Recovery of cytomegalovirus (CMV)–specific T-cell–mediated immunity after allogeneic hematopoietic stem cell transplantation (SCT) is critical for protection against CMV disease. The study used fluorochrome-conjugated tetrameric complexes of HLA-A2 molecules loaded with the immunodominant NLVPMVATV (NLV) peptide derived from the CMV protein pp65 to quantify A2-NLV–specific CD8+ T cells in partially T-cell–depleted grafts administered to 27 HLA-A*0201+ patients and to monitor recovery of these T cells during the first 12 months after SCT. None of the 9 CMV-seronegative patients became infected with CMV, whereas 14 of 18 CMV-seropositive patients developed CMV antigenemia after SCT. CMV-seropositive recipients of grafts from CMV-seronegative donors required more preemptive treatment with ganciclovir (GCV) than those of grafts from CMV-seropositive donors (3 [1-6] versus 1 [0-3] courses, respectively; P = .009). The number of A2-NLV–specific CD8+ T cells in the grafts correlated inversely with the number of preemptive GCV courses administered (r = −0.61; P = .01). None of the 9 CMV-seronegative patients mounted a CMV-specific immune response as measured by monitoring A2-NLV–specific CD8+ T cells after SCT. Thirteen of 14 CMV-seropositive patients without CMV disease recovered these T cells. In spite of preemptive GCV treatment, CMV disease developed in 4 patients, who all failed to recover A2-NLV–specific CD8+ T cells after SCT(P = .002). Thus, enumeration of HLA-restricted, CMV-specific CD8+ T cells in the grafts and monitoring of these T cells after SCT may constitute a rapid and sensitive tool to identify SCT recipients at risk for developing CMV disease.
Introduction
Progressive cytomegalovirus (CMV) infection in organ and hematopoietic stem cell transplant (SCT) recipients is strongly related to T-cell immunodeficiency after transplant. Recovery of cytotoxic T-lymphocyte (CTL) activity against CMV-infected fibroblasts in peripheral blood samples of SCT recipients is associated with resolution of active CMV infection.1 With the use of more sensitive culture techniques for the detection of HLA-restricted, CMV-specific CD4+ and CD8+ T-cell responses, a correlation between regeneration of CMV-specific T cells and protection from CMV disease has been shown.2 3
The structural CMV protein pp65 has been identified as a major source of immunodominant CTL epitopes.4,5 The fact that pp65-specific CTLs are maintained at very high frequency in healthy CMV carriers6 suggests that, even in immunocompetent hosts, CMV reactivation occurs intermittently but remains subclinical because of rapid control by the host's immune response. Recently, it has become possible to directly enumerate antigen-specific CD8+T cells by using so-called tetramers.7 8 Such a tetrameric complex is formed by 4 synthetic and biotinylated peptide-loaded HLA molecules that are centrally linked by a fluorochrome-conjugated streptavidin molecule, which can be visualized by flow cytometry. Here, we used HLA-A2 tetramers loaded with the immunodominant NLVPMVATV (NLV) peptide derived from pp659 to quantify CD8+, HLA-A2-NLV–specific T cells in the partially T-cell–depleted grafts administered to HLA-A*0201+ patients and to study the kinetics of regeneration of these T cells after SCT. Specifically, we addressed whether or not the number of transplanted CD8+, A2-NLV–specific T cells and their kinetics of regeneration correlated with protection against progressive CMV infection and CMV disease.
Patients and methods
Patients
Between 1997 and 2000, 27 consecutive HLA-A*0201+(hereafter abbreviated as A2) recipients received a partially T-cell–depleted SCT from a genotypically HLA-matched sibling or an HLA-A, B, DRB1-matched unrelated donor (MUD) according to institutional review board-approved protocols (Table1). Patients were considered “standard risk” in case of a diagnosis of acute myelogenous leukemia in first complete remission, acute lymphoblastic leukemia in first complete remission, chronic myeloid leukemia in first chronic phase, or untreated severe aplastic anemia. All other diagnoses were considered high risk.
All patients were prepared for SCT by using cyclophosphamide (120 mg/kg body weight) and total body irradiation (6 Gy on each of 2 successive days with partial shielding of the lungs for a total lung dose of 2 × 4.5 Gy). Antithymocyte globulin was administered to all 7 MUD SCT recipients as part of the conditioning regimen to enhance immunosuppression. T-cell depletion was performed by sheep erythrocyte rosetting in 15 patients and by selection of CD34+ cells in 12 patients, followed by T-cell add-back, as to have the grafts contain 0.2 × 106 T cells/kg body weight of the recipient. All patients received additional prophylaxis for graft-versus-host disease (GVHD) by using cyclosporine (3 mg/kg/d) from days −3 to +120 relative to SCT. Acute and chronic GVHD were classified according to severity and were treated as described elsewhere,10 as was transfusion support and prophylaxis of bacterial and fungal infections.
Diagnostic tests for CMV infection and CMV disease
CMV seropositivity was assessed by detection of immunoglobulin (Ig)M and IgG antibodies to CMV late antigen.11 The presence of CMV-specific IgG antibodies in healthy blood donors, in SCT donors, or in patients before SCT was taken as a marker for CMV carrier status. CMV antigenemia was monitored weekly in SCT recipients from days 0 to +150 and at longer intervals thereafter by using immunocytochemical detection of pp65 antigen in leukocytes.12 Test results were considered positive in case of 1 or more positively stained leukocyte. Patients with a positive pp65 antigenemia test were monitored twice weekly until pp65+ leukocytes had become undetectable. CMV disease was diagnosed on the basis of an inflammatory process because of CMV, confirmed either by the immediate early antigen assay or CMV cultures, and combined with the presence of typical cytopathic effects in histologic preparations if biopsies were available.10
Ganciclovir therapy
Preemptive ganciclovir (GCV) therapy (5 mg/kg intravenously twice daily) was started if CMV antigenemia tests had revealed 4 or more pp65+ leukocytes per test and was discontinued after 2 successive negative test results.10 CMV disease was treated with a combination of GCV and CMV-specific immunoglobulins.13
Enumeration of CD8+ T lymphocytes binding
HLA-A2, NLV tetramers
Venous blood samples collected in sodium heparin were collected at 2, 3, 6, 9, and 12 months after SCT. As reference, venous blood samples were obtained from 29 controls (ie, 20 apparently healthy laboratory workers and 9 SCT donors). From the latter, the blood samples were obtained before mobilization or bone marrow donation. Absolute CD8+ T-cell counts were assessed within 6 hours after venipuncture; mononuclear cells were isolated from the remainder of the sample, cryopreserved in medium containing 10% dimethyl sulfoxide, and stored in liquid N2. CD8+ T lymphocytes were enumerated by using a 3-color, single-platform, whole-blood immunostaining technique.14 The following monoclonal antibodies were used: CD45 (clone 2D1 conjugated with fluorescein isothiocyanate [FITC]; BD Biosciences [BD], San Jose, CA), CD8 (clone SK1 conjugated with phycoerythrin [PE]; BD) and TCR PAN α/β (clone BMA031 conjugated with PE-Cy5; Immunotech, Marseille, France). Of each sample, 50 000 leukocytes were acquired using a FACSCalibur flow cytometer (BD). During data analysis, CD8+ T lymphocytes were defined as events with low to medium forward light scatter (FSC), low sideward light scatter (SSC), CD45+, TCRαβ+, and CD8+.
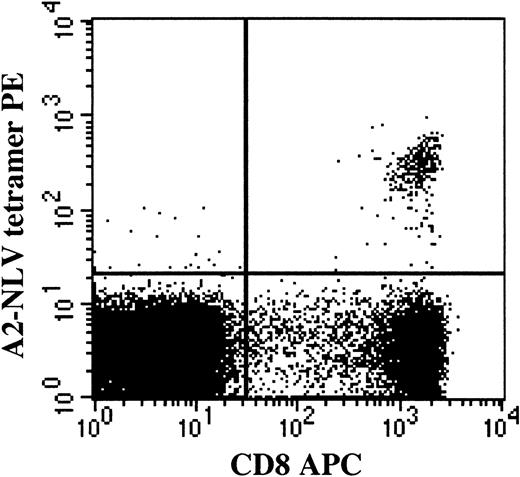
The proportion of CD8+ T cells binding HLA-A2 NLV (A2-NLV) tetramers was assessed on cryopreserved and thawed mononuclear cell suspensions and reference SCT samples. The A2-NLV tetramer, conjugated with PE, was produced by Beckman-Coulter, Immunomics Division (Marseille, France), and validated as described elsewhere.9 After thawing, cells were incubated with A2-NLV tetramer and CD8 (clone SK1 conjugated with allophycocyanin). After 1 wash, cells were resuspended in phosphate-buffered saline (PBS) containing 1 μg/mL 7-aminoactinomycin D (Sigma, St Louis, MO). Following acquisition of 20 000 living CD8+ lymphocytes (defined as having low to intermediate FSC, low SSC, as well as being 7-AAD− and CD8+), the proportion of living CD8+ T cells binding the A2-NLV tetramer was assessed, and the absolute number of circulating A2-NLV–specific CD8+ T cells was calculated from the proportion of CD8+ T cells binding A2-NLV tetramers and the simultaneously obtained absolute CD8+ T-cell count. Of note, the A2-NLV tetramer-binding T cells were CD8bright and not CD8dim (Figure1). The lower limit of detection was 0.1% A2-NLV–specific CD8+ T cell (fraction of viable CD8+ T cells) and, in absolute counts, 1 A2-NLV–specific CD8+ T cell/μL blood. In the stem cell grafts, the number of transplanted A2-NLV–specific CD8+ T cells/kg body weight of the recipient was calculated from the proportion of CD3+ T cells coexpressing CD8 and binding A2-NLV tetramers plus the simultaneously established number of CD3+ T cells/kg transplanted.
T lymphocytes binding the HLA-A2 NLVPMVATV (A2-NLV) tetramer are CD8bright and not CD8dim.
Following acquisition of a list mode data file containing 20 000 viable CD8+ lymphocytes, viable cells were selected on the basis of 7-AAD negativity (Gate 1). Among the viable mononuclear cells, CD8+ lymphocytes were selected on the basis of CD8 expression and a low sideward scatter signal (Gate 2). Lymphocytes fulfilling the criteria of Gates 1 and 2 are displayed in the figure. The percentage of A2-NLV–specific CD8+ T lymphocytes was assessed and expressed as a fraction of viable CD8+lymphocytes by using quadrant statistics. PE, phycoerythrin; APC, allophycocyanin.
T lymphocytes binding the HLA-A2 NLVPMVATV (A2-NLV) tetramer are CD8bright and not CD8dim.
Following acquisition of a list mode data file containing 20 000 viable CD8+ lymphocytes, viable cells were selected on the basis of 7-AAD negativity (Gate 1). Among the viable mononuclear cells, CD8+ lymphocytes were selected on the basis of CD8 expression and a low sideward scatter signal (Gate 2). Lymphocytes fulfilling the criteria of Gates 1 and 2 are displayed in the figure. The percentage of A2-NLV–specific CD8+ T lymphocytes was assessed and expressed as a fraction of viable CD8+lymphocytes by using quadrant statistics. PE, phycoerythrin; APC, allophycocyanin.
Statistical methods
For nonparametric statistical analyses, Wilcoxon test and Spearman rank correlation test were used. Fisher exact test (2-sided) was used to analyze the 2-by-2 tables. P values < .05 were considered significant.
Results
CMV serology before SCT and CMV infection
Nine patients were CMV seronegative before SCT and 3 of them received grafts from a CMV-seropositive donor. None of these 9 patients became infected with CMV after SCT (Table2).
Eighteen patients were CMV seropositive (Table 2); 5 of them received SCT from a CMV-seronegative donor and all of these 5 patients reactivated their CMV as evidenced by the appearance of pp65+ leukocytes in the blood at a median of 25 days (range: 22-47) after SCT (Table 3). These 5 patients received preemptive GCV therapy, and recurrence of CMV antigenemia required multiple courses of GCV treatment in 4 of them. Three patients developed CMV disease at 106, 236, and 253 days after SCT. CMV disease was fatal in 2 of these 3 patients, who died of CMV encephalitis (patient 4) and CMV pneumonitis (patient 5). CMV infection involved the bone marrow in patient 3 with early-onset disease (ie, day 106), but this syndrome resolved on treatment with GCV and CMV-specific immunoglobulins.
Nine of 13 CMV-seropositive recipients of SCT from a CMV-seropositive donor developed CMV antigenemia after SCT; pp65+ leukocytes became detectable at a median of 49 days (range: 17-355) after SCT (Table 3). Seven of these 9 patients received preemptive GCV therapy, and recurrence of CMV antigenemia required multiple courses of GCV treatment in 3 of these 7 patients. One patient (6) developed CMV disease of the bone marrow at 38 days after SCT, which was followed by secondary graft failure; ultimately, this patient died of CMV encephalitis despite prolonged GCV therapy.
More preemptive treatment with GCV had to be administered to CMV-seropositive recipients of grafts from CMV-seronegative donors than to those of grafts from CMV-seropositive donors (3 [1-6] versus 1 [0-3] courses, respectively; P = .009). CMV disease occurred more frequently after SCT from a CMV-seronegative than from a CMV-seropositive donor (3 of 5 versus 1 of 13 patients, respectively;P = .04). The higher incidence of CMV antigenemia and disease after SCT from CMV-seronegative versus CMV-seropositive donors indicates that CMV-specific T lymphocytes in the graft may protect against progressive CMV infection (see below).
Enumeration of A2-NLV–specific CD8+ T lymphocytes
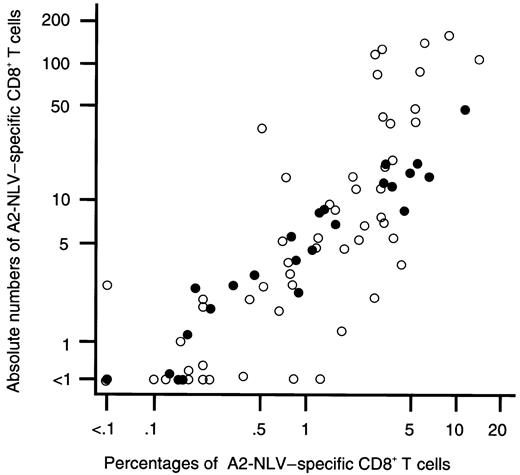
No A2-NLV–specific CD8+ T cells were detectable in the peripheral blood of 8 CMV-seronegative SCT donors tested. When expressed in absolute counts, A2-NLV–specific CD8+ T cells were above the detection limit (ie, ≥ 1/μL) in 21 of 29 CMV-seropositive SCT donors or healthy laboratory workers. The median value was 3/μL (range: < 1-46). When expressed as a fraction of CD8+ T cells, A2-NLV–specific CD8+ T cells were above the detection limit (ie, ≥ 0.1% of CD8+ T cells) in 23 of the 29 CMV-seropositive individuals. The median value was 0.7% (range: < 0.1%-11.3%) of CD8+ T cells. The enumeration results expressed in relative and absolute numbers were strongly and positively correlated, both in healthy CMV-seropositive individuals and in SCT recipients (overall, Spearman rank correlation coefficient r = 0.94; Figure2). Therefore, results are only presented in absolute numbers in the following sections.
The percentages and absolute numbers of A2-NLV–specific CD8+ T cells in peripheral blood are strongly and positively correlated.
On the x axis, the percentage of A2-NLV–specific CD8+ T cells (expressed as a fraction of total CD8+ cells) is shown; on the y axis, the absolute number of A2-NLV–specific CD8+ T cells (expressed in counts per microliter). Data are from 29 blood samples of 29 healthy HLA-A*0201+, CMV-seropositive SCT donors or laboratory workers (●) and from 71 blood samples obtained from 18 HLA-A*0201+, CMV-seropositive SCT recipients (○). Overall, Spearman rank correlation test is r = 0.94 (P < .0001). Logarithmic scales were used for the x and y axes to compress the figure.
The percentages and absolute numbers of A2-NLV–specific CD8+ T cells in peripheral blood are strongly and positively correlated.
On the x axis, the percentage of A2-NLV–specific CD8+ T cells (expressed as a fraction of total CD8+ cells) is shown; on the y axis, the absolute number of A2-NLV–specific CD8+ T cells (expressed in counts per microliter). Data are from 29 blood samples of 29 healthy HLA-A*0201+, CMV-seropositive SCT donors or laboratory workers (●) and from 71 blood samples obtained from 18 HLA-A*0201+, CMV-seropositive SCT recipients (○). Overall, Spearman rank correlation test is r = 0.94 (P < .0001). Logarithmic scales were used for the x and y axes to compress the figure.
Repopulation kinetics of A2-NLV–specific CD8+ T lymphocytes after SCT
The repopulation kinetics of total CD8+ T cells is shown in Figure 3 and that of the subset of A2-NLV–specific CD8+ T cells in Figure4. These data are separately shown for 9 CMV-seronegative patients (Figure 3A, Figure 4A), in 4 CMV-seropositive patients without antigenemia (Figure 3B, Figure4B), in 10 CMV-seropositive patients developing CMV antigenemia but no CMV disease (Figure 3C, Figure 4C), and in 4 patients with CMV disease after SCT (Figure 3D, Figure 4D). The rates of repopulation of total CD8+ T cells (Figure 3) in these 4 groups was not significantly different, although, among patients with active CMV infection, CD8+ T-cell repopulation in the group with CMV disease (Figure 3D) lagged behind that of the group with CMV antigenemia but without CMV disease (Figure 3C). However, differences between these latter 2 groups became significant when A2-NLV–specific CD8+ T cells were studied (Figure 4).
Repopulation of total CD8+ T cells after allogeneic, T-cell–depleted SCT.
(A) CMV-seronegative patients (n = 9); (B) CMV-seropositive patients without CMV antigenemia after SCT (n = 4); (C) CMV-seropositive patients developing CMV antigenemia but no CMV disease (n = 10); (D) CMV-seropositive patients developing CMV antigenemia and CMV disease (n = 4). Logarithmic scales were used for the y axes to compress the figure. For each patient group, the median values per time point are connected with a line. In each panel, the upper and lower horizontal lines indicate the 95th and 5th percentiles of 29 healthy CMV-seropositive HLA-A2+ individuals, respectively. SCT, stem cell transplantation.
Repopulation of total CD8+ T cells after allogeneic, T-cell–depleted SCT.
(A) CMV-seronegative patients (n = 9); (B) CMV-seropositive patients without CMV antigenemia after SCT (n = 4); (C) CMV-seropositive patients developing CMV antigenemia but no CMV disease (n = 10); (D) CMV-seropositive patients developing CMV antigenemia and CMV disease (n = 4). Logarithmic scales were used for the y axes to compress the figure. For each patient group, the median values per time point are connected with a line. In each panel, the upper and lower horizontal lines indicate the 95th and 5th percentiles of 29 healthy CMV-seropositive HLA-A2+ individuals, respectively. SCT, stem cell transplantation.
Repopulation of A2-NLV–specific CD8+ T cells after allogeneic, T-cell–depleted SCT.
In each panel, the horizontal line indicates the 95th percentile of 29 healthy CMV-seropositive (SCT) HLA-A2+ individuals. Among CMV-seropositive recipients with CMV antigenemia (C,D), differences in A2-NLV–specific CD8+ T-cell counts between patients without and with CMV disease were significant between 2 and 9 months after SCT (P values between .001 and .05 using Wilcoxon test). During that period, differences in total CD8+ T-cell counts between patients without and with CMV disease were not significant. See further the legend to Figure 3.
Repopulation of A2-NLV–specific CD8+ T cells after allogeneic, T-cell–depleted SCT.
In each panel, the horizontal line indicates the 95th percentile of 29 healthy CMV-seropositive (SCT) HLA-A2+ individuals. Among CMV-seropositive recipients with CMV antigenemia (C,D), differences in A2-NLV–specific CD8+ T-cell counts between patients without and with CMV disease were significant between 2 and 9 months after SCT (P values between .001 and .05 using Wilcoxon test). During that period, differences in total CD8+ T-cell counts between patients without and with CMV disease were not significant. See further the legend to Figure 3.
A2-NLV–specific CD8+ T cells remained undetectable in 8 of the 9 CMV-seronegative patients, irrespective of whether the donor was CMV seropositive or not. In 1 patient, A2-NLV–specific CD8+ T cells just reached the detection limit (ie, 1 cell/μL) at 3 and 6 months, to become undetectable again at 9 and 12 months after SCT (Figure 4A).
All but 1 of 14 CMV-seropositive patients without CMV disease recovered their A2-NLV–specific CD8+ T cells (ie, these cells became detectable at 2 or more occasions). The group of 10 patients developing CMV antigenemia after SCT showed a reconstitution of A2-NLV–specific CD8+ T cells to higher (ie, supranormal) levels (Figure 4C) than the group of 4 patients without CMV antigenemia in which A2-NLV–specific CD8+ T cells stayed within the normal range and even remained undetectable in 1 of them (patient 16; Figure 4B).
Most strikingly, A2-NLV–specific CD8+ T cells remained undetectable throughout the entire follow-up of 3 of the 4 patients developing CMV disease, whereas A2-NLV–specific CD8+ T cells were detected only once in the fourth patient (5) (ie, 2 cells/μL on day 82) but were undetectable again when CMV disease developed at day 236 (Figure 4D). Thus, CMV-seropositive SCT recipients who developed CMV viremia but reconstituted their A2-NLV–specific CD8+ T cells had a significantly lower frequency of CMV disease than those who did not recover such T cells (1 of 11 versus 0 of 3 patients, respectively; P = .01).
The following parameters did not significantly influence the repopulation kinetics of total CD8+ T cells (all 27 patients) or A2-NLV–specific CD8+ T cells (18 CMV-seropositive patients) at the time points studied: SCT donor type (ie, sibling versus unrelated), stem cell source (ie, bone marrow versus peripheral blood), technique of T-cell depletion (ie, sheep erythrocyte rosetting versus CD34+ cell selection), and donor CMV serology (ie, positive versus negative) (data not shown).
Transfer of A2-NLV–specific CD8+ T lymphocytes with the grafts
No A2-NLV–specific CD8+ T cells were detectable in the grafts of 5 CMV-seronegative donors tested (Table 3). The partially T-cell–depleted grafts from 10 of the 13 CMV-seropositive donors tested contained a median of 1.6 × 103 A2-NLV–specific CD8+ T cells/kg body weight of the recipient (range: 0.2-11.3), whereas such T cells could not be detected in the remaining 3 grafts. Of note, 2 grafts containing 0.8 and 4.6 × 103A2-NLV–specific CD8+ T cells/kg, respectively, were administered to CMV-seronegative recipients, who did not recover these T cells during 12 months of follow-up.
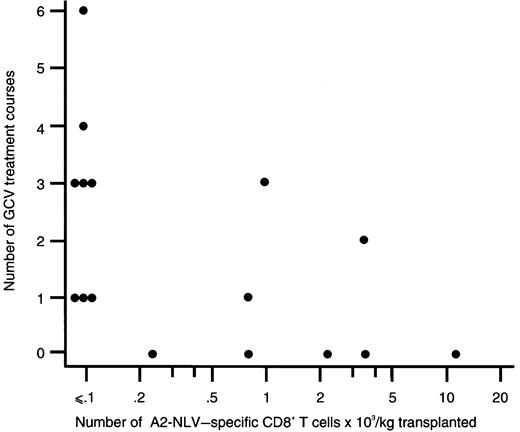
Among CMV-seropositive patients, all 8 recipients of grafts without detectable A2-NLV–specific CD8+ T cells needed preemptive GCV treatment versus 3 of 8 recipients of grafts with detectable A2-NLV–specific CD8+ T cells (P = .03). Furthermore, the number of A2-NLV–specific CD8+ T cells transferred with the graft was inversely correlated with the number of preemptive GCV treatment courses administered (r = −0.61;P = .01; Figure 5). Among CMV-seropositive SCT recipients, CMV disease occurred in 4 of 8 patients (50%) receiving a graft without detectable A2-NLV–specific CD8+ T cells (ie, < 0.1 × 103 cells/kg), and none of the 8 patients receiving a graft with detectable A2-NLV–specific CD8+ T cells developed CMV disease(P = .08).
The number of A2-NLV–specific CD8+ T cells transplanted and the number of preemptive ganciclovir courses administered to the respective SCT recipients are inversely correlated.
The number of A2-NLV–specific CD8+ T cells transplanted was expressed as the number of transplanted T cells × 103 per kg body weight of the recipient; a logarithmic scale was used for the x axis to compress the figure. Spearman rank correlation coefficient is −0.61 (P = .01). GCV, ganciclovir.
The number of A2-NLV–specific CD8+ T cells transplanted and the number of preemptive ganciclovir courses administered to the respective SCT recipients are inversely correlated.
The number of A2-NLV–specific CD8+ T cells transplanted was expressed as the number of transplanted T cells × 103 per kg body weight of the recipient; a logarithmic scale was used for the x axis to compress the figure. Spearman rank correlation coefficient is −0.61 (P = .01). GCV, ganciclovir.
Discussion
The use of HLA-A*0201 tetramers loaded with the CMV pp65-derived NLV peptide enabled us to monitor CMV-specific T-cell immunity by quantitating A2-NLV–specific CD8+ T lymphocytes in HLA-A2+ recipients of an allogeneic, partially T-cell–depleted SCT. Specifically, we studied whether or not quantification of CMV-specific CD8+ T cells in stem cell grafts and monitoring of their rate of recovery after SCT would predict the occurrence of progressive CMV infection and, in particular, CMV disease. Although the number of patients in our study was small (ie, 27, of which 18 were CMV seropositive), several of our conclusions are supported by the recent report of Cwynarski et al15 who studied CMV-specific T-cell reconstitution using tetramer technology in 13 recipients of non–T-depleted sibling SCT and in 11 recipients of unrelated SCT who had undergone in vivo T-cell depletion using the Campath 1H monoclonal antibody.
Our key findings are the following: (1) failure to recover A2-NLV–specific CD8+ T cells after SCT by CMV-seropositive recipients developing CMV viremia was associated with the development of CMV disease; (2) the number of A2-NLV–specific CD8+ T cells in the grafts administered to CMV-seropositive SCT recipients is inversely correlated with the number of recurrent CMV infections after SCT, requiring preemptive GCV treatment; and (3) among CMV-seropositive recipients, recurrent CMV antigenemia and CMV disease occurred significantly more frequently after SCT from a CMV-seronegative than from a CMV-seropositive donor.
Our results indicate that protection against progressive CMV infection and CMV disease is being conferred by (1) CMV-specific memory T lymphocytes transferred with the grafts and (2) the development of a CMV-specific cellular immune response after SCT. In line with our findings, Cwyniarski et al15 found that reconstitution of CMV-specific CD8+ T cells to levels more than 10 × 103/μL protected against CMV disease. These combined results confirm earlier studies based on functional assays, ie, cytotoxicity assays to monitor CMV-specific CD8+ T cells2,3 and lymphoproliferation assays to monitor CMV-specific CD4+ T cells.3 16
With respect to early recovery of the CMV-specific immune response, Cwynarski et al15 found, in CMV-seropositive patients, that CMV-specific CD8+ T-cells became detectable in all of 12 recipients of SCT from a CMV-seropositive donor (between 21 and 116 days after SCT), whereas CMV-specific CD8+ T cells regenerated only in 1 of 5 recipients of SCT from a CMV-seronegative donor (at 149 days after SCT). These data are consistent with earlier findings, ie, that CMV-seropositive recipients of SCT from CMV-seropositive donors regenerate their CMV-specific helper T-cell3,17 and cytotoxic T-cell responses3more quickly than recipients of SCT from CMV-seronegative donors. In our study, monitoring was started only at 2 months after SCT, and, from that time point onward, no significant effect of donor CMV serology on the rate of CMV-specific CD8+ T-cell reconstitution was apparent. Remarkably, in some of our SCT recipients with CMV antigenemia (even including one recipient of a SCT from a CMV-seronegative donor [patient 1]), as well as in some of Cwynarski's patients,15 CMV-specific CD8+ T cells had already regenerated to levels well above the normal range at 2 months after SCT. These results are consistent with the hypotheses that, apart from memory T cells in the graft, naive T cells may mount a primary immune response against CMV, and/or residual recipient-derived memory T cells may contribute to the regeneration of CMV-specific T cells after SCT.
With respect to long-term recovery of the CMV-specific immune response, we observed that A2-NLV–specific CD8+ T cells reconstituted to lower levels in CMV seropositive patients without (or with very late) CMV antigenemia as compared with those developing CMV antigenemia early after SCT (but without CMV disease). In addition, we did not observe regeneration of A2-NLV–specific CD8+ T cells in CMV-seronegative recipients of grafts from CMV-seropositive donors. Again, these findings are consistent with those of Cwynarski et al15 and suggest that CMV antigenemia (ie, active CMV infection) is needed to stimulate the expansion of A2-NLV–specific CD8+ T cells. In line with these observations, Li et al3 observed in their randomized study of GCV versus placebo as prophylaxis for active CMV infection that GCV prophylaxis until day 100 after SCT was associated with delayed recovery of CMV-specific T-helper and CTL responses and suggested a reduced stimulation of CMV-specific memory T cells because of suppression of CMV replication by GCV. Although this mechanism may also have been operational in our patients, we also propose that a delayed recovery of CMV-specific T-cell immunity per se, especially after SCT from a CMV-seronegative donor, may have caused increased CMV replication and antigenemia, necessitating preemptive GCV therapy.
The structural CMV protein pp65 has been identified as a major source of immunodominant CTL epitopes,4,5 among which the NLV peptide constitutes the major epitope presented by HLA-A2.5,18 The fact that quantification of CD8+ T cells specific for this single peptide only, yielded already highly informative data in this group of HLA-A2+patients emphasizes the immunodominance of the NLV peptide in the HLA-A2–restricted immune response against CMV. However, the NLV peptide does not stimulate CD8+ T cells efficiently in all HLA-A2+ CMV carriers.19 This situation may be due to (1) the varying frequency of CTL specific for a given peptide as a function of the HLA type of the individual and (2) the role of other CMV-encoded proteins than pp65 as targets for the immune response against CMV. In this context it is relevant that CD8+ CTLs specific for the CMV immediate early protein 1, transcribed very early in the course of active CMV infection, are found in similarly high frequencies as CTL specific for pp65, which is a structural protein of the virion and whose expression does not require transcription of viral DNA.19 20 These results may explain our observation that approximately one quarter of healthy CMV-seropositive HLA-A2+ individuals had no detectable A2-NLV–specific CD8+ T cells. Also, this situation may have been the case in our patient 7 who regenerated her A2-NLV–specific CD8+T cells only to very low levels but did not develop CMV disease in spite of recurrent high-level CMV antigenemia.
The advantage of flow cytometric assays over the conventional culture-based assays such as cytotoxicity, lymphoproliferation, and even ELISPOT assays are reduced time requirements, lower costs, and higher accuracy and precision (ie, higher amenability to standardization). A major restriction of tetramer-based enumeration of antigen-specific CD8+ T cells is that each combination of peptide and presenting HLA molecule requires a unique reagent, so that a comprehensive analysis of CMV-specific CTL would require the use of multiple tetramers. An attractive alternative is the flow cytometric functional evaluation of antigen-specific CD4+ and CD8+ T cells by induction of intracellular cytokine after short-term (ie, 6-18 hours) stimulation.21,22 The evaluation of patients using protein-spanning cocktails of CMV-derived peptides (to study CD4+ T-helper cells and CD8+CTL)23 or CMV viral lysate (to study CD4+ T cells)21 may widen the applicability of antigen-specific immune monitoring as exemplified in Cwyniarski's15 and our studies.
In conclusion, the absence of A2-NLV–specific CD8+ T cells in the grafts and the impaired recovery of these T cells after SCT was associated with persistent CMV antigenemia and CMV disease in HLA-A2+, CMV-seropositive recipients of T-cell–depleted grafts reactivating the virus. The value for individual patient management of rapid assessments of CMV-specific immune reconstitution should be addressed in further prospective studies. Specifically, we envision that this strategy may enable us to identify those patients who may benefit from preemptive therapy, in particular the adoptive transfer of CMV-specific T lymphocytes for prevention of late CMV disease.
We are grateful to Mrs N. de Leeuw, Mrs J. Doekharan-van der Sluis, and Mr R. Kester for their technical assistance.
C.T. is an employee of Beckman-Coulter, Immunomics Division, whose potential product iTag tetramers includes the tetramer studied in the present work (A2-NLV).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jan W. Gratama, Department of Clinical and Tumor Immunology, University Hospital, Daniel den Hoed Cancer Center, P.O. Box 5201, 3008 AE Rotterdam, The Netherlands; e-mail:gratama@immh.azr.nl.