In an attempt to improve induction chemotherapy for older patients with acute myeloid leukemia (AML),1314 patients were randomized to 1 of 3 induction treatments for 2 courses of DAT (daunorubicin, cytarabine, and thioguanine) 3 + 10, ADE (daunorubicin, cytarabine, and etoposide) 10 + 3 + 5, or MAC (mitoxantrone-cytarabine). The remission rate in the DAT arm was significantly better than ADE (62% vs 50%; P = .002) or MAC (62% vs 55%;P = .04). This benefit was seen in patients younger and older than 70 years. There were no differences between the induction schedules with respect to overall survival at 5 years (12% vs 8% vs 10%). A total of 226 patients were randomized to receive granulocyte colony-stimulating factor (G-CSF) or placebo as supportive care from day 8 after the end of treatment course 1. The remission rate or survival were not improved by G-CSF, although the median number of days to recover neutrophils to 1.0 × 109/L was reduced by 5 days. Patients who entered remission (n = 371) were randomized to stop after a third course (DAT 2 + 7) or after 6 courses, ie, a subsequent COAP (cyclophosphamide, vincristine, cytarabine, and prednisolone), DAT 2 + 5, and COAP. The relapse risk (81% vs 73%), disease-free survival (16% vs 23%), and overall survival at 5 years (23% vs 22%) did not differ between the 3-course or 6-course arms. In addition to a treatment duration randomization, 362 patients were randomized to receive 12-month maintenance treatment with low-dose interferon, but no benefit was seen with respect to relapse risk, disease-free survival, or overall survival.
Introduction
The treatment of acute myeloid leukemia (AML) in older patients has not improved significantly in recent years compared with the considerable progress made in younger patients.1-7 This difference is probably multifactorial and relates to differences in the biology of the disease in older patients as exemplified by a higher proportion of patients with an adverse karyotype, a more frequent expression of a chemoresistant phenotype, and an increased frequency of disease evolution from a pre-existing and perhaps unrecognized myelodysplasia. The presence of comorbidity means that older patients, ie, patients older than 60 years, are less able to withstand intensive chemotherapy, with the consequence that the patients recruited to trials of intensive therapy are a selected minority of patients with the disease in this age group. In the United Kingdom Medical Research Council (MRC) AML trials database, in patients entered into trials between 1970 and 1990 there was a modest improvement in remission rate, probably attributable to improved supportive care, but the long-term survival improvement has been modest and much less than in younger patients and remains poor. Several aspects of treatment require improvement. Many studies in older patients have demonstrated that initial complete remission rates are around 45% to 55%, and the relapse risk of remitters is around 80% to 85%.1-6 We have previously demonstrated in older patients considered fit for chemotherapy that more intensive treatment in remission induction with a 3 + 10 schedule of daunorubicin, cytarabine, and thioguanine (DAT), while not improving the proportion of patients who enter remission, improves long-term survival with, in total, a reduced requirement in the amount of supportive care as measured by hospital days, red cell and platelet support, and days on antibiotics when compared with a more gentle DAT 1 + 5 schedule.8 Similar observations have been confirmed in other studies.4 Arguably, more effective induction therapy should not only reduce the proportion of patients with resistant disease—about 25% of older patients in our experience—but improve remission duration. In younger patients who receive aggressive chemotherapy, the induction deaths consequent upon cytopenia have been reduced to less than 10%, while 25% of older patients fail induction due to failure of supportive care.7,8 There has been muchoptimism that hemopoietic growth factors could reduce the period of neutropenic risk and thereby lead to an improved remission rate and survival. To date several trials have failed to deliver this aspiration despite curtailing the duration of neutropenia.9-15
In this trial we report a comparison between 3 induction schedules aimed at improving initial response: our traditional DAT 3 + 10 schedule versus the same schedule but with etoposide substituted for thioguanine (ADE), versus a mitoxantrone-cytarabine combination (MAC). During this trial we conducted a placebo-controlled trial of granulocyte colony-stimulating factor (G-CSF), used as supportive care after the first course of induction treatment, in an effort to reduce treatment-related mortality and thereby increase the rate of remission.
Postinduction treatment in younger patients has become intensive, and it appears that a total of 4 or 5 courses may be optimum. However, consolidation is less well tolerated in older patients, so the appropriate treatment has yet to be developed. In our previous MRC AML8 trial, which recruited patients from 1978 to 1983, 6 versus 2 consolidation courses of DAT were compared and failed to show a survival difference.1 In that trial, patients who remained in remission for 12 months were randomized to receive a further 3 months of monthly maintenance or 4 courses of late intensification with COAP (cyclophosphamide, vincristine, cytarabine, and prednisolone). The COAP schedule was well tolerated and reduced the number of deaths and, for this reason, was compared with the more intensive MAZE (M-amsacarine, 5-azacytidine, and etoposide) as consolidation in the MRC AML9 trial, which recruited patients between 1984 and 1990.16 Although MAZE reduced the relapse risk overall, it was associated with an excess of deaths in remission and was poorly tolerated in patients older than 55 years. The addition of maintenance in that trial provided no benefit.
Here we attempt to define the number of consolidation courses that are necessary in the older patient by comparing a total of 3 courses with a total of 6 courses incorporating 2 courses of COAP and 1 of DAT.
At the initiation of this study, interferon-α (IFN-α) was becoming widely established as an effective agent in chronic myeloid leukemia which, at low dose, was well tolerated in older patients. To assess the value of IFN-α as maintenance, patients were randomized to receive or not receive IFN-α for one year after completion of allocated chemotherapy, which represents the first major randomized trial of IFN-α in AML.
Given the lack of progress in treatment of the older patient, there is an issue of whether there are subgroups of patients who benefit more than others and who should continue to be offered intensive chemotherapy and, by implication, there are groups of patients in whom the current approach is more likely to shorten life and who should be offered alternative treatment approaches. We have achieved this in younger patients based on a limited number of prognostic factors, including cytogenetics, which have been prospectively validated and are now used to make treatment decisions.17 18 In addition to patients considered fit for chemotherapy and who therefore may enter trials, estimated to be around 10% of older patients in our experience, substantial numbers of patients are not offered an intensive approach. Little is known about the outcome for such patients or whether their quality of life can be improved by developing improved nonintensive treatments.
