The exposure of internal glycoprotein (GP) IIb/IIIa receptors has been proposed to explain the incomplete inhibition of aggregation of thrombin receptor–activating peptide (TRAP)-stimulated platelets by abciximab. However, a marked and rapid externalization of GPIIb/IIIa was also observed upon stimulation with 30 μM adenosine diphosphate (ADP). ADP-induced fibrinogen binding was completely inhibited by 10 μg/mL abciximab, 30 nM tirofiban, or 3 μg/mL eptifibatide, while fibrinogen binding induced by 100 μM TRAP was inhibited only by 50%. Interestingly, striking differences in fibrinogen binding kinetics in ADP- versus TRAP-stimulated platelets were observed. ADP-induced fibrinogen binding was much slower than that of abciximab. These differences in the fibrinogen binding rate were due to differential GPIIb/IIIa activation kinetics because the actual fibrinogen binding rate (measured by adding fibrinogen after platelet activation) was similar in ADP- and TRAP-stimulated platelets. Thus, the TRAP-induced GPIIb/IIIa activation rate would allow significant amounts of fibrinogen to occupy externalized GPIIb/IIIa receptors even in the presence of the inhibitor.
Introduction
Abciximab inhibits fibrinogen binding to activated glycoprotein (GP) IIb/IIIa receptors.1 At standard therapy regimens, a largely complete (≥ 90%) blockade of GPIIb/IIIa receptors is maintained during the infusion of the drug.2 Although interpatient variation in response to abciximab is recognized,3 GPIIb/IIIa receptor blockade usually results in a complete inhibition of adenosine diphosphate (ADP)-induced platelet aggregation. However, when platelets, isolated from patients during abciximab infusion, are stimulated with strong agonists such as thrombin or thrombin receptor–activating peptide (TRAP), platelet aggregation is only partially inhibited.3,4 It has been proposed that, despite internalization of abciximab into the internal pool of GPIIb/IIIa,5 a significant amount of unblocked internal GPIIb/IIIa receptors becomes exposed on the platelet surface upon stimulation with strong platelet agonists and these receptors may mediate platelet aggregation. However, when platelets are stimulated with ADP, α-granule proteins such as CD62P are usually externalized.6 One would therefore expect that ADP stimulation would also result in GPIIb/IIIa externalization. Thus, we sought alternative explanations for the differential inhibition of platelet fibrinogen binding by abciximab in platelets stimulated with weak versus strong agonists.
Study design
Preparation of platelets
Platelet-rich plasma was prepared from citrated (1:7) blood by centrifugation at 1000g for 45 seconds. Platelets were pelleted by centrifugation at 1200g for 30 seconds and resuspended in HEPES-Tyrode buffer (134 mM NaCl, 12 mM NaHCO3, 2.9 mM KCl, 2 mM CaCl2, 0.36 mM NaH2PO4, 1 mM MgCl2, 5 mM HEPES, 5 mM glucose, 0.5 mg/mL bovine serum albumin, pH 7.4).
GPIIb/IIIa receptor expression
Washed platelets (30 × 109/L) were stimulated with 30 μM ADP (Sigma, Deisenhofen, Germany) or 100 μM TRAP (SFLLRN, Bachem, Bubendorf, Switzerland) at 37°C. At different time points after stimulation, cells were fixed with paraformaldehyde (1%) for 10 minutes followed by incubation with saturating concentrations of anti-CD41–phycoerythrin, anti-CD61–fluorescein isothiocyanate, or the respective isotypic control antibodies (Coulter-Immunotech, Marseille, France) for 15 minutes. Then, samples were diluted with Isotone and analyzed on an Epics-XL cytometer (Beckman Coulter, Krefeld, Germany). The platelet population was identified on its forward and side scatter distribution, which was validated by staining with anti-CD42b antibodies (Dako, Hamburg, Germany). Detectors were set to logarithmic amplification, and 30 000 platelets were analyzed using the System II software. In additional experiments, platelet-rich plasma was incubated with 10 μg/mL abciximab (ReoPro, Centocor, Leiden, The Netherlands) for 15 minutes at 37°C. Washed platelets were isolated and resuspended in abciximab-free buffer prior to stimulation and flow cytometry.
