | 1.7 | 8.6 | 51.7 ± 2.5 | 57.7 ± 2.7 |
| 24 hours | 4.6 | 15.9 | 1.7 | 8.8 | 59.5 ± 4.1 | 66.3 ± 4.6 |
| Adhesion time . | Adherent layer cells injected (× 106) . | Ratio of adherent layer cells to fresh female cells* . | Flask equivalent injected . | Ratio of input male equivalent cells to total female cells† . | Actual % male chimerism‡ . | Ratio of actual to expected engraftment‡ . |
|---|---|---|---|---|---|---|
| Short engraftment time | ||||||
| 20 minutes | 15.0 | 15 | 1.8 | 2.5 | 4.5 ± 0.5 | 6.3 ± 0.7 |
| 40 minutes | 17.8 | 17.8 | 1.8 | 2.5 | 10.8 ± 3.3 | 15.2 ± 4.7 |
| 1 hour | 19.5 | 19.5 | 1.8 | 2.4 | 18.5 ± 3.2 | 26.2 ± 4.5 |
| Long engraftment time | ||||||
| 20 minutes | 15.0 | 15 | 1.8 | 2.5 | 10.9 ± 3.3 | 15.2 ± 4.6 |
| 40 minutes | 17.8 | 17.8 | 1.8 | 2.5 | 17.2 ± 2.3 | 24.2 ± 3.2 |
| 1 hour | 12.3 | 17.3 | 1.7 | 6.0 | 63.7 ± 3.0 | 79.4 ± 6.9 |
| 6 hours | 6.7 | 16 | 1.7 | 8.6 | 51.7 ± 2.5 | 57.7 ± 2.7 |
| 24 hours | 4.6 | 15.9 | 1.7 | 8.8 | 59.5 ± 4.1 | 66.3 ± 4.6 |
Results of 4 experiments (3 to 5 recipients per group per experiment) are shown combined. Short engraftment is 2 weeks; long engraftment is 8 to 11 weeks.
Ratio of the number of adherent layer cells to fresh female cells injected (calculated at the time of transplantation).
Ratio of the number of input male equivalent cells to total female cells injected (calculated retrospectively by means of fluorescence in situ hybridization results of adherent layer female percentage).
Mean percentage ± SEM.
Short-term and long-term engraftment at different adhesion times.
Fresh male marrow suspensions were inoculated onto pre-established female stromal layers and allowed to adhere for 20 minutes (0.3 hours), 40 minutes (0.7 hours), 1 hour, 6 hours, or 24 hours before being harvested for transplantation. The 6-hour and 24-hour adhesion times were performed only for long-term engraftment. Recipient bone marrow was analyzed 2 weeks later for short-term engraftment and 8 to 11 weeks later for long-term engraftment. The ratios of actual to expected engraftment are shown. Results of 4 experiments are shown, with 3 to 5 recipients per group per experiment. ░, short-term engraftment; ▪, long-term engraftment. *For short-term engraftment, the result for 20-minute group is significantly lower than for the 1-hour (P = .014), but there is no significant difference between the 20-minute and 40-minute groups or between the 40-minute and 1-hour groups. For long-term engraftment, the 1-, 6-, and 24-hour groups are not significantly different from each other. The 20-minute and 40-minute groups are not significantly different from each other, but **both are significantly lower than for the 1-, 6-, and 24-hour groups (P < .0006).
Short-term and long-term engraftment at different adhesion times.
Fresh male marrow suspensions were inoculated onto pre-established female stromal layers and allowed to adhere for 20 minutes (0.3 hours), 40 minutes (0.7 hours), 1 hour, 6 hours, or 24 hours before being harvested for transplantation. The 6-hour and 24-hour adhesion times were performed only for long-term engraftment. Recipient bone marrow was analyzed 2 weeks later for short-term engraftment and 8 to 11 weeks later for long-term engraftment. The ratios of actual to expected engraftment are shown. Results of 4 experiments are shown, with 3 to 5 recipients per group per experiment. ░, short-term engraftment; ▪, long-term engraftment. *For short-term engraftment, the result for 20-minute group is significantly lower than for the 1-hour (P = .014), but there is no significant difference between the 20-minute and 40-minute groups or between the 40-minute and 1-hour groups. For long-term engraftment, the 1-, 6-, and 24-hour groups are not significantly different from each other. The 20-minute and 40-minute groups are not significantly different from each other, but **both are significantly lower than for the 1-, 6-, and 24-hour groups (P < .0006).