In summary, the aims of this study were first to improve remission induction by comparing 3 induction regimens and to evaluate the benefit of using G-CSF in supportive care. Secondly, we aimed to define the required number of total treatment courses by comparing a short (3 courses) versus long (6 courses) approach and sought to investigate the role of a 12-month maintenance schedule of low-dose IFN-α. Finally, we used this large data set to preliminarily define subgroups of patients who may benefit from future attempts to improve on the existing intensive approach or who should be considered for palliative treatment or novel nonintensive approaches.
Patients, materials, and methods
Patients
Between November 1990 and June 1998, 1314 patients were entered into the MRC AML11 trial by 258 clinicians from 138 centers, mainly in the United Kingdom but with 2 centers in the Republic of Ireland (14 patients) and 1 in New Zealand (12 patients) also collaborating.
The trial was initially designed for patients aged 56 years and older with the then concurrent MRC AML10 trial recruiting patients up to 55 years. At the end of 1994, the AML10 trial was succeeded by MRC AML12 and the age threshold for AML11 was raised to 60 years and older. Patients younger than 60 years were, however, permitted to enter this study if they were not considered suitable for the more intensive therapy employed in either the AML10 or AML12 trials.
Patients with any form of de novo or secondary AML were eligible. Secondary AML was defined as AML either following prior cytotoxic chemotherapy or radiotherapy for other cancers or subsequent to a preceding hematologic disorder. Patients with blast crisis of previously documented Philadelphia chromosome–positive chronic myeloid leukemia were not eligible. The trial required approval of each institutional ethics review committee and required patients to give informed consent.
Treatment
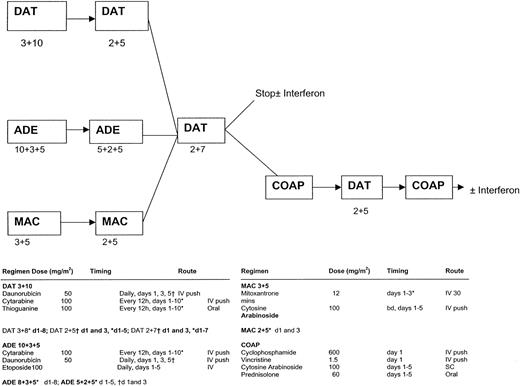
There were 3 randomized comparisons within the trial (Figure1; the figure also gives details of all the chemotherapy schedules used in AML11). The first was between DAT, ADE, MAC as induction therapy. Treatment was allocated in a 1:1:2 ratio (DAT:ADE:MAC). All but 3 of the patients in AML11 were entered into this comparison. Patients were scheduled to receive 2 courses of their allocated regimen, with the second course being a truncated version of the first. The second course was scheduled to commence after neutrophils and platelets had recovered to 1.0 × 109/L and 100 × 109/L, respectively. Patients who were in remission after 2 courses of induction treatment received a course of consolidation therapy with DAT 2 + 7. The second randomization was between short consolidation (DAT 2 + 7 only) versus long consolidation where, in addition to DAT 2 + 7, the 3 further courses (COAP, DAT, and COAP) were given, ie, 3 versus 6 courses of therapy in total. The third randomization, which was performed at the same time as the second, was between IFN-α maintenance for one year versus no maintenance. Patients allocated to IFN-α were scheduled to receive recombinant IFN-alfa 2a (Roferon-A) at a dose of 3 × 106units 3 times per week, with dose reductions permitted if necessary and with IFN-α being stopped temporarily if the neutrophil count fell below 1.0 × 109/L or the platelet count fell below 50 × 109/L. From January 1993, those with a morphologic diagnosis of acute promyelocytic leukemia were eligible for the MRC ATRA trial in which 40 older patients were randomized to receive either a short (5-day) course of all-trans-retinoic acid (ATRA) prior to initiating chemotherapy versus the addition of ATRA to induction chemotherapy until the achievement of complete remission (CR). ATRA was given in a daily oral dose of 45 mg/m.2This study has been fully reported previously.19
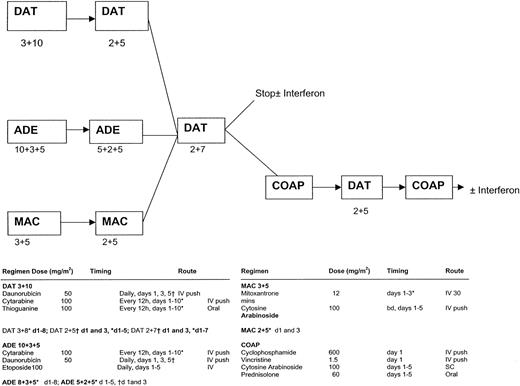
MRC-AML11 protocol flow chart.
DAT 3 + 10: daunorubicin 50 mg/m2 slow intravenous (IV) push on days 1, 3, and 5; cytarabine 100 mg/m2 12-hourly IV push on days 1 to 10; thioguanine 100 mg/m2 12-hourly orally on days 1 to 10. ADE 10 + 3 + 5: daunorubicin 50 mg/m2 slow IV push on days 1, 3, and 5; cytarabine 100 mg/m2 12-hourly IV push on days 1 to 10; etoposide (VP-16) 100 mg/m2 IV (1-hour infusion) on days 1 to 5. MAC: mitozantrone 12 mg/m2 IV (30-minute infusion) on days 1 to 3; cytarabine 100 mg/m2 12-hourly IV push on days 1 to 5. DAT 2 + 5: as DAT 3 + 10 but daunorubicin on days 1 and 3 only and cytarabine and thioguanine on days 1 to 5 only. ADE 5 + 2 + 5: as ADE 10 + 3 + 5 but daunorubicin on days 1 and 3 only and cytarabine on days 1 to 5 only. MAC 2 + 5: as MAC 3 + 5 but mitozantrone on days 1 and 3 only. DAT 2 + 7: as DAT 3 + 10 but daunorubicin on days 1 and 3 only and cytarabine and thioguanine on days 1 to 7 only. COAP: cyclophosphamide 600 mg/m2 IV on day 1; vincristine 1.5 mg/m2 (maximum 2 mg/m2) on day 1; cytarabine 100 mg/m2 subcutaneous injection on days 1 to 5; prednisolone 60 mg/m2 orally on days 1 to 5.
