In normal T-cell development interleukin-7 (IL-7) functions as an antiapoptotic factor by regulating bcl-2 expression in immature thymocytes and mature T cells. Similar to what occurs in normal immature thymocytes, prevention of spontaneous apoptosis by IL-7 in precursor T-cell acute lymphoblastic leukemia (T-ALL) cells correlates with up-regulation of bcl-2. IL-7 is also implicated in leukemogenesis because IL-7 transgenic mice develop lymphoid malignancies, suggesting that IL-7 may regulate the generation and expansion of malignant cells. This study shows that in the presence of IL-7, T-ALL cells not only up-regulated bcl-2 expression and escaped apoptosis but also progressed in the cell cycle, resulting in sequential induction of cyclin D2 and cyclin A. Down-regulation of p27kip1 was mandatory for IL-7–mediated cell cycle progression and temporally coincided with activation of cyclin-dependent kinase (cdk)4 and cdk2 and hyperphosphorylation of Rb. Strikingly, forced expression of p27kip1 in T-ALL cells not only prevented cell cycle progression but also reversed IL-7–mediated up-regulation of bcl-2 and promotion of viability. These results show for the first time that a causative link between IL-7–mediated proliferation and p27kip1 down-regulation exists in malignant T cells. Moreover, these results suggest that p27kip1 may function as a tumor suppressor gene not only because it is a negative regulator of cell cycle progression but also because it is associated with induction of apoptosis of primary malignant cells.
Introduction
In normal T-cell development interleukin-7 (IL-7) plays a nonredundant role as an antiapoptotic factor by regulating bcl-2 expression in immature thymocytes and mature T cells.1 Mice deficient in IL-7 receptor (IL-7R) are lymphopenic because of a defect in cell expansion at an early stage of differentiation, and the few mature T cells that develop are functionally impaired.2 Both defects are completely rescued by overexpression of the antiapoptotic bcl-2gene.3,4 Similarly, mice deficient in IL-7 exhibit an early defect in lymphopoiesis, and developmental transition of their immature thymocytes to a T-cell committed fate is accompanied by a striking loss of Bcl-2 protein expression and an increased proportion of cells in G0/G1 stage of the cell cycle.5,6 Culture of immature thymocytes from IL-7–deficient mice with IL-7 caused up-regulation of bcl-2 protein and cell survival, indicating that, during T-cell lineage developmental transition and prior to T-cell antigen receptor rearrangement, IL-7–mediated signals are linked to an antiapoptosis mechanism and cell cycle progression.6
T-cell acute lymphoblastic leukemia (T-ALL) results from clonal expansion of hemopoietic progenitors that have undergone malignant transformation at distinct stages of differentiation, and they may retain certain features of their normal counterparts. Indeed earlier studies determined that similar to normal immature thymocytes, leukemic blasts from a number of patients with T-ALL express functional IL-7Rs.7 Subsequently, it was reported that IL-7 was stimulatory on blast colony formation and DNA synthesis, suggesting that IL-7 may play an important regulatory role in the biology of T-ALL.7,8 More recently, it was determined that IL-7 prevents spontaneous apoptosis of pre–T-ALL cells and this effect correlates with up-regulation of bcl-2.9 IL-7 is produced in the bone marrow and the thymic stroma, and, thus, it is present in the microenvironments in which the malignant T cells develop. Importantly, IL-7 has also been implicated in leukemogenesis because IL-7–transgenic mice develop lymphoid malignancies.1Therefore, the question arises whether IL-7 is involved not only in promotion of viability but also in clonal expansion of T-ALL cells.
In the present study we show that, during culture with IL-7, primary T-ALL cells not only escaped apoptosis but also progressed to the S+G2/M phase of the cell cycle. IL-7 induced sequential expression of cyclin D2 and cyclin A and dramatically increased the enzymatic activity of cyclin-dependent kinases (cdks) cdk4, cdk6, and cdk2. This event temporally correlated with hyperphosphorylation of Rb and progression to the S phase of the cell cycle. Among the cip/kip and INK family cdk inhibitors, only p27kip1 was dramatically decreased at the time of cdk2 activation and S phase progression. Down-regulation of p27kip1 had a causative role in IL-7–mediated cell cycle progression, because forced sustained expression of p27kip1 prevented activation of cdk2, hyperphosphorylation of Rb, and cell cycle progression. Importantly, viability studies revealed that forced sustained expression of p27kip1 during culture with IL-7 not only prevented cell cycle progression but also prevented IL-7–mediated bcl-2 expression and reversed the effect of IL-7 on the promotion of viability. Thus, our results show that IL-7 mediates both bcl-2 expression, leading to prevention of apoptosis, and cell cycle progression by down-regulating p27kip1 cdk inhibitor in primary T-ALL cells.
Materials and methods
Primary T-ALL samples and immunophenotypic analysis
T-ALL cells were obtained from the peripheral blood and/or the bone marrow of patients with high leukemia involvement (85%-100%). Informed consent and approval by the institutional review board was obtained for all sample collections. Samples were enriched by density centrifugation over Ficoll-Hypaque and then washed twice in RPMI-1640 supplemented with 10% (vol/vol) fetal bovine serum (FBS) and 2 mM L-glutamine (further referred to as RPMI-10 media). Expression of cell surface molecules was determined by direct labeling using standard methodology.10 Fc receptors were blocked by incubation with mouse immunoglobulin before the addition of the specific monoclonal antibodies (mAbs). The mAbs used were fluorescein isothiocyanate (FITC)-conjugated anti-CD3 and anti-CD2 and phycoerythrin (PE)-conjugated anti-CD4, CD5, CD7, as well as anti-CD8, CD19, CD13, and CD33. Irrelevant isotype-matched antibodies were used as negative controls. Samples were analyzed in a Coulter Elite or XL flow cytometer, and data were acquired in listmode files. At least 5000 positive events were measured for each sample.
In vitro culture
Cells isolated by density centrifugation over Ficoll-Hypaque were cultured in 24-well plates as 2 × 106 cells/mL at 37°C with 5% CO2 in RPMI-10 without any cytokine (medium alone) or with 10 ng/mL IL-7 (Endogen, Woburn, MA). At the indicated time points, cells were harvested and processed as indicated in the following descriptions for assessment of viability, cell cycle progression, and preparation of lysates for Western blotting and immunoprecipitation.
Proliferation assays
Cells were cultured in flat-bottom 96-well plates as 2 × 106 cells/mL at 37°C with 5% CO2 in RPMI-10 without any cytokine or with 10 ng/mL IL-7 for the indicated time points. Cells were incubated with 3H-thymidine (27 × 10−3 mBq/well) for 16 hours prior to harvest. DNA synthesis, as measured by 3H-thymidine incorporation, was assessed using a liquid scintillation counter. Proliferation index was calculated as thymidine incorporation of IL-7–cultured cells over thymidine incorporation of medium-alone cultured cells.
Assessment of cell viability
Quantitative determination of viability of the malignant cells after culture under different conditions was performed by using an Annexin V-based apoptosis detection kit and the manufacturer's protocol (R&D Systems, Flanders, NJ). Briefly, cells were suspended in the appropriate binding buffer, stained with FITC-conjugated Annexin V and propidium iodide at room temperature for 15 minutes, and subsequently analyzed by flow cytometry.
Cell cycle analysis
Determination of the percentage of cells at each stage of the cell cycle was performed by assessment of DNA content after staining with propidium iodide as described before.11Briefly, 5 × 105 cells per sample were resuspended in 0.5 mL phosphate-buffered saline (PBS) and then fixed with ice-cold 80% ethanol. Propidium iodide was added at a final concentration of 2.5 μg/mL, ribonuclease A was added at 50 μg/mL, and samples were incubated for 30 minutes at 37°C in the dark. Flow cytometric analysis was performed by using Lysis II (Becton Dickinson, Mountain View, CA) and/or XL2 software, and analysis of cell cycle histograms was carried out using ModFit LT (Verity, Topsham, ME) or WinCycle DNA Analysis software (Phoenix Flow Systems, San Diego, CA).
Determination of malignant origin of IL-7–responsive cells
To determine the clonality of IL-7–responsive cells 2 approaches were undertaken. In the first approach, cells from patients for which patient-specific T-cell receptor (TCR) rearrangements were identified were cultured with IL-7. After 72 hours of culture S+G2/M cells were sorted by flow-activated cell sorter (FACS) using Hoechst 33342 dye (Sigma-Aldrich, St Louis, MO), and the presence of specific rearrangement was examined by reverse transcriptase-polymerase chain reaction (RT-PCR) as previously described.12 PCR products were electrophoresed through 2% agarose gels containing ethidium bromide and visualized under UV light. Normal T cells and cells from other T-ALL patients were used as negative controls. In the second approach, for patients without specific rearrangements but with low or negative CD3 expression, cells were cultured with IL-7. After 72 hours of culture, absence of CD3 expression of cells in S+G2/M was assessed by flow cytometry, using propidium iodide and FITC-conjugated CD3 (Pharmingen, San Diego, CA).
Intracellular staining
Expression of Bcl-2 protein family members, Bcl-2, Bcl-xL, Bax, and Bad was assessed at 72 hours of culture by intracellular staining. Cells were fixed in 0.1% formaldehyde for 30 minutes at 4°C, washed in PBS, and resuspended in 1× Perm/Wash Solution (Pharmingen). The antibodies used were mouse monoclonal FITC-conjugated anti–Bcl-2 (DAKO, Glostrup, Denmark) and Bad (Transduction Laboratories, Lexington, KY), and rabbit polyclonal purified anti–Bcl-xL (Santa Cruz Biotechnology, Santa Cruz, CA) and Bax (Transduction Laboratories). FITC-conjugated goat antirabbit (Southern Biotechnology Associates, Birmingham, AL) was used as secondary antibody. Irrelevant isotype-matched antibodies were used as negative controls. Propidium iodide was added to each sample to distinguish live from apoptotic cells. Samples were analyzed by flow cytometry (XL2 software; Beckman-Coulter, Fullerton, CA). Results were expressed as the ratio of mean fluorescence intensity (MFI) of the specific antibody stain over the MFI of the negative control antibody.
Immunoblotting, immunoprecipitation, and in vitro kinase reactions
Following the indicated conditions and time intervals of culture, cell lysates were prepared, and equal amounts of protein (50 μg/sample) were analyzed by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred onto nitrocellulose membranes, and immunoblotted with the indicated mAbs or antiserum. Cyclin A, cyclin D2, cyclin E, cdk2, cdk4, cdk6, and p16INK4a antiserum were purchased from Santa Cruz Biotechnology, p21cip1 mAb from Upstate Biotechnology (Lake Placid, NY), and p27kip1 mAb from Transduction Laboratories. To examine the phosphorylation status of Rb, proteins were analyzed by 6% SDS-PAGE, transferred onto nitrocellulose membrane, and blotted with Rb-specific mAb (Pharmingen). After immunoblotting with mAbs or antiserum, immunodetection was performed by incubation with horseradish peroxidase-conjugated anti–mouse immunoglobulin g (IgG; 1:5000) or anti–rabbit IgG (1:10 000; Promega, Madison, WI) as indicated by the host origin of the primary antibody and developed by chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ). Stripping and reprobing of the immunoblots were done as described.13
For in vitro kinase reactions, immunoprecipitations were done by using equal amounts of protein (200 μg/sample) with anti-cdk2–specific antiserum agarose conjugate (Santa Cruz Biotechnology). In vitro kinase reactions were performed by using histone H1 (Sigma) as exogenous substrate, according to described protocol.14 After immunoprecipitation with anti-cdk4–specific antiserum, in vitro kinase reactions were performed by using Rb–glutathione-S-transferase (Santa Cruz Biotechnology) as exogenous substrate as described.14 Reactions were analyzed by 10% SDS-PAGE, transferred to polyvinylidene fluoride (PVDF) membrane, and exposed to x-ray film.
Rapamycin and VP22/p27kip1 fusion protein
Rapamycin was purchased from Sigma-Aldrich and was added in the culture at a final concentration of 100 nM. VP22 fusion proteins allow expression of recombinant proteins in cells without directly transfecting them.15 Forced expression of exogenous p27kip1 was achieved by using a VP22/p27kip1fusion protein produced in COS cells as previously described.16 Because VP22/p27kip1 fusion protein is tagged with 6× histidine, it allows preparation of purified fusion protein from cell lysates of COS transfectants by the use of nickel-chelated agarose columns (Pierce, Rockford, IL). Cells were cultured as described earlier with or without IL-7 plus VP22/p27kip1 or VP22 control (1:10 vol/vol) and/or 100 nM rapamycin.
Bcl-2 antisense oligonucleotides
Bcl-2 antisense and scrambled control oligonucleotides (Biomol Research Laboratories, Plymouth Meeting, PA) were added to the culture at 100 nM as a complex with Lipofectin (Life Technologies [Gibco], Gaithersburg, MD), according to a method adapted from the manufacturer's protocol. Briefly, cells were washed twice in AIM V serum-free medium, cultured in 24-well plates, and immediately spun down. Oligonucleotides were resuspended in sterile double-distilled H2O and incubated for 15 minutes with Lipofectin and AIM V medium at room temperature, before adding to the cell culture. As an additional control sterile ddH2O was used without any oligonucleotide. Cells with oligonucleotide mix were incubated at 37°C for 4 hours. The cells were harvested, washed, and recultured in RPMI with 10% FCS with the same concentration of oligonucleotide mix as previously used. Bcl-2 protein expression, viability, and proliferation were assessed at 72 hours of culture.
Results
IL-7 promotes viability of T-ALL cells by up-regulating bcl-2 protein expression
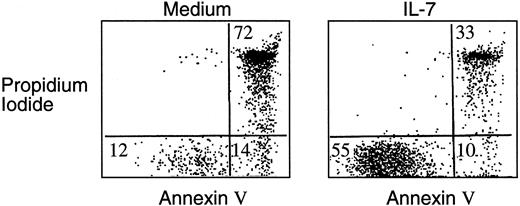
Highly enriched leukemic cells were isolated from the bone marrow or peripheral blood of pediatric patients with T-ALL. Of the 21 patients analyzed, all were positive for the pan–T-cell marker CD7 as well as for CD2 and/or CD5. Eight patients were CD3− and the remaining had a more mature phenotype and expressed this marker on the surface. On the basis of these findings, patients were classified according to the maturation stage of the malignant T cells17 (Table 1). No expression of the B-lineage differentiation antigen CD19 or the myeloid lineage markers CD13 and CD33 was detected (data not shown). Responsiveness of leukemic cells to IL-7 was determined by inhibition of spontaneous apoptosis during in vitro culture. Ten of 12 patients studied had a significant response to IL-7 as determined by increased viability assessed by annexin V and propidium iodide staining followed by FACS analysis, as shown in Figure 1. The effect of IL-7 in promotion of viability was obvious at 24 hours of culture, and maximal difference in viability between media and IL-7 cultures was observed at 72 hours. Dose-response curves in responding samples indicated that concentration of 10 ng/mL provided the saturating amount of IL-7 required to induce maximal response. Therefore, all subsequent experiments were performed at this time interval, using the optimal concentration of IL-7.
IL-7 prevents apoptosis of T-ALL cells during in vitro culture.
T-ALL cells were cultured either in media alone or in the presence of IL-7. After 72 hours of culture, cells were harvested and stained with annexin V and propidium iodide as described in “Materials and methods,” and viability was determined by FACS analysis. Results obtained from one representative patient are shown and were similar to those observed in 10 of 12 patients studied.
IL-7 prevents apoptosis of T-ALL cells during in vitro culture.
T-ALL cells were cultured either in media alone or in the presence of IL-7. After 72 hours of culture, cells were harvested and stained with annexin V and propidium iodide as described in “Materials and methods,” and viability was determined by FACS analysis. Results obtained from one representative patient are shown and were similar to those observed in 10 of 12 patients studied.
Analysis of Bcl-2, Bcl-xL, Bad, and Bax protein expression by flow cytometry revealed that all proteins were detected in primary T-ALL cells (data not shown). Culture with IL-7 increased Bcl-2 expression as determined by the increase in MFI compared with culture with media alone (Table 2), a finding consistent with previous results.9 In contrast, no changes in bcl-xL, bad, or bax expression correlated with culture in IL-7 and IL-7–mediated promotion of viability of T-ALL (data not shown). To determine whether up-regulation of bcl-2 was simply a correlative event or whether it had a causative role in IL-7–mediated promotion of viability of T-ALL cells, bcl-2 antisense oligonucleotides were added during culture of T-ALL cells with IL-7. As shown in Table2, coculture with Bcl-2 antisense oligonucleotides prevented IL-7–mediated bcl-2 protein up-regulation and reversed IL-7–mediated in vitro survival of T-ALL cells. These results indicate that up-regulated bcl-2 had a critical functional significance for the IL-7–mediated promotion of viability of T-ALL cells.
IL-7 promotes cell cycle progression of T-ALL cells
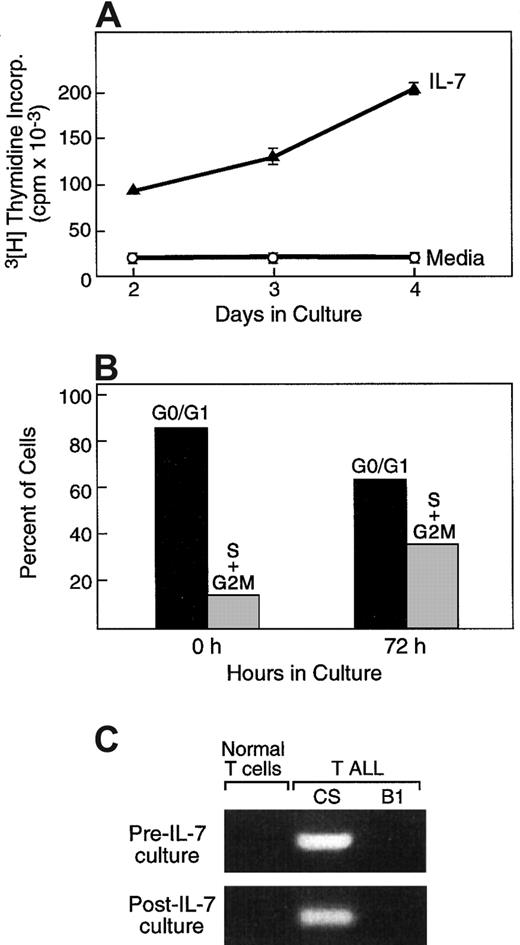
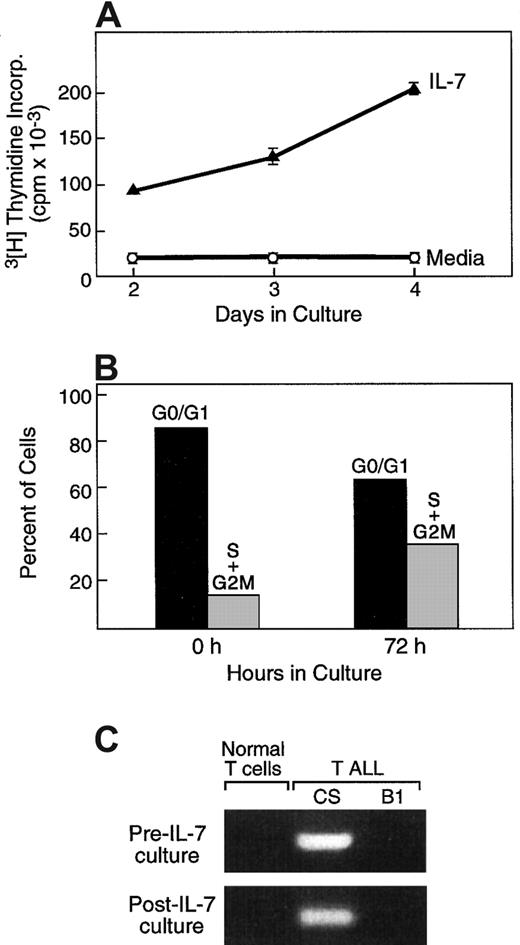
As mentioned above, besides promotion of viability, IL-7/IL-7R–mediated signals have a critical role in cell expansion at early stages of T-cell differentiation.5,6 18 To determine whether, besides increased survival, IL-7 might mediate clonal expansion of primary T-ALL cells, we examined DNA synthesis following culture of leukemic cells with either media or with IL-7. As shown in Figure 2A for one representative case, in 14 of 21 tested patients, addition of IL-7 resulted in increased DNA synthesis compared with media alone, as determined by assessment of3H-thymidine incorporation at various time intervals of culture. The mean value of the proliferation index among these 14 patients was 17.3 and the median value was 8.5 (range, 2.8-78.8). In contrast to the response of primary T-ALL cells to IL-7, no proliferation was observed when primary peripheral blood T lymphocytes from healthy volunteer donors were examined (data not shown). To examine whether increased 3H-thymidine incorporation in IL-7 cultures was only due to the higher percentage of viable T-ALL cells or, alternatively, represented cell expansion, cell cycle analysis was performed. Culture with IL-7 resulted in the increase of cells in the S and G2/M phases of the cell cycle (Figure 2B), demonstrating that IL-7 not only prevented apoptosis but also induced proliferation of primary T-ALL cells.
IL-7 promotes cell cycle progression in T-ALL cells.
(A) T-ALL cells were cultured for 72 hours in media alone or in the presence of IL-7, and proliferation was examined by thymidine incorporation. (B) At the same time interval IL-7–cultured cells were isolated, and the percentage of cells at each phase of the cell cycle was examined as described in “Materials and methods.” (C) Cells cycling in response to IL-7 are of malignant origin. After culture with IL-7, cells at the S+G2/M phases of the cell cycle were isolated by cell sorting and examined by RT-PCR by using patient-specific primers for the detection of a specific clonal TCR rearrangement identified at diagnosis. Results from one representative patient (P4) among 3 patients studied are shown. Primers used were specific for patient P4 and generated a PCR product from P4 (CS) but not from patient P2 (B1) or from normal T cells.
IL-7 promotes cell cycle progression in T-ALL cells.
(A) T-ALL cells were cultured for 72 hours in media alone or in the presence of IL-7, and proliferation was examined by thymidine incorporation. (B) At the same time interval IL-7–cultured cells were isolated, and the percentage of cells at each phase of the cell cycle was examined as described in “Materials and methods.” (C) Cells cycling in response to IL-7 are of malignant origin. After culture with IL-7, cells at the S+G2/M phases of the cell cycle were isolated by cell sorting and examined by RT-PCR by using patient-specific primers for the detection of a specific clonal TCR rearrangement identified at diagnosis. Results from one representative patient (P4) among 3 patients studied are shown. Primers used were specific for patient P4 and generated a PCR product from P4 (CS) but not from patient P2 (B1) or from normal T cells.
To confirm that proliferating cells in the in vitro cultures were of malignant origin and not of normal T cells contaminating the sample, 2 approaches were undertaken. First, cycling cells at the S+G2/M phases of the cell cycle were isolated by cell sorting and examined by PCR using patient-specific primers for the detection of a specific clonal TCR rearrangement identified at diagnosis. Such specific rearrangements were identified in the cycling cells, confirming that these cells were of malignant origin and were absent in cells from other patients or from healthy donors (Figure 2C). Second, for patients without specific rearrangements but with low or negative CD3 expression, cells at the S+G2/M phases were sorted after 72 hours of IL-7 culture, and absence of CD3 expression was assessed by flow cytometry, using propidium iodide and FITC-conjugated anti-CD3. This immunophenotypic finding of the cells undergoing cell cycle progression during culture with IL-7 was identical to that of fresh leukemic cells at the time of diagnosis (data not shown). Therefore, both approaches determined that the proliferating cells during in vitro culture with IL-7 were of T-ALL origin.
IL-7 mediates up-regulation of cyclins and activation of cdks in T-ALL cells
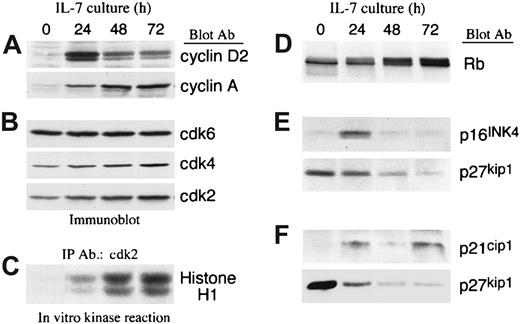
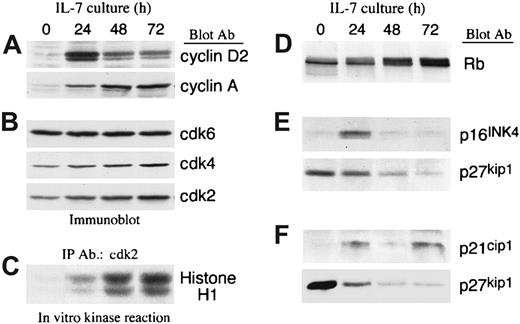
The transition from the G0/G1 to the S phase of the cell cycle is affected by exogenous factors and is positively regulated by cyclin/cdk complexes, which phosphorylate various intracellular substrates including Rb.19 Inactivation of Rb because of its phosphorylation results in release of E2F transcription factors, thereby allowing them to initiate DNA binding and transcription of S-phase genes.20-22 To determine the molecular mechanism(s) by which IL-7 mediated cell cycle progression of T-ALL cells, we examined the expression of cyclins, the expression and activation of cdks, and the expression of cdk inhibitors. IL-7 led to up-regulation of cyclin D2 and cyclin A in a sequential manner. Cyclin D2, which is an early G1 cyclin, peaked at 24 hours of culture, whereas cyclin A, which is an S-phase cyclin, was maximal at later time points of culture (Figure 3A).
IL-7 mediates up-regulation of cyclins and activates cyclin-dependent kinases due to down-regulation of cyclin dependent kinase inhibitor p27kip1 in T-ALL cells.
(A,B) T-ALL cells were cultured with IL-7 for the indicated time intervals, lysates were analyzed by SDS-PAGE and immunoblotted with an antibody specific for cyclin D2. Blots were stripped and reprobed sequentially with antibodies specific for cyclin A, cdk6, cdk4, and cdk2. Representative results from 1 among 4 patients studied are shown. (C) From the same samples immunoprecipitations were performed with anti-cdk2–specific antiserum agarose conjugate and in vitro kinase reactions were done by using Histone H1 as exogenous substrate. Reactions were analyzed by 10% SDS-PAGE, transferred to PVDF membrane and exposed to x-ray film. (D) Lysates from the same samples were analyzed by 6% SDS-PAGE, transferred on nitrocellulose membrane, and immunoblotted with mAb specific for Rb. (E,F) Samples from 2 individual patients were cultured for various time intervals in the presence of IL-7 and examined for the expression of p16INK4, p21cip1, and p27kip1 by immunoblot. Representative results from 2 among 6 patients studied are shown.
IL-7 mediates up-regulation of cyclins and activates cyclin-dependent kinases due to down-regulation of cyclin dependent kinase inhibitor p27kip1 in T-ALL cells.
(A,B) T-ALL cells were cultured with IL-7 for the indicated time intervals, lysates were analyzed by SDS-PAGE and immunoblotted with an antibody specific for cyclin D2. Blots were stripped and reprobed sequentially with antibodies specific for cyclin A, cdk6, cdk4, and cdk2. Representative results from 1 among 4 patients studied are shown. (C) From the same samples immunoprecipitations were performed with anti-cdk2–specific antiserum agarose conjugate and in vitro kinase reactions were done by using Histone H1 as exogenous substrate. Reactions were analyzed by 10% SDS-PAGE, transferred to PVDF membrane and exposed to x-ray film. (D) Lysates from the same samples were analyzed by 6% SDS-PAGE, transferred on nitrocellulose membrane, and immunoblotted with mAb specific for Rb. (E,F) Samples from 2 individual patients were cultured for various time intervals in the presence of IL-7 and examined for the expression of p16INK4, p21cip1, and p27kip1 by immunoblot. Representative results from 2 among 6 patients studied are shown.
IL-7 slightly increased expression of cdk2 and cdk4 and had no effect on expression of cdk6 (Figure 3B). Importantly, in contrast to the minimal effect on the expression of these cdks, IL-7 induced a striking activation of cdk4 (data not shown) and cdk2 (Figure 3C) as determined by in vitro kinase reaction. To determine the in vivo significance of these findings we examined the phosphorylation status of endogenous Rb, which is one of the most critical substrates of the enzymatic activity of cdks in vivo. Culture with IL-7 resulted in hyperphosphorylation of Rb (Figure 3D), indicating that IL-7–induced cdk activation occurred in vivo.
IL-7 activates cdk2 and promotes cell cycle progression in T-ALL cells because of down-regulation of cdk inhibitor p27kip1
The enzymatic activation of cdks is regulated by cdk inhibitors, which include members of the kip/cip and the INK family. Because IL-7 induced a dramatic increase in the enzymatic activity of the cdks, despite the minimal effect in their protein expression, we examined whether IL-7 influenced the expression of cdk inhibitors. Among these cdk inhibitors, p21cip1, p16INK4a, and p27kip1 were detected. p27kip1 was detected in all patients studied (6 of 6). In contrast, p16INK4a was detected only in 1 patient and p21cip1 was detected in 3 patients. When p16INK4a and p21cip1 were detectable, they were up-regulated during culture with IL-7 (Figure3E,F). In contrast, p27kip1 was significantly down-regulated during IL-7 culture in all cases (Figure 3E,F).
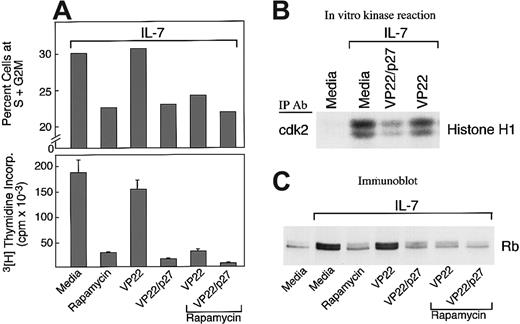
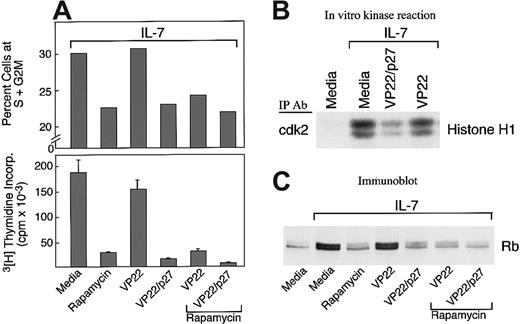
To determine whether down-regulation of p27kip1 had a causative role in IL-7–mediated cell cycle progression, 2 approaches were undertaken: First, T-ALL cells were cultured with IL-7 in the presence of rapamycin, which prevents down-regulation of endogenous p27kip1.23,24 Second, T-ALL cells were cultured with IL-7 in the presence of VP22/p27kip1 fusion protein, which is capable of translocating recombinant p27kip1 into the nucleus of cocultured cells, thereby leading to forced sustained expression of p27kip1.16 As shown in Figure4A, rapamycin, VP22/p27kip1fusion protein, or their combination significantly inhibited IL-7–mediated cell cycle progression and cellular proliferation. Moreover, VP22/p27kip1 fusion protein, but not VP22 control protein, inhibited IL-7–induced cdk2 activation (Figure 4B). Consistent with the effects of rapamycin and VP22/p27kip1on cell cycle progression and cellular proliferation (Figure 4A) and the effect of VP22/p27kip1 on cdk2 activation (Figure 4B), analysis of the phosphorylation status of endogenous Rb revealed that rapamycin, VP22/p27kip1, or their combination reversed hyperphosphorylation of Rb induced by IL-7 (Figure 4C). Thus, sustained increased expression of p27kip1 prevents IL-7–mediated cdk2 activation, DNA synthesis, and progression through the cell cycle. These results strongly indicate that down-regulation of p27kip1 has a causative role in IL-7–mediated activation of cdk2, resulting in subsequent hyperphosphorylation of Rb and cell cycle progression.
Effects of rapamycin and p27kip1.
(A) Rapamycin and forced expression of p27kip1 by VP22/p27kip1 fusion protein prevent IL-7–mediated increase of T-ALL cells in the S+G2M phase and DNA synthesis. T-ALL cells were cultured with IL-7 either alone or in the presence of rapamycin, VP22/p27kip1 fusion protein, VP22 control protein, or the indicated combinations. Cultures were examined for percentage of cells at the S+G2M phases of the cell cycle and for DNA synthesis by thymidine incorporation as described in Materials and methods. Representative results from 1 among 3 patients studied are shown. (B) VP22/p27kip1 prevents IL-7–mediated activation of cdk2. T-ALL cells were cultured for 72 hours under the indicated conditions, and activation of cdk2 was determined in cell lysates by in vitro kinase reactions by using Histone H1 as exogenous substrate. Reactions were analyzed by SDS-PAGE, transferred to PVDF membrane, and exposed to x-ray film. (C) Rapamycin, VP22/p27kip1, and their combination prevent IL-7–mediated phosphorylation of Rb. T-ALL cells were cultured for 72 hours under the indicated conditions, analyzed by 6% SDS-PAGE, transferred on nitrocellulose membrane, and immunoblotted with mAb specific for Rb.
Effects of rapamycin and p27kip1.
(A) Rapamycin and forced expression of p27kip1 by VP22/p27kip1 fusion protein prevent IL-7–mediated increase of T-ALL cells in the S+G2M phase and DNA synthesis. T-ALL cells were cultured with IL-7 either alone or in the presence of rapamycin, VP22/p27kip1 fusion protein, VP22 control protein, or the indicated combinations. Cultures were examined for percentage of cells at the S+G2M phases of the cell cycle and for DNA synthesis by thymidine incorporation as described in Materials and methods. Representative results from 1 among 3 patients studied are shown. (B) VP22/p27kip1 prevents IL-7–mediated activation of cdk2. T-ALL cells were cultured for 72 hours under the indicated conditions, and activation of cdk2 was determined in cell lysates by in vitro kinase reactions by using Histone H1 as exogenous substrate. Reactions were analyzed by SDS-PAGE, transferred to PVDF membrane, and exposed to x-ray film. (C) Rapamycin, VP22/p27kip1, and their combination prevent IL-7–mediated phosphorylation of Rb. T-ALL cells were cultured for 72 hours under the indicated conditions, analyzed by 6% SDS-PAGE, transferred on nitrocellulose membrane, and immunoblotted with mAb specific for Rb.
Down-regulation of p27kip1 is mandatory for induction of bcl-2 expression and promotion of viability by IL-7 in T-ALL cells
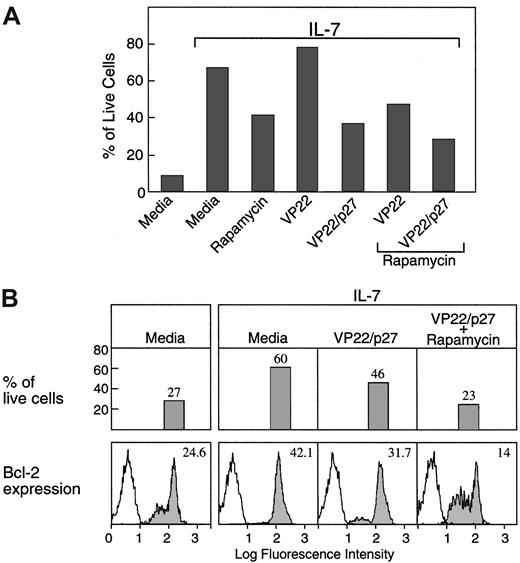
Propidium iodide staining for analysis of cell cycle progression revealed that addition of rapamycin, VP22/p27kip1, or their combination not only diminished the ability of IL-7 to induce cell cycle progression but also diminished the ability of IL-7 to promote survival of T-ALL cells during culture (Figure5A).
Down-regulation of p27kip1 is mandatory for induction of bcl-2 expression and promotion of viability by IL-7 in T-ALL cells.
(A) T-ALL cells were cultured for 72 hours under the indicated conditions, and the percentage of viable cells was examined by propidium iodide staining followed by FACS analysis as described in Materials and methods. (B) T-ALL cells isolated from the indicated culture conditions were examined for viability as above and for expression of bcl-2 protein levels by intracellular staining and by FACS analysis, as described in Materials and methods.
Down-regulation of p27kip1 is mandatory for induction of bcl-2 expression and promotion of viability by IL-7 in T-ALL cells.
(A) T-ALL cells were cultured for 72 hours under the indicated conditions, and the percentage of viable cells was examined by propidium iodide staining followed by FACS analysis as described in Materials and methods. (B) T-ALL cells isolated from the indicated culture conditions were examined for viability as above and for expression of bcl-2 protein levels by intracellular staining and by FACS analysis, as described in Materials and methods.
Because IL-7 promoted viability of T-ALL cells by up-regulating expression of bcl-2, we examined whether forced sustained expression of p27kip1, which diminished the ability of IL-7 to promote survival of T-ALL cells, affected expression of bcl-2. As shown in Figure 5B, VP22/p27kip1 alone or in combination with rapamycin inhibited IL-7–induced up-regulation of bcl-2 protein expression as determined by flow cytometry. These results show that down-regulation of p27kip1 is mandatory not only for cell cycle progression but also for up-regulation of bcl-2 expression and promotion of viability of T-ALL cells in response to IL-7.
Discussion
IL-7 was initially identified and cloned on the basis of its ability to induce proliferation of B-cell precursors in the absence of stromal cells.25 However, IL-7 is also expressed in the thymus and has been shown to stimulate the growth of immature double-negative and mature single-positive thymocytes in thymic organ cultures.6,25-27 In addition, IL-7/IL-7R signaling plays a critical role in the maintenance of the peripheral T-cell pool, because the few peripheral T cells seen in the IL-7R−/− mice show impaired response to both TCR-dependent and TCR-independent stimuli because of decreased frequency and clonogenicity of T cells.2,18 In humans, defective IL-7R expression results in T−B+natural killer+ severe combined immunodeficiency.28 29 These data implicate that IL-7 plays a fundamental role at most stages of T-cell development.
Expression of IL-7R during T-cell ontogeny coincides with the expression of bcl-2,30 and the lack of IL-7 signaling results in reduction of endogenous bcl-2 levels both in thymocytes and in mature T cells.18 Strikingly, the impaired functions of IL7R−/− T cells are normalized by the introduction of bcl-2 transgene.3,4 However, although the bcl-2 transgene rescued T cells from the defects of IL-7 deficiency and allowed proliferative response to T-cell mitogenic signals, bcl-2 itself does not stimulate proliferation and cannot substitute for signals generated during thymic selection driven by TCR/major histocompatibility complex interaction.4 Furthermore, expression of bcl-2 inhibits proliferation of stimulated T cells.31-34 Therefore, although the IL-7/IL-7R system plays a critical role in the maintenance of bcl-2 levels in developing thymocytes and peripheral T cells, the mechanism by which IL-7 induces T-cell proliferation is not mediated by bcl-2. Our present study shows that IL-7 directly mediates cell cycle progression and proliferation on T-ALL cells by down-regulating the cdk inhibitor p27kip1. p27kip1 regulates the G1-S transition by stoichiometric inhibition of cyclin E-cdk2 holoenzyme. p27kip1 also binds to cyclin D/cdk4 and cyclin D/cdk6 complexes and inhibits their activity, whereas, in actively proliferating cells, p27kip1 is sequestered by cyclin D-cdk4 complexes. This interaction helps ensure that D-type cyclins and E-type cyclins are sequentially activated in the G1 phase.35
Our present study shows that IL-7 mediates not only up-regulation of bcl-2 and increased survival but also cell cycle progression and proliferation of T-ALL cells. The key step required for the induction of both increased viability and cell cycle progression by IL-7 is the down-regulation of p27kip1 cdk inhibitor. Down-regulation of p27kip1 temporally coincided with a dramatic increase in the enzymatic activity of cdk4 and cdk2, hyperphosphorylation of Rb, and progression to the S phase of the cell cycle. Down-regulation of p27kip1 was mandatory for IL-7–mediated cell cycle progression, because rapamycin, which prevents p27kip1down-regulation and forced expression of p27kip1 in T-ALL cells, inhibited IL-7–mediated cdk2 activation, Rb hyperphosphorylation, and cell cycle progression. Moreover, rapamycin and forced expression of p27kip1 also significantly diminished the effect of IL-7 on the promotion of viability by preventing IL-7–mediated up-regulation of bcl-2, suggesting that IL-7–mediated survival is achieved, at least in part, through down-regulation of p27kip1.
Extensive studies during the past few years provided compelling evidence that p27kip1 has a critical role in carcinogenesis, because p27kip1-deficient and hemizygote mice develop spontaneous tumors.36 Moreover, in human tumors the levels of p27kip1 protein strongly correlate with prognosis and are controlled by posttranscriptional mechanisms that regulate protein expression in the absence of p27kip1gene mutation.37,38 Our present studies show that IL-7 induced proliferation of T-ALL cells by down-regulating p27kip1 protein levels and support the notion that p27kip1 might function as a tumor suppressor gene not only because it works as a negative regulator of cell cycle progression but also because it is associated with induction of apoptosis. Studies on IL-7 transgenic mice have shown that IL-7 perturbs thymic T-cell development and leads to the absence of CD3−CD4+CD8+ double-positive thymocytes, resulting in a remarkable increase in the percentage of single-positive CD3+CD4+ or CD3+CD8+ thymocytes, a phenotype very rare among normal thymocytes. Moreover, IL-7 transgene promotes malignant transformation of lymphocytes, resulting in the development of lymphomas.1 On the basis of these findings it has been suggested that, as a biologic effector, IL-7 not only perturbs the development of lymphoid cells but also promotes the growth of such populations at risk for malignant transformation.1Importantly, IL-7 is produced by bone marrow and thymic stroma.25 Therefore, it is present in the microenvironments in which the malignant T cells develop and may play an important role in the acquisition of selective growth advantage of the leukemic cells.
Although the molecular pathways of cytokine signaling and their influence on viability and malignant transformation are poorly understood, the early signaling events triggered by engagement of the IL-7Rs are directly linked to activation of protein tyrosine kinase activity, protein tyrosine phosphorylation, activation of phosphatidylinositol 3-kinase (PI-3K), and inositol phospholipid turnover in human fetal thymocytes and T-lineage ALL blasts.39,40 PI-3K is involved in the regulation of the Forkhead family of transcription factors,41 whereas IL-7R ligation directly activates Jak kinases and transcription factors of the STAT family, most notably STAT5.40 Although most studies have shown that p27kip1 expression is mainly regulated at the posttranscriptional level by controlling degradation of this protein,42-44 a small number of studies have proposed that p27kip1 may also be regulated at the transcriptional level.45,46 Interestingly, the later study has also implicated the increased expression of p27kip1 in the induction of cell death.46 This mechanism involves PI-3K–mediated activation of Forkhead transcription factors, which become phosphorylated and function as repressors of p27kip1transcription. However, STAT proteins regulate p27kip1expression at a posttranscriptional level, and the decrease of p27kip1 abundance correlates with the ability of cytokines to induce both progression from G1 to the S phase of the cell cycle and malignant transformation.47
There is extensive evidence that during the G1-S transition p27kip1 protein levels change dramatically because of ubiquitin-targeted degradation of p27kip1 by the 26S proteasome.43 Like other G1-S regulatory proteins, p27kip1 must undergo phosphorylation on Thr187, which lies within the cdk/mitogen-activated protein kinase (MAPK) consensus site, to be targeted by the ubiquitin ligase complex.48Cyclin/cdk holoenzymes can phosphorylate this residue, and it has been proposed that cyclin E/cdk2 performs this function in vivo.49 Other studies support a critical role of Ras and the MAPK in the phosphorylation of p27kip1.50IL-7 is known to mediate active signaling events by Jak family kinases that are linked to the IL-7R as well as by PI-3K, subsequently leading to Ras/MAPK activation.51 Therefore, IL-7 may regulate direct phosphorylation and ubiquitination of p27kip1, leading to its association with other regulatory components of the ubiquitination complex, a process required for subsequent degradation of p27kip1.
Further studies will be required to elucidate the role of each one of these signaling pathways in IL-7–mediated survival and proliferation of T-ALL cells. Such studies will determine whether these pathways are equally involved in IL-7–induced down-regulation of p27kip1 and up-regulation of bcl-2 or whether each pathway has a selective or predominant role in regulating these 2 downstream molecular events. Regardless of the mechanism, our results show for the first time that a causative link between IL-7–mediated proliferation and p27kip1 down-regulation exists in malignant T cells. More importantly, sustained increase of p27kip1 expression in T-ALL cells not only prevents their proliferation but also promotes apoptosis by preventing IL-7–mediated up-regulation of bcl-2. Further understanding of the mechanisms involved in this process could lead to improved therapeutic strategies aimed at achieving high levels of p27kip1 expression in T-ALL blasts, perhaps with a gene therapy approach or by the use of cdk inhibitors that are currently being broadly developed.52
We thank Dr John Donovan for assistance on the designing of patient-specific primers and Alla Berezovskaya and Thomas Keenan for technical support.
Supported by grants AI 43552, HL 54785, AI 41584, and CA 68484 from the National Institutes of Health. J.B. is supported by a grant from Programa PRAXIS XXI, Fundacao Para a Ciencia e a Tecnologia, Portugal.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Vassiliki A. Boussiotis, Dana-Farber Cancer Institute, Mayer 547, 44 Binney St, Boston, MA 02115; e-mail:vassiliki_boussiotis@dfci.harvard.edu.