The long-term (8- to 11-week) engraftment capability of adherent male cells was determined after adhesion times of 20 minutes (0.3 hours), 40 minutes (0.7 hours), 1 hour, 6 hours, and 24 hours (Figure 4). The ratios of actual to expected engraftment for the 20-minute and 40-minute groups were not significantly different from each other, with values of 15.2% ± 4.6%, and 24.2% ± 3.2%, respectively. However both were significantly lower than for the 1-hour, 6-hour, and 24-hour groups (P < .0006). The 1-hour, 6-hour, and 24-hour groups were not significantly different from one another, with values of 79.4% ± 6.9%, 57.7% ± 2.7%, and 66.3% ± 4.6%, respectively. The average ratio of actual to expected engraftment for all long-term recipients of adherent cells harvested at adhesion times of 1 hour or greater was 70.7% ± 4.3%.
Short- and long-term engraftment differed significantly for cells harvested after 1 hour of adhesion time, with ratios of actual to expected engraftment of 26.2% ± 4.5% and 79.4% ± 6.9%, respectively (P = .0005). There was no difference between short- and long-term engraftment for recipients of cell layers harvested after 20 minutes or 40 minutes of adhesion.
Discussion
We studied the in vitro homing of HSCs in fresh whole bone marrow suspensions to pre-established Dexter-type murine LTBMC stromal layers. We first established the details and feasibility of the assay system, then performed experiments examining the numbers of whole bone marrow cells that did and did not home as well as the numbers of engraftable HSCs that homed and the timing of their homing. We have shown that the in vitro homing of HSCs occurs quickly and that stromal adhesion enriches HSCs from a whole bone marrow population.
Since the cells in suspension were expected to adhere to the stromal layer, we anticipated that the supernatant cell counts would drop, and they did so rapidly. Further, as expected, the percentage of male cells in the adherent layer increased from 20 minutes of adhesion to 6 hours. However, neither the total adherent layer cell counts nor the number of male cells in the adherent layer increased in proportion to the decrease in the supernatant. Further, the percentage of male cells in the adherent layer decreased between 6 and 24 hours. In addition, the absolute number of male cells in the adherent layer also decreased from 1 to 24 hours, when only 3% of the total test male cells were recovered in the adherent layer. However, 70% of test HSCs were present in the adherent layer at 24 hours; therefore, adhesion to stroma enriched HSCs by preserving them while other cells died off.
Because the proportion of male cells in the adherent layer varied among groups and experiments, we did not believe it was possible to directly compare chimerism results. Therefore, we developed the ratio of actual to expected engraftment (Table 1) to reflect the proportion of test male HSCs that adhered to the stromal layer in each experiment, thus standardizing the engraftment results to allow comparison among experiments. Table 2 shows how the calculations helped to correct for differences in the adherent layer in different experiments. Further, the ratio is of biological interest because it shows the proportion of HSCs in an in vitro bone marrow suspension that homed at various adhesion times. The calculation of the ratio of actual to expected engraftment involves 2 important assumptions. First, we assumed that female cells in the stromal layer would compete in a manner similar to an equivalent number of fresh cells. This was based on our results in other studies49 showing that although the total cell number in 4-week-old LTBMCs is less than input, the proportion of engrafting HSCs remains the same as in fresh marrow. We further assumed that the recipients' marrow would be entirely of graft origin (ie, no endogenous recovery). This is the theoretical ideal for the competitive repopulation assay in lethally irradiated recipients; however, in reality, in our experiments there does appear to be a small proportion (approximately 5%) of host recovery, so the expected engraftment calculated in this way may be slightly high, resulting in a slightly decreased ratio of actual to expected engraftment. However, the inaccuracy should not be more than a few percent in any case.