MRC-AML11 protocol flow chart.
DAT 3 + 10: daunorubicin 50 mg/m2 slow intravenous (IV) push on days 1, 3, and 5; cytarabine 100 mg/m2 12-hourly IV push on days 1 to 10; thioguanine 100 mg/m2 12-hourly orally on days 1 to 10. ADE 10 + 3 + 5: daunorubicin 50 mg/m2 slow IV push on days 1, 3, and 5; cytarabine 100 mg/m2 12-hourly IV push on days 1 to 10; etoposide (VP-16) 100 mg/m2 IV (1-hour infusion) on days 1 to 5. MAC: mitozantrone 12 mg/m2 IV (30-minute infusion) on days 1 to 3; cytarabine 100 mg/m2 12-hourly IV push on days 1 to 5. DAT 2 + 5: as DAT 3 + 10 but daunorubicin on days 1 and 3 only and cytarabine and thioguanine on days 1 to 5 only. ADE 5 + 2 + 5: as ADE 10 + 3 + 5 but daunorubicin on days 1 and 3 only and cytarabine on days 1 to 5 only. MAC 2 + 5: as MAC 3 + 5 but mitozantrone on days 1 and 3 only. DAT 2 + 7: as DAT 3 + 10 but daunorubicin on days 1 and 3 only and cytarabine and thioguanine on days 1 to 7 only. COAP: cyclophosphamide 600 mg/m2 IV on day 1; vincristine 1.5 mg/m2 (maximum 2 mg/m2) on day 1; cytarabine 100 mg/m2 subcutaneous injection on days 1 to 5; prednisolone 60 mg/m2 orally on days 1 to 5.
Between November 1994 and January 1997, 226 patients participated in a randomized placebo-controlled trial of G-CSF (Lenograstim) given at a daily dose of 293 μg by subcutaneous injection starting on day +8 after the end of course 1 chemotherapy and continuing until neutrophil recovery to 0.5 × 109/L or for a maximum of 10 days if the neutrophil count had not recovered by this time. There was an equal distribution of entrants between the induction arms.
Definitions of end points
A normocellular bone marrow aspirate containing less than 5% leukemic blast cells and showing evidence of normal maturation of other marrow elements was the criterion for the achievement of CR. The persistence of myelodysplastic features did not exclude the diagnosis of CR. Remission failures were classified by the investigating clinician as due either to induction death (ID), ie, related to treatment and/or hypoplasia, or as resistant disease (RD), ie, related to the failure of therapy to eliminate the disease (including partial remissions with 5%-15% blasts). Where the clinician's evaluation was not available, deaths within 30 days of entry were classified as ID and deaths at more than 30 days as RD.
The following definitions are also used: Overall survival (OS) is the time from entry to death; for remitters, disease-free survival (DFS) is the time from CR to first event (either relapse or death in CR); for remitters, the relapse risk (RR) is the cumulative probability of relapse ignoring (ie, censoring at) death in first CR; and death in first CR is the cumulative probability of dying in first CR ignoring relapse.
Statistical methods
Randomizations were balanced by minimization. The protocol specified that the primary comparison for the induction randomization would be of the mitozantrone-containing regimen (MAC) versus the daunorubicin-containing regimens (DAT and ADE), with a subsidiary comparison of DAT versus ADE. However, because there is evidence (see “Results”) that the outcomes with DAT and ADE are different, it would be inappropriate to combine them for comparison with MAC; therefore, 3 primary comparisons are presented: DAT versus ADE, DAT versus MAC, and ADE versus MAC. Remission rates and reasons for failure were compared using standard χ2 tests. Kaplan-Meier life tables were constructed for survival data and were compared by means of the log-rank test, with surviving patients being censored at June 1, 2000, when follow-up was complete for all but 15 patients (1%) (the small number of patients lost to follow-up are censored at the date they were last known to be alive). All percentage values quoted in the text for survival, DFS, and RR are at 5 years. Hematologic recovery and hospital stay were compared by means of the log-rank test. Toxicity and supportive care requirements were compared by means of the Wilcoxon test. All P values are 2-tailed. All analyses are “intention-to-treat,” ie, all randomized patients were included irrespective of protocol compliance.
Results
Induction randomization
Patient characteristics.
The presenting features of the patient population are shown in Table 1. Both the mean and median ages were 66 years. Of the 20 patients younger than age 56 years, 12 were aged 55 years, 7 were aged 52 to 54 years, and 1 was aged 44 years. Of the 7 patients aged 80 years or older, 4 were aged 80 years, with one each aged 82, 85, and 91 years. Central nervous system involvement was only reported in 5 patients. Performance status was defined by the World Health Organization scale. Secondary leukemia was defined on the basis of a history of previous chemotherapy or radiotherapy (n = 44) or of a previously documented antecedent hematologic diagnosis (myelodysplasia, n = 181; myeloproliferative condition, n = 21; other disorders, n = 31; or unspecified, n = 22). Cytogenetic information was available in 1065 (79%) patients and is reported in detail elsewhere (see accompanying article by Grimwade et al,20 page 1312). Favorable karyotype was defined as t(8;21), t(15;17), or inv(16) irrespective of the presence of additional changes. Patients with complex changes (at least 5 unrelated abnormalities) were defined as adverse risk, while the remainder, including patients with normal karyotype, were regarded as intermediate risk.
Compliance with treatment allocation.
Information on compliance with allocated induction therapy is available for 95% of patients (94% DAT, 95% ADE, 95% MAC). Compliance was excellent for course 1, with 96% of patients (96% DAT, 95% ADE, 97% MAC) starting their allocated treatment. Twenty-six patients (6 DAT, 9 ADE, 11 MAC) did not commence chemotherapy, while 22 patients (7 DAT, 7 ADE, 8 MAC) received other therapy. Noncompliant patients are included in the analysis.
Remission rate.
The overall CR rate was 55%, with failure rates of 19% due to ID and 26% due to RD. The CR rate of patients allocated to DAT (62%) was significantly better than that of patients allocated to ADE (50%,P = .002) or MAC (55%, P = .04) (Table2). As in our other studies, the protocol did not specify peripheral blood recovery to 1.5 × 109/L of neutrophils or 100 × 109/L of platelets as in the National Cancer Institute critera.21 However, at least 95% and 92% of patients who met the protocol definition of CR also met these criteria at some point during therapy, with 87% and 85% meeting them after course 1. The reasons for the inferior CR rates with ADE and MAC were different (Table 2): With ADE there was an excess of IDs, with MAC there was more RD. No interactions of treatment effect with age were observed (Table 2).
Toxicity and supportive care.
Courses 1 and 2 were evaluated for toxicity supportive care and hemopoietic recovery. There were no important differences in nonhematologic toxicity or for the number of days taken to recover neutrophil and platelet counts between the treatments after course 1 or 2, although neutrophil recovery was slower in the MAC arm. The supportive care requirements are detailed in Table3.
The impact of G-CSF on the 226 patients randomized from this study will be reported in full elsewhere. Despite an average reduction of neutropenic days (< 1.0 × 109/L), by 5 days there was no significant difference in remission rate between G-CSF or placebo overall (58% vs 51%; P = 0.4) or within the DAT, ADE, or MAC induction arms. G-CSF did not improve OS compared with placebo (15% vs 18% at 3 years, P = 1.0).
Outcome after complete remission.
For all patients who entered CR, the DFS was 15%, the RR was 82%, and the actuarial risk of death in remission was 15%. Of the 57 patients who died in first CR, 35 died within 200 days of remission, usually from treatment-related causes (mainly infection). The 22 deaths beyond that point were due to various causes: infection (3), hemorrhage (3), cardiac failure (6), other cancers (3), other causes (4), and unknown causes (3). There were no significant differences between DAT, ADE, or MAC with respect to deaths in first remission, RR, or DFS (Table 4).
Overall survival.
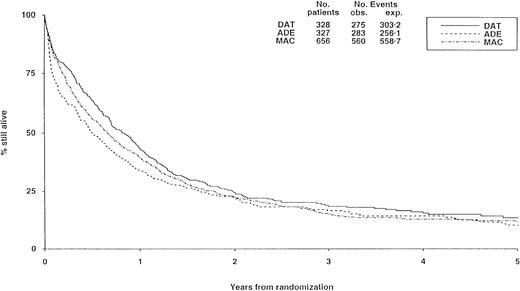
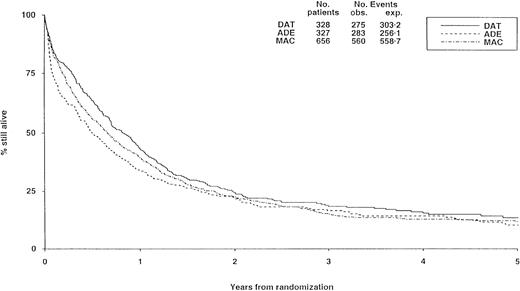
There were no substantial differences in long-term survival between the 3 induction arms (Figure 2), although survival was significantly worse with ADE than with DAT (P = .02), but differences between DAT and MAC (P = .1) and between ADE and MAC (P = .2) were not significant.
Survival from randomization by induction treatment.
Survival at 5 years is 12% for DAT, 8% for ADE, and 10% for MAC. Under number of events, obs. indicates the number observed in each arm; exp., the number expected (from log-rank analysis).
Survival from randomization by induction treatment.
Survival at 5 years is 12% for DAT, 8% for ADE, and 10% for MAC. Under number of events, obs. indicates the number observed in each arm; exp., the number expected (from log-rank analysis).
Consolidation and maintenance randomizations
Patient characteristics.
The features at diagnosis of the patients who were randomized between short versus long consolidation and between IFN-α maintenance versus no maintenance are shown in Table 5.
Compliance with treatment allocation.
Of the 186 patients randomized to long consolidation, this was started in 156 patients. Of these, 114 received all 3 courses, 22 received 2 courses, and 20 received 1 course. Seventeen patients did not start consolidation, while information on consolidation received is not available for the remaining 13 patients allocated to long consolidation. Among patients allocated to short consolidation, 4 received further unscheduled chemotherapy in first CR.
Of the 182 patients allocated to IFN-α, 41 are known not to have started the treatment. This was largely seen in patients allocated to long chemotherapy, where 55% started IFN-α compared with 94% in the short chemotherapy arm. Of the 131 patients starting IFN-α, 42 completed the designated 12 months, 33 completed no more than 2 months, 26 completed 3 to 5 months, 18 completed 6 to 8 months, and 7 completed 9 to 11 months (5 not known). No patients on the control arm are known to have received IFN-α.
Outcome.
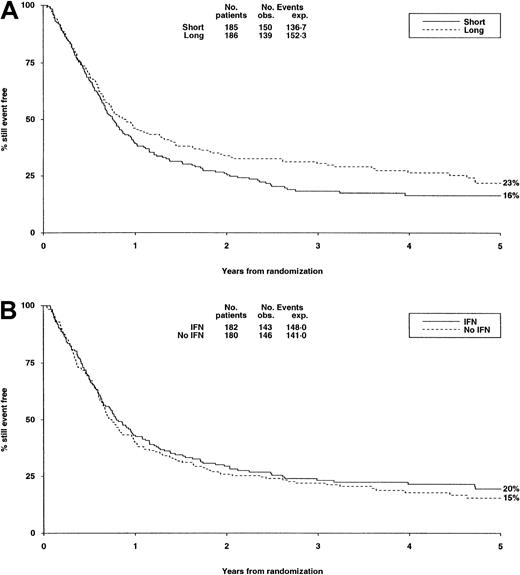
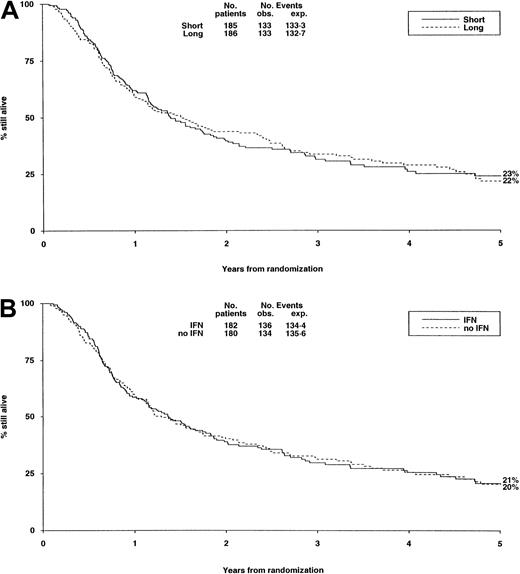
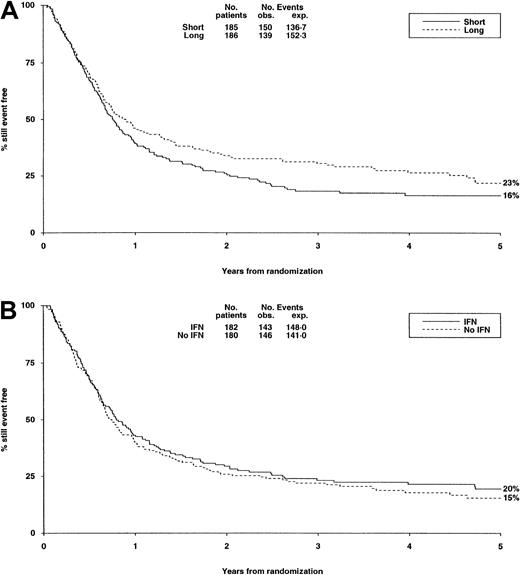
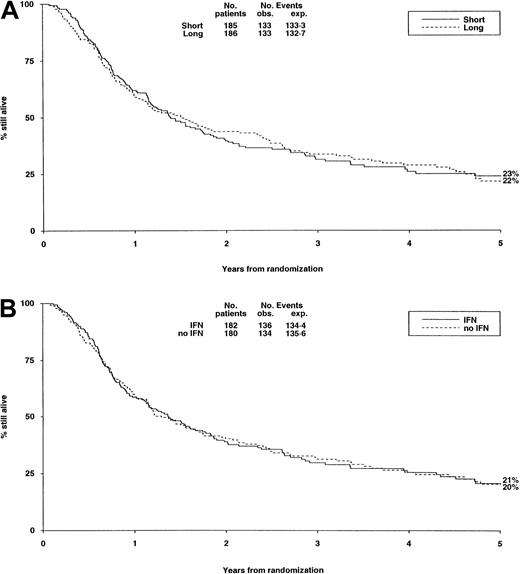
There were no significant differences in either randomization with respect to deaths in first CR, RR, DFS (Table6, Figure3A,B), or OS (Figure4).
DFS from randomization by consolidation treatment.
DFS at 5 years is 16% for short and 23% for long consolidation (A) and 20% for IFN and 15% for no IFN (B). Under number of events, Obs. indicates the number observed in each arm; Exp., the number expected (from log-rank analysis).
DFS from randomization by consolidation treatment.
DFS at 5 years is 16% for short and 23% for long consolidation (A) and 20% for IFN and 15% for no IFN (B). Under number of events, Obs. indicates the number observed in each arm; Exp., the number expected (from log-rank analysis).
Survival from randomization by consolidation treatment.
Survival at 5 years is 23% for short and 22% for long consolidation (A) and 21% for IFN and 20% for no IFN (B).
Survival from randomization by consolidation treatment.
Survival at 5 years is 23% for short and 22% for long consolidation (A) and 21% for IFN and 20% for no IFN (B).
Factors that predict outcome
Parameters that were found to be highly significantly associated with the achievement of remission in multivariate analysis were cytogenetic group, presenting white blood count (WBC), age, secondary leukemia, performance status, and French-American-British (FAB) type M3 (Table 7). OS was influenced by cytogenetics, presenting WBC, age, secondary leukemia, and performance status. Details of the cytogenetics of this trial are reported elsewhere.20 Only 6% of cases were in the favorable cytogenetic group, but the OS was 34% whereas the 11% known to have adverse cytogenetics had a survival of 2%. WBC became influential at 100 × 109/L, below which survival was 15% and above which it was 7%. Patients younger than 70 years had a 16% OS compared with 11% for patients aged at least 70 years. Secondary leukemia had a similar remission rate to de novo disease (53% vs 57%) but was significantly worse if it developed from preceding myelodysplasia (42%). Patient sex or disease FAB group, apart from FAB M3, were not influential on outcome.
Discussion
A total of 70% of patients with AML are older than 60 years, and the 1- to 2-year survival reported in various clinical trials ranged between 10% and 15%. There is little evidence that treatment has improved survival rates in the last 25 years although remission rates have gradually increased, most probably reflecting improved supportive care. This contrasts sharply with the situation in younger patients, in whom both remission rates and survival has improved substantially as a consequence of developing more intensive schedules.7 22-24The overall results reported in this trial are unexceptional and do not provide any evidence to suggest that treatment can be improved overall by the strategies tested. In older patients it is difficult to compare one trial with another or, indeed, to extrapolate the results of a trial to an individual patient in the clinic because of the selected nature of patients who enter trials. Most trial protocols offer an intensive approach to treatment for which patients may not be considered medically fit or into which patients are willing to be recruited. Most (71%) entrants into this trial were younger than 70 years and only 7% older than 75 years, which is clearly unrepresentative of the AML population as a whole. Age 60 years is often, although arbitrarily, used as a cutoff to define “older” patients with AML. Because chronological age is not necessarily a good indicator of biological age and fitness for therapy, AML11 did not specify fixed age limits. If analysis is restricted to patients aged 60 years or older, the results and their interpretation do not alter in any important fashion. Similarly, because patients with acute promyelocytic leukemia still need chemotherapy in addition to ATRA, it is appropriate to include them in studies comparing different regimens even though they are usually considered separately from other forms of AML. Their exclusion from AML11 would not alter the results.
There are therefore several issues to be addressed. First, chemotherapy needs to be improved both in induction and postinduction phases. Second, we need to establish in which subsets of patients this is likely to be achievable. Third, we need to determine in which patients an intensive approach is unsuitable and in whom treatment is shortening life. In this respect, end points other than those used to measure disease response should be given equal importance. Fourth, even if progress is made in these areas, there will still be a substantial group of older patients in whom improved nonintensive approaches need to be developed.
In this trial we attempted to improve outcome for patients considered fit for intensive treatment by testing newer induction schedules (ADE and MAC) against our traditional DAT 3 + 10 protocol, which had been established in our previous AML9 trial to be superior to a more gentle DAT 1 + 5 schedule in older patients. It was hoped that the replacement of thioguanine with etoposide would be more effective, but this proved not to be the case. Similarly randomized comparisons have previously suggested that mitoxantrone was more efficacious than daunorubicin with the possible additional advantage of being less cardiotoxic, which could be a useful feature in patients in this age group.25 In this trial the DAT schedule was significantly better overall and in all patient age subgroups. This does not necessarily mean that the third drug is of value—only that there is no important difference between thioguanine and etoposide, which confirms our extensive experience in younger patients.7 The DAT protocol was also superior to the MAC schedule, but the protocol design did not permit a direct comparison of daunorubicin and mitoxantrone because of the confounding effect of an extra 5 days treatment with cytosine and the presence of thioguanine in the DAT schedule. Other studies have directly addressed this issue, with some evidence to suggest a benefit for mitoxantrone when compared with daunorubicin, but in one major recent study the daunorubicin dose was only 30 mg/m5.
One difference between the initial response in older and younger patients is the higher ID rate in older patients. A contributing factor may be poorer tolerance to neutropenia as opposed to the duration of neutropenia—which is not different. As part of a major placebo-controlled trial in more than 800 patients of G-CSF in AML given as supportive treatment, we were unable to demonstrate in patients older than 60 years any impact on remission rate or survival despite observing the expected reduction in the duration of neutropenia and number of days on antibiotics or in hospital. These latter features may be important in terms of overall quality of life, which was not assessed in this study.
Despite a superiority in remission rate with DAT, we found no subsequent difference in RR, DFS, or OS between the 3 induction schedules. In the previous AML8 and AML9 trials, the COAP combination had proved partially effective and tolerable as consolidation compared with a more intensive MAZE combination which, although able to reduce RR, was poorly tolerated in older patients. For this reason, COAP was taken forward as the standard approach to postinduction therapy and we were able to demonstrate that a total of 3 courses was as effective as 6. Because DAT 2 + 5 and COAP as used here are not intensive treatments by current standards, it is conceivable that there are subgroups of patients who were undertreated. For example, patients with a favorable karyotype when treated with the same induction schedule as younger patients of the same karyotype had a remission rate of 70%, which was not significantly inferior to that of younger patients (90%)—an observation noted in studies of the German Collaborative Group.3 However, the RR was considerably greater (58%) when compared with younger patients given a more intensive consolidation treatment (35%).20 26 This might infer that the relative weakness of postinduction treatments accounted for this difference.
In this trial design we chose not to pursue the evaluation of maintenance chemotherapy because our previous trials had shown no benefit.1,8 Given the preliminary results available at the time this trial was planned of IFN-α in chronic myeloid leukemia, we chose to conduct the first major assessment of IFN-α as maintenance treatment in AML. Disappointingly, we have not been able to show any reduction in RR or improvement in survival. It is possible that the chosen dose of 3 × 106 units 3 times per week was inadequate but, because IFN-α is less well tolerated in older patients, it may not be practical to study a higher dose. Perhaps IFN-α was not given a fair chance because patients were randomized after course 3, and half of those randomized had to receive 3 further courses of chemotherapy before starting the IFN-α allocation. Overall, about a third of patients allocated to IFN-α did not receive it, but this was largely found in patients allocated to 6 courses of chemotherapy who had 55% compliance, compared with those allocated to 3 courses where compliance was 94%. Even in the latter group there was no evidence of benefit (DFS was 21% vs 11%, P = .15; OS was 26% vs 20%, P = 0.4). Although the planned dose was low, some patients found it unacceptable and stopped treatment early. Maintenance treatment has been shown by others to be of benefit in 3 collaborative groups.2,5,27 Because the recent European Organization for Research and Treatment of Cancer–Haemato-Oncology Collaborative Group for Adults-Netherlands (EORTC-HOVON) AML9 trial was able to show a small but significant survival advantage using low-dose Ara-C maintenance,5 further development of a maintenance schedule for particular patients may be fruitful.
It is clear that, just as is apparent in younger patients, AML is heterogeneous disease in the older patient. Prognostic factors, particularly cytogenetics, have become of central importance in treatment decisions in younger patients.26,28,29 However, these factors only became evident as treatment improved. Treatment options are more limited because of comorbidity in older patients. Young patients seldom elect a palliative approach but some older patients, even though they might be fit for intensive treatment, opt for a palliative approach. Many older patients do not enter current clinical trials, because the trials tend to offer only an intensive approach to treatment.30 Even in patients considered fit for treatment, the lack of therapeutic progress over the last 20 years raises the important issue of determining who benefits from current treatment approaches and who are the patients whose life may be shortened by an intensive treatment. In the former group, improved treatment with curative intent might be possible, whereas in the latter group and for most older patients more useful palliative approaches are needed until novel treatments emerge.
In an attempt to define patients who may benefit from intensive chemotherapy, we identified a number of parameters that influenced treatment outcome. Cytogenetic information was obtained in 1065 (79%) patients in this study and are reported in detail elsewhere. It has long been known that one of the explanations of the inferior response in older patients was the different proportions of the favorable and unfavorable cytogenetic groups. Older patients with a favorable karyotype have a similar CR rate but higher RR, possibly because of inferior postinduction treatment in addition to the features of age. Patients with adverse cytogenetics composed 11% in this study and had a low remission rate (26%) and extremely poor survival (2%). There is certainly doubt about the justification of offering such patients currently available intensive treatment approaches. Even if remission is achieved, it is only temporary. This would sustain an argument to identify these patients at diagnosis and avoid the toxicity and indignity of intensive treatment. Many centers cannot obtain cytogenetic data promptly, but there may be a case for the use of rapid assessment techniques to identify these patients. Only one study involving 60 patients prospectively evaluated a strategy of immediate conventional chemotherapy versus a wait-and-see approach using mild therapy with hydroxyurea or cytarabine.4 The survival in the conventionally treated cases was twice as long as with the palliative approach. This has been taken to mean that conventional chemotherapy should be offered to older patients. That trial posed an extremely important question that remains relevant nearly 20 years later, where such a study is still justified in patients known to have a particularly adverse prognostic profile.
Based on a prognostic factor analysis of patients in this trial, the features available at diagnosis that predict a lower chance of achieving a CR are WBC more than 100 × 109/L, age over 70 years, secondary leukemia, and poor performance score. Patients with these features will not benefit from the induction approach used in this study. Cytogenetic definition of adverse prognosis will not usually be available to assist in this decision but became very useful in predicting response to postinduction treatment if the patient achieved CR. Such risk profiling could be useful to target subgroups of patients, but no patients in this age category can be considered to have a satisfactory prognosis.
The options to improve conventional chemotherapy are limited. Substitution of daunorubicin with a newer drug such as idarubicin has not been shown to be beneficial in older patients.31 The frequency of P glycoprotein expression in older patients and its correlation to treatment response offer a potential target to improve the effectiveness of daunorubicin.32 Several drugs are capable of doing this. The proof of principle is provided in Southwest Oncology Group study in relapsed disease where the addition of cyclosporin A significantly improved DFS and OS.33The cyclosporine analog PSC-833 is less nephrotoxic and myelosuppressive and is active in vitro at concentrations achievable in vivo. Initial studies in older patients have been associated with increased toxicity, but further studies are needed to evaluate this agent.34,35 There may be benefit in evaluating cytarabine or anthracycline dose levels in the older patients considered suitable for the intensive approach. Older patients will not tolerate high-dose cytarabine, but the dose response seen in younger patients may be available at intermediate doses. This question is now being investigated in our current AML14 trial together with PSC-833 modulation of daunorubicin. Few studies have formally attempted to evaluate a low-dose approach to treatment. In one study of an oral schedule versus a conventional approach, survival was superior with the oral schedule but, as is often the case, this could partly be explained by a poorer than expected result in the conventional arm.36 A novel approach is possible by targeting treatment using immunoconjugates, which have been shown to be effective as single agents in relapsed disease, with a relatively favorable toxicity profile,37 but require prospective evaluation as firstline treatment.
We thank the clinicians who entered their patients into MRC AML11 for their support; Rachel Clack, Jill Crowther, Sarah Cullip, Cathy Hope, Sue Knight, and Angela Radley for data management; and Siân Edwards for preparing the manuscript.
The following institutions and collaborators participated in AML11 (*members of the MRC Adult Leukaemia Working Party).
Aberdeen Royal Infirmary (D. J. Culligan,* A. A. Dawson, D. J. King, J. Tighe, H. G. Watson); Addenbrooke's Hospital (A. R. Green,* R. Marcus, J. K. H. Rees*); Alexandra Hospital (D. Obeid); Ards Hospital (A. Kyle); Arrowe Park Hospital (T. J. Deeble, D. W. Galvani); Ashford Hospital (A. S. Laurie); Auckland Hospital (P. J. Browett,* R. Varcoe*); Barnsley District General Hospital (J. P. Ng); Bassetlaw Hospital (B. Paul); Bedford Hospital (D. T. Howes); Belfast City Hospital (Z. R. Desai, T. C. M. Morris); Birmingham Heartlands Hospital (C. Fegan, M. J. Leyland, D. W. Milligan*); Bradford Royal Infirmary (L. A. Parapia, A. T. Williams); Bristol Royal Infirmary (G. L. Scott); Central Middlesex Hospital (S. Davies, K. Ryan); Cheltenham General Hospital (E. Blundell, R. G. Dalton); Christchurch Hospital (D. N. J. Hart); City Hospital (D. Bareford); Clinical Trial Service Unit (R. Gray,* R. Peto,* S. Richards,* K. Wheatley*); Conquest Hospital (J. Beard, S. G. Weston-Smith); Corbett Hospital (S. A. El-Tamtamy); Countess of Chester Hospital (J. V. Clough, E Rhodes); Crosshouse Hospital (J. G. Erskine, P. Vosylius); Darlington Memorial Hospital (P. J. Williamson); Derbyshire Royal Infirmary (A. McKernan, D. C. Mitchell); Derriford Hospital (J. A. Copplestone, A. Prentice*); Dumfries & Galloway Royal Infirmary (A. Stark); Dundee Teaching Hospitals (P. Cachia, A. Heppleston, M. J. Pippard); Ealing Hospital (U. M. Hegde); Eastbourne District General Hospital (P. A. Gover, R. J. Grace); Edgware General Hospital (D. Harvey); Epsom General Hospital (L. Jones, M. J. Semple); Falkirk District Royal Infirmary (A. D. J. Birch); Farnborough Hospital (A. K. Lakhani, I. R. Samaratunga); Frenchay Hospital (P. J. Whitehead); George Eliot Hospital (M. N. Narayanan); Glan Clwyd District General Hospital (D. R. Edwards, D. I. Gozzard); Glasgow Royal Infirmary (R. Chopra,* I. M. Franklin*); Gloucester Royal Hospital (S. Chown, J. Ropner); Good Hope Hospital (M. S. Hamilton, J. Tucker); Greenwich District Hospital (R. M. Ireland); Guy's Hospital (K. G. A. Clark, S. A. Schey*; Hammersmith Hospital (J. Apperley, J. M. Goldman*); Harrogate District Hospital (A. G. Bynoe, M. W. McEvoy); Hemel Hempstead General Hospital (J. F. M. Harrison); Hillingdon Hospital (R. Jan-Mohamed, R. Kaczmarski); Horton General Hospital (I. J. Durrant*); Huddersfield Royal Infirmary (C. Carter); Ipswich Hospital (N. J. Dodd,* C. N. Simpson); Kidderminster General Hospital (M. L. Lewis); King George Hospital (N. Akhtar); King's College Hospital (H. Hambley, G. Mufti*); Law Hospital (T. L. Allan, J. D. Browning, G. Helenglass); Leeds General Infirmary (J. A. Child,* G. J. Morgan, D. R. Norfolk, G. M. Smith, D. Swirsky*); Leicester Royal Infirmary (C. S. Chapman, A. E. Hunter,* R. M. Hutchinson,* V. E. Mitchell, J. K. Wood); Lincoln County Hospital (M. A. Adelman, D. R. Prangnell); Lister Hospital (C. Tew, S. M. Watkins); Manchester Royal Infirmary (J. A. Liu Yin*); Manor Hospital (G. P. Galvin); Milton Keynes General Hospital (E. J. Miller, D. J. Moir, D. M. White); Monklands District General Hospital (E. J. Fitzsimons, J. A. Murphy, R. Soutar, W. Watson); New Cross Hospital (A. MacWhannell); Norfolk and Norwich Hospital (A. J. Black,* A. M. Deane, J. Leslie, G. E. Turner*); North Devon District Hospital (B. Attock); North Hampshire Hospital (D. L. Aston, A. E. Milne); North Staffs Hospital Centre (P. M. Chipping); Northampton General Hospital (M. E. Haines, J. R. Y. Ross, S. S. Swart); Northwick Park Hospital (C. D. L. Reid, P. Skacel); Nottingham City Hospital (N. H. Russell*); Nottingham University Hospital (J. M. Davies, G. Dolan); Oxford Radcliffe Hospital (C. Bunch,* P. Emerson,* T. J. Littlewood,* J. S. Wainscoat); Pembury Hospital (D. S. Gillett, C. Taylor); Peterborough District Hospital (S. A. Fairham, M. Sivakumuran, J. Z. Wimperis); Pilgrim Hospital (S. Sobolewski, V. M. Tringham); Pinderfields General Hospital (M. C. Galvin, P. Hillmen); Pontefract General Infirmary (R. Sibbald); Poole Hospital (A. J. Bell, A. Worsley); Queen Alexandra Hospital (T. Cranfield, M. Ganczakowski); Queen Elizabeth Hospital, Birmingham (J. A. Holmes, J. A. Murray); Queen Elizabeth Hospital, Norfolk (P. Coates, J. Keidan); Queen Mary's Sidcup (S. Bowcock, S. Rassam); Rotherham District General (H. F. Barker, P. C. Taylor); Royal Bournemouth Hospital (T. J. Hamblin,* H. Myint, D. G. Oscier); Royal Chesterfield Hospital (D. J. Clark, R. Collin); Royal Cornwall Hospital (M. D. Creagh, A. R. Kruger, M. Patterson); Royal Devon and Exeter Hospital (M. V. Joyner, R. Lee, M. A. Pocock); Royal Free Hospital (A. V. Hoffbrand,* A. B. Mehta, H. G. Prentice,* K. Yong); Royal Hallamshire Hospital (J. T. Reilly, E. Vandenberghe,* D. A. Winfield*); Royal Hants. County Hospital (W. O. Mavor); Royal Infirmary of Edinburgh (C. A. Ludlam, A. C. Parker*); Royal Liverpool University Hospital (P. Chu, R. E. Clark,* C. R. M. Hay); Royal London Hospital (A. C. Newland*); Royal Marsden Hospital (D. Catovsky,* R. L. Powles*); Royal Shrewsbury Hospital (M. J. O'Shea); Royal Surrey County Hospital (G. Robbins); Royal Sussex County Hospital (J. Duncan); Royal United Hospital (C. R. J. Singer, J. G. Smith); Royal Victoria Hospital, Belfast (J. M. Bridges,* F. G. C. Jones,* E. E. Mayne, M. F. McMullin*); Royal Victoria Infirmary (P. Hamilton,* S. G. O'Brien*); Salisbury District Hospital (H. F. Parry); Sandwell General Hospital (S. I. Handa, P. J. Stableforth); Scunthorpe General Hospital (S. Jalihal, R. Stewart); Seacroft Hospital (S. M. Rajah); Singleton Hospital (S. Al-Ismail, M. S. Lewis); South Tyneside Hospital (A. M. Hendrick); Southampton University Hospital (A. Duncombe, A. Provan, O. S. Roath,* A. G. Smith*); Southmead Hospital (R. S. Evely, J. Hows, R. R. Slade); St Alban's City Hospital (E. J. Gaminara); St George's Hospital (S. E. Ball, D. H. Bevan); St Helier Hospital (J. Behrens, J. Mercieca); St James Hospital (P. V. Browne, S. R. McCann,* I. Temperley*); St James's University Hospital (D. L. Barnard, B. A. McVerry); St Mary's Hospital, London (S. H. Abdalla, B. J. Bain); St Mary's Hospital, Portsmouth (P. J. Green); St Richard's Hospital (P. C. Bevan, P. Stross); St Thomas' Hospital (R. Carr, T. C. Pearson*); Staffordshire General Hospital (T. A. J. Phaure, P. Revell); Stirling Royal Infirmary (D. M. Ramsay); Stobhill Hospital (R. L. C. Cumming, R. B. Hogg); Stoke Mandeville Hospital (A. M. O'Hea, S. M. Sheerin, A. Watson); Sunderland Royal Infirmary (P. J. Carey*); Torbay Hospital (B. Murphy*); University College Hospital, Galway (E. L. Egan, M Murray); University College Hospital, London (S. Devereux, A. H. Goldstone,* D. C. Linch,* K. G. Patterson, J. B. Porter); University Hospital Lewisham (N. Mir); University Hospital of Wales (A. K. Burnett,* W. P. Chairman, S. H. Lim, C. Poynton, J. A. Whittaker*); University of Birmingham (I. C. M. MacLennan*); Victoria Infirmary (R. A. Sharp, P. J. Tansey*); Walsgrave Hospital (R. I. Harris, M. J. Strevens); Walton Hospital (J. H. Martindale, P. A. Stevenson); Wansbeck General Hospital (I. Neilly); Warwick Hospital (S. Basu, P. E. Rose); West Middlesex Hospital (R. G. Hughes, M. Sekhar); West Suffolk Hospital (P. Harper); Western General Hospital (N. C. Allan,* P. Ganly, M. J. Mackie, P. Shepherd*); Wexham Park Hospital (N. Bienz, C. Hatton, P. H. Mackie); Whipps Cross Hospital (C. C. Anderson, C. DeSilva); Whiston Hospital (J. Tappin); Whittington Hospital (N. E. Parker); William Harvey Hospital (D. G. Wells); Wycombe General Hospital (R. Aitchison, S. Kelly, J. K. Pattinson); York District Hospital (L. R. Bond); Ysbyty Gwynedd (H. E. T. Korn, D. H. Parry).
A complete list of the participants in the trial and their institutions is given in an at the end of this article.
Submitted November 21, 2000; accepted April 4, 2001.
Reprints:A. K. Burnett, Dept of Haematology, University of Wales College of Medicine, Heath Park, Cardiff, CF14 4XN, United Kingdom; e-mail: burnettak@cardiff.ac.uk.