Fibrinogen binding
Fibrinogen binding was measured in 30 × 109/L washed platelets using Oregon Green–labeled fibrinogen (Molecular Probes, Eugene, OR). In preliminary studies, binding specificity was verified by competition experiments with unlabeled fibrinogen (Sigma). For the measurement of fibrinogen binding, Oregon Green–labeled fibrinogen (final concentration 0.5 mg/mL) mixed with unlabeled fibrinogen (final concentration 3 mg/mL) to achieve physiologic fibrinogen concentrations was added prior to platelet stimulation. The reaction was stopped at different time points by dilution (1:200) with Isotone. Fibrinogen binding was immediately quantified by flow cytometry. To measure the actual fibrinogen binding rate (independently of GPIIb/IIIa externalization/activation kinetics), platelets were first stimulated for 15 minutes followed by addition of Oregon Green–labeled fibrinogen and flow cytometry.
Abciximab binding
Abciximab was biotinylated using the FluoReporter Mini-Biotin-XX Protein Labeling Kit (Molecular Probes) according to the manufacturer's instructions. Functional activity of biotinylated abciximab was verified by inhibition of anti-CD41 binding. Biotinylated abciximab inhibited anti-CD41 binding with the same potency as did native abciximab (data not shown). For the measurement of abciximab binding rate, 150 × 109/L (150 000/μL) washed platelets were incubated with 3 μg/mL biotinylated abciximab. At different time points, the reaction was stopped by dilution (1:200) with Isotone. Immediately after dilution, platelets were incubated with saturating concentrations of streptavidin-phycoerythrin for 10 minutes, and abciximab binding was measured by flow cytometry.
Results and discussion
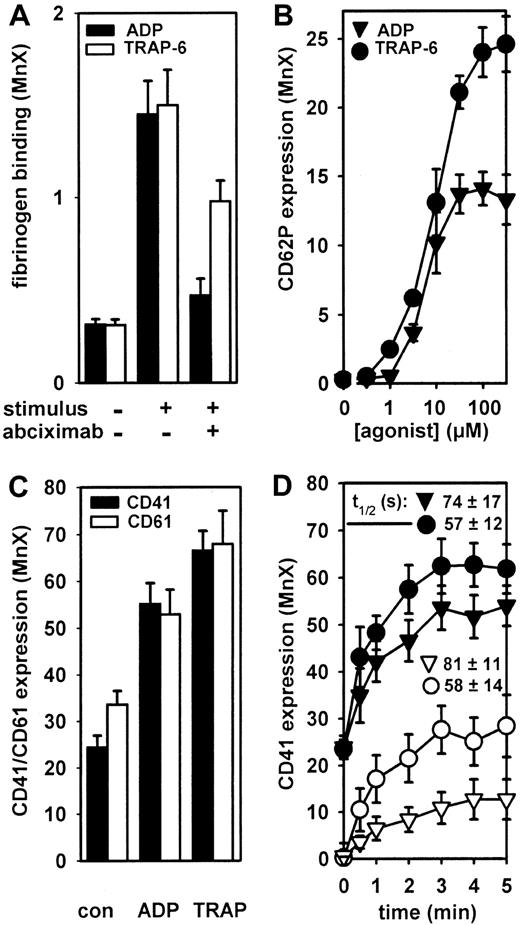
ADP-induced fibrinogen binding was concentration-dependently inhibited by abciximab. A complete inhibition was seen with 10 μg/mL abciximab (Figure 1A). In contrast, at the same abciximab concentration, only a partial (about 50%) inhibition was seen when TRAP was used to stimulate fibrinogen binding (Figure 1A). Only a partial (about 50%) inhibition of TRAP-induced fibrinogen binding was also observed with 30 nM tirofiban or 3 μg/mL eptifibatide (data not shown).
Complete inhibition of ADP-induced fibrinogen binding by abciximab despite GPIIb/IIIa receptor externalization.
(A) Fibrinogen binding induced by 30 μM ADP and 100 μM TRAP was measured in washed platelets 10 minutes after stimulation. Abciximab (10 μg/mL) was preincubated for 15 minutes prior to stimulation. (B) Washed platelets were stimulated with 0.3 to 300 μM ADP and 0.3 to 300 μM TRAP for 15 minutes, and surface expression of CD62P was measured. (C) Washed platelets were stimulated with 30 μM ADP and 100 μM TRAP for 15 minutes, and the expression levels of CD41 and CD61 were measured. (D) Washed platelets were stimulated with 30 μM ADP (▾) and 100 μM TRAP (●) for different time periods, and the expression of CD41 was measured. The time course of anti-CD41 binding was also measured in washed platelets pretreated for 10 minutes with 10 μg/mL abciximab after stimulation with 30 μM ADP (▿) and 100 μM TRAP (○). To obtain relative GPIIb/IIIa externalization rates, data from panel C were transformed to relative increased values (maximal expression at 5 minutes, 100%) and the externalization half-times (t1/2) were deduced from each individual experiment. Data are means ± SEM from 4 to 6 independent experiments. The externalization half-times were not significantly different (P > .05, ANOVA-Bonferroni).
Complete inhibition of ADP-induced fibrinogen binding by abciximab despite GPIIb/IIIa receptor externalization.
(A) Fibrinogen binding induced by 30 μM ADP and 100 μM TRAP was measured in washed platelets 10 minutes after stimulation. Abciximab (10 μg/mL) was preincubated for 15 minutes prior to stimulation. (B) Washed platelets were stimulated with 0.3 to 300 μM ADP and 0.3 to 300 μM TRAP for 15 minutes, and surface expression of CD62P was measured. (C) Washed platelets were stimulated with 30 μM ADP and 100 μM TRAP for 15 minutes, and the expression levels of CD41 and CD61 were measured. (D) Washed platelets were stimulated with 30 μM ADP (▾) and 100 μM TRAP (●) for different time periods, and the expression of CD41 was measured. The time course of anti-CD41 binding was also measured in washed platelets pretreated for 10 minutes with 10 μg/mL abciximab after stimulation with 30 μM ADP (▿) and 100 μM TRAP (○). To obtain relative GPIIb/IIIa externalization rates, data from panel C were transformed to relative increased values (maximal expression at 5 minutes, 100%) and the externalization half-times (t1/2) were deduced from each individual experiment. Data are means ± SEM from 4 to 6 independent experiments. The externalization half-times were not significantly different (P > .05, ANOVA-Bonferroni).
Interestingly, both TRAP and ADP significantly stimulated the externalization of CD62P (Figure 1B). Consistently, externalization of GPIIb/IIIa receptors was also observed with both agonists (Figure 1C). Although the relative velocity of ADP-induced GPIIb/IIIa externalization appears to be somewhat slower than with TRAP, this difference was not statistically significant (Figure 1D).
To verify that the observed increases in CD41 fluorescence intensity were due to GPIIb/IIIa externalization, additional experiments were carried out with platelets pretreated with 10 μg/mL abciximab. In these platelets, all surface GPIIb/IIIa receptors were blocked by abciximab because, after washing, anti-CD41 binding was completeley inhibited (Figure 1C). When these platelets were stimulated with ADP or TRAP, anti-CD41 binding increased in a time-dependent manner, indicating externalization of unblocked GPIIb/IIIa receptors (Figure1C).
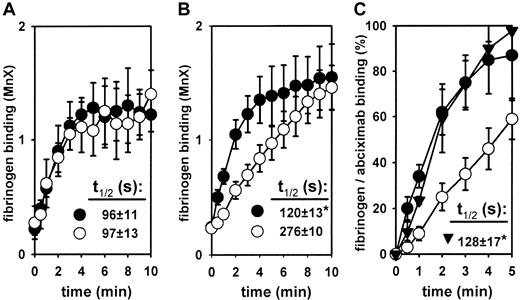
These data suggest that GPIIb/IIIa externalization per se cannot explain the differential inhibition of fibrinogen binding by GPIIb/IIIa antagonists in ADP- versus TRAP-stimulated platelets. In the study of Gawaz et al,4 only an incomplete inhibition of TRAP-induced platelet aggregation was seen even immediately after an abciximab bolus. At this time, abciximab concentrations of about 1 to 3 μg/mL are usually achieved.2 At these concentrations, abciximab almost completely blocks GPIIb/IIIa receptors. For example, at 3 μg/mL abciximab, anti-CD41 binding was reduced by 88% ± 3% (at 1000 × 109/L platelets), by 97% ±1% (at 300 × 109/L platelets), and by 99% ± 1% (at 100 × 109/L platelets). Thus, at therapeutic plasma concentrations, abciximab should be able to block externalized GPIIb/IIIa receptors even at supraphysiologic platelet counts. However, because GPIIb/IIIa externalization is a very rapid process, the kinetics of abciximab versus fibrinogen binding should determine the inhibition of externalized GPIIb/IIIa receptors. We have therefore measured fibrinogen binding kinetics upon stimulation with ADP and TRAP, respectively. First, the fibrinogen binding rate was determined after addition of fluorescent fibrinogen to platelets stimulated for 15 minutes in the absence of fibrinogen (Figure2A). In these experiments, no agonist-dependent differences in fibrinogen binding kinetics were observed. In a second series of experiments, the fibrinogen binding rate was measured in platelets stimulated in the presence of fibrinogen, mimicking the physiologic way of fibrinogen binding. In these experiments, striking differences were observed depending on the agonist used. The TRAP-induced fibrinogen binding rate was much higher as compared with ADP (Figure 2B). At 10 minutes, however, the same degree of fibrinogen binding was achieved with both stimuli.
Fibrinogen binding kinetics in ADP- versus TRAP-stimulated platelets as compared with the abciximab binding rate.
(A) The time course of fibrinogen binding induced by 30 μM ADP (○) and 100 μM TRAP (●) was measured in washed platelets stimulated with the agonists for 15 minutes at 37°C in the absence of fibrinogen, followed by addition of labeled fibrinogen. (B) The time course of fibrinogen binding induced by 30 μM ADP (○) and 100 μM TRAP (●) was measured in washed platelets in the presence of labeled fibrinogen. (C) The abciximab (3 μg/mL) (▴) binding rate was measured in washed platelets. To superimpose abciximab and fibrinogen binding kinetics in platelets stimulated by 30 μM ADP (○) and 100 μM TRAP (●), the results are presented as a percentage of maximal binding (100% fibrinogen binding = binding at 10 minutes of stimulation with TRAP; 100% abciximab binding = binding at 10 minutes of incubation time). Data are means ± SEM from 4 to 6 independent experiments. Externalization and binding half-times (t1/2) were deduced from each individual experiment. *P < .05 versus the ADP-stimulated fibrinogen binding rate (P < .05, ANOVA-Bonferroni).
Fibrinogen binding kinetics in ADP- versus TRAP-stimulated platelets as compared with the abciximab binding rate.
(A) The time course of fibrinogen binding induced by 30 μM ADP (○) and 100 μM TRAP (●) was measured in washed platelets stimulated with the agonists for 15 minutes at 37°C in the absence of fibrinogen, followed by addition of labeled fibrinogen. (B) The time course of fibrinogen binding induced by 30 μM ADP (○) and 100 μM TRAP (●) was measured in washed platelets in the presence of labeled fibrinogen. (C) The abciximab (3 μg/mL) (▴) binding rate was measured in washed platelets. To superimpose abciximab and fibrinogen binding kinetics in platelets stimulated by 30 μM ADP (○) and 100 μM TRAP (●), the results are presented as a percentage of maximal binding (100% fibrinogen binding = binding at 10 minutes of stimulation with TRAP; 100% abciximab binding = binding at 10 minutes of incubation time). Data are means ± SEM from 4 to 6 independent experiments. Externalization and binding half-times (t1/2) were deduced from each individual experiment. *P < .05 versus the ADP-stimulated fibrinogen binding rate (P < .05, ANOVA-Bonferroni).
Thus, the initial binding rate of abciximab should critically determine its inhibitory effects on fibrinogen binding. The measurement of abciximab binding revealed a binding rate that was significantly higher than the ADP-induced fibrinogen binding rate (Figure 2B). Thus, upon stimulation with ADP, abciximab should be able to block externalized GPIIb/IIIa receptors before fibrinogen would bind. In contrast, the TRAP-induced fibrinogen binding rate was comparable to the binding rate of abciximab and would allow significant amounts of fibrinogen to occupy externalized GPIIb/IIIa receptors prior to their blockade by abciximab.
Taken together, although rapid GPIIb/IIIa externalization does occur with both weak and strong platelet stimuli, differences in GPIIb/IIIa activation kinetics, resulting in a differential fibrinogen binding rate, may provide an additional explanation for the differential inhibition of ADP- versus TRAP-stimulted fibrinogen binding by GPIIb/IIIa inhibitors.
The authors thank Thomas Hohlfeld for helpful suggestions, Kerstin Freidel for excellent technical assistance, and Erika Lohmann for competent secretarial help.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Karsten Schrör, Institut für Pharmakologie und Klinische Pharmakologie, Heinrich-Heine-Universität, Moorenstr. 5, D-40225 Düsseldorf, Germany; e-mail: kschroer@uni-duesseldorf.de.