Long-term engraftment was significantly higher than short-term engraftment for 1 hour of adhesion time. One possible explanation is that a substantial portion of the competition was provided by female HSCs residing in the stromal layer, and in other experiments,49 we observed that 4-week-old LTBMCs have significantly better short-term than long-term engraftment capability. Thus, in these experiments, the competition may be more robust at short engraftment times. This issue could be avoided by using irradiated stromal layers; however, we specifically chose to use unirradiated stroma as it has been shown that irradiation changes the nature of established stromal layers37,39 and we wished our cultures to resemble in vivo marrow as closely as possible. Alternatively, the increased long-term engraftment could reflect differential in vitro homing of different progenitors and HSCs. Indeed, if homing is a characteristic activity of true HSCs, then long-term engrafting cells would be expected to adhere better in this assay. Finally, if a single population of stem cells is responsible for all engraftment, then HSCs in different functional phases of that single population may have adhered differently. Our group has previously shown that a reversible engraftment defect occurs in certain phases of the cell cycle, indicating plasticity of the engraftment phenotype.50Further, the adhesion receptor profile of murine hematopoietic progenitors and cell lines changes with cytokine incubation and cell cycle transit, resulting in changes in fibronectin adhesion and possibly other changes that may affect homing and engraftment.51 Thus, differential adhesion in this homing assay may very well reflect such plasticity related to cell cycle phase or perhaps to circadian rhythm or other, as yet undefined, changes in HSC phenotype.
The adhesion of both short-term and long-term engrafting HSCs was already substantial at 20 minutes and increased up to 1 hour. Adhesion of long-term engrafting HSC plateaued after 1 hour. This indicates that adhesion of engraftable HSCs increases during the first hour of contact with stroma; that maximum adhesion takes place within 1 hour of contact; and that the HSCs are maintained as viable, engraftable cells for at least 24 hours. After 1 hour of adhesion, the ratio of actual to expected engraftment for all long-term recipients indicated that approximately 2 out of 3 test HSCs had homed in vitro. The finding of rapid in vitro homing is concordant with the results of other in vitro adhesion studies27 as well as with our own finding that 82% ± 7% of lineage-negative, rhodamine-dull, Hoechst-dull (lin−rholowHolow) cells adhered to individual stromal cells within 1 hour of contact.52Further, other studies have shown that in vivo homing plateaus within the first few hours after transplantation16 19 also agreeing with the results we present here.
These data demonstrate the usefulness of this model system for the study of stem-stromal adhesion interactions, allowing further dissection of the mechanism of HSC homing and exploration of possible manipulations of the process. The assay system can be used to study stromal adhesion of various populations of bone marrow cells under various conditions of engraftment. Different stromal systems could be used, including irradiated LTBMC stroma, irradiated or nonirradiated cell lines, and explant stromal cultures from other organs. Likewise, other HSC populations could be used, including post-5FU marrow, cytokine-stimulated marrow, various purified populations (eg, lin−rholowHolow, lin−stem cell antigen+), and cells from other organs or tumors. Various manipulations of the adhesion step, such as addition of adhesion-receptor blocking antibodies, can be easily done. This is the first description of a functional assay for in vitro homing of engraftable stem cells to complex stroma.
Todd Bonnici, Jane Carlson, Mark Dooner, Caron Engstrom, Anna Fraioli, Bernice Fraioli, Houri Habibian, Marguerite Joly, Lizhen Pang, Judy Reilly, and Kimberly Werme for technical assistance, and Joanne Wuu for assistance with statistical evaluation of the data.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Angela E. Frimberger, Department of Research, Roger Williams Medical Center, 825 Chalkstone Ave, Providence, RI 02908; e-mail: afrimberger@hotmail